Abstract
Purpose
To compare pathologic and healthy tendons using shear-wave elastography (SWE).
Methods
A systematic review with meta-analysis was done searching Pubmed and EMBASE up to September 2022. Prospective, retrospective and cross-sectional studies that used SWE in the assessment of pathologic tendons versus control were included. Our primary outcome were SWE velocity (m/s) and stiffness (kPa). Methodological quality was assessed by the methodological index for non-randomized studies (MINORS). We used the mean difference (MD) with corresponding 95% confidence intervals (CIs) to quantify effects between groups. We performed sensitivity analysis in case of high heterogeneity, after excluding poor quality studies according to MINORS assessment. We used Grades of Recommendation, Assessment, Development and Evaluation to evaluate the certainty of evidence (CoE).
Results
Overall, 16 studies with 676 pathologic tendons (188 Achilles, 142 patellar, 96 supraspinatus, 250 mixed) and 723 control tendons (484 healthy; 239 contralateral tendon) were included. Five studies (31.3%) were judged as poor methodological quality. Shear-wave velocity and stiffness meta-analyses showed high heterogeneity. According to a sensitivity analysis, pathologic tendons had a lower shear wave velocity (MD of − 1.69 m/s; 95% CI 1.85; − 1.52; n = 274; I2 50%) compared to healthy tendons with very low CoE. Sensitivity analysis on stiffness still showed high heterogeneity.
Conclusion
Pathological tendons may have reduced SWE velocity compared to controls, but the evidence is very uncertain. Future robust high-quality longitudinal studies and clear technical indications on the use of this tool are needed.
Protocol
PROSPERO identifier: CRD42023405410
Clinical relevance statement
SWE is a relatively recent modality that may increase sensitivity and diagnostic accuracy of conventional ultrasound imaging promoting early detection of tendinopathy. Non-negligible heterogeneity has been observed in included studies, so our findings may encourage the conduct of future high-quality longitudinal studies which can provide clear technical indications on the use of this promising tool in tendon imaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tendinopathies are multifactorial disorders affecting tendons, mainly associated to overuse, which result in pain, reduced function and exercise tolerance [1]. Tendinopathy is quite common, with increasing prevalence worldwide [2]. Diagnosis is based on clinical and imaging evaluation, and early recognition is important to minimize patient pain and disability [3]. Ultrasonography is the gold standard technique to image tendons [4,5,6,7], while conventional radiography and magnetic resonance may be helpful to rule out other causes of pain [8,9,10,11,12,13,14]. Shear-wave elastography (SWE) is an ultrasound technique that allows measuring the elasticity properties of tissues, by assessing the displacement of particles after an acoustic beam is transmitted from an ultrasound transducer [15, 16]. Despite SWE has been introduced first to evaluate other tissues (liver, breast, kidney, etc.) [17,18,19,20], several authors have investigated the potential of SWE in musculoskeletal disorders. For what concerns tendinopathies, interesting studies have been focused on the value of SWE, particularly about the evaluation of the supraspinatus, Achilles, and patellar tendons. As a matter of fact, tendons present a characteristic elasticity coefficient that is related to their structure and mechanical properties. It can be impacted by degenerative modifications, including an increased concentration of collagen type III fibers and fibrocartilaginous changes, due to microtrauma, overload, and vascular alterations, which result in different elasticity patterns and decreased tendon stiffness [21]. In this setting, SWE may support B-mode ultrasound to detect tendon disease and may be used as an imaging biomarker to assess treatment response of tendinopathies. To date, evidence supports the use of sonoelastography as a first-choice imaging modality in Achilles tendinopathy, medial and lateral epicondylitis [4]. Nevertheless, published results about the application of SWE in tendinopathies are still sparse and no clear SWE stiffness and velocity cut-off values have been established to differentiate pathologic and normal tendons. Hence, this systematic review and meta-analysis was performed aiming to firstly investigate differences in pathologic and healthy tendons’ characteristics assessed by SWE. Secondarily, we aim to assess intra- and inter-observer reproducibility of SWE modulus and SWE velocity in tendon evaluations.
Methods
Study design
We conducted this systematic review of the literature according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [22, 23]. The protocol was registered in the International Prospective Register of Systematic Reviews database (PROSPERO identifier: CRD42023405410). Full PRISMA and MOOSE checklists can be found in Additional file 1.
Literature search strategy
Our search was done on PubMed and Embase databases from inception to September 2022. Further, the references of included studies and reviews were thoroughly checked to find eligible publications. The keywords “tendon” or “tendinopathy” and “shear wave” were used. We limited our search strategy to English language. We screened all studies by title and abstract, then the full text of eligible studies was checked for review, as well the references to identify any additional publications to be included. The literature search was carried on by one reviewer and checked by a senior researcher, with two and 10 years’ experience, respectively.
Inclusion and exclusion criteria
We applied the following inclusion criteria: (i) involvement of human participants; (ii) studies reporting outcome data of SWE velocity (m/s) or stiffness (kPa) of tendons affected by tendinopathy and normal tendons used as healthy controls (iii)Prospective, retrospective and cross-sectional studies dealing with the evaluation of tendons by means of SWE. Exclusion criteria were: (i) studies on patients subjected to surgery; (ii) studies reporting no quantitative SWE values; (iii) studies that used strain elastography; (iv) case report, case series involving < 10 subjects, narrative reviews, guidelines, consensus statements, editorials, letters, comments, or conference abstracts.
Data extraction
Data concerning these parameters were collected and analyzed:
-
(a)
General characteristics of the paper: first author, year of publication, study design;
-
(b)
study population characteristics: number of patients and controls, age, gender, and tendon status (normal vs abnormal);
-
(c)
measurement methods: position (rest, tension), SWE scan protocol and any intervention;
-
(d)
outcomes:
-
(a)
SWE modulus (kPa) and SWE velocity (m/s) of pathologic tendons and healthy tendons;
-
(b)
inter-observer reproducibility measured by intraclass correlation coefficient (ICC);
-
(c)
intra-observer reproducibility of measurements done in different sessions measured by ICC.
-
(a)
For what concerns prioritization of outcome data, we adopted the following a priori strategies:
-
when both longitudinal and axial values were reported, only longitudinal values have been considered;
-
when both baseline and after-treatment values were reported, only baseline values have been considered;
-
when measurements were done with the subject lying in different positions, only the neutral position has been considered;
-
when both proximal, mid and distal scans of tendons were reported, only mid-proximal scans have been considered;
-
when active and passive tendon measurements were reported, only active ones were considered.
-
we considered studies as cross-sectional given that we focused on a specific time point (i.e., we considered only baseline values when dealing with interventional studies).
Quality assessment
Two researchers evaluated study quality and differences were solved after discussion. The Methodological index for non-randomized studies (MINORS) was used [24] to evaluate quality of the observational studies, which was adapted to the cross-sectional observation at baseline removing item 6 and 7 that required follow assessment for judgement. Our adapted MINORS contains 10 items, and the quality of studies was scored from 0 to 20. All discrepancies were resolved through discussion. For each item, a score of 0 means that it was not described in the paper, 1 means that it was described inadequately, and 2 mean that it was described measured by adequately. Studies with inadequate control groups (i.e., contralateral tendon as controls) and missing baseline equivalence between groups were judged as proxy for poor methodological quality.
Statistical analysis
Studies characteristics were resumed in tables using the number of tendons assessed as unit of analysis [25]. If at least two papers showed the same outcome, data from each study were pooled in a meta-analysis using a random effect model [26]. We used the mean difference (MD) with 95% confidence interval (CI) in case the same measurement method was used. Statistical heterogeneity was evaluated through the I2 statistic [27]. We also performed subgroup analysis for each tendon (i.e., Achilles, patellar) with the relative estimated effect. In case of high heterogeneity (> 75%), we explored methodological issues and we carried on a sensitivity analysis after excluding poor quality studies [25]. We used Review Manager (RevMan) software version 5.3 for this meta-analysis.
Certainty of evidence
We used the five Grades of Recommendation, Assessment, Development and Evaluation (GRADE) [28] to evaluate the certainty of evidence for each study in the meta-analysis through the GRADEpro software (https://www.gradepro.org). When more than 10 studies were included in meta-analysis, we evaluated the publication bias using funnel plot [29] and Egger’s tests [30].
Results
Study selection
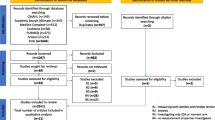
Excluding all duplicates, 292 records were screened and 233 were discarded. The text of the remaining 59 potentially eligible paper was checked, 43 of which were not in accordance with our inclusion criteria. Last, we included 16 studies (reported in Additional file 2). Figure 1 shows the flow diagram of our study selection.
General characteristics
Overall, we included 16 studies with 676 pathological tendons (188 Achilles, 142 patellar, 96 supraspinatus, 250 mixed) and 723 control tendons (484 healthy; 239 contralateral tendons). Six were prospective studies, 8 case control, 1 cross-sectional and 1 mixed retrospective and prospective published between 2015 and 2022. Mean age of subjects was 43 years, with the median percentage of males being 58% (IQR 30–86%). SWE was applied with longitudinal scan in all studies. Table 1 reports the general characteristics of included papers.
Quality assessment
Table 2 reports the quality of all included studies. The median MINORS assessment score was 17 out of 20 (IQR 16–18). Five studies (31.3%) had a poor methodological quality.
Shear modulus (kPa)
Overall, 11 studies assessed this outcome with 8 including 371 pathologic tendons and 313 contralateral/healthy tendons providing useful outcome data. Meta-analysis showed substantial heterogeneity (I2 = 99%) (Fig. 2). According to a sensitivity analysis, excluding three studies [31,32,33] with poor methodological quality, heterogeneity was still substantial (I2 = 99%;) and remained unresolved.
Meta-analysis on shear modulus. Legend: APL, abductor pollicis longus; CET, common extensor tendon; CFT, common flexor tendon; CI, confidence interval; EPB, extensor pollicis brevis; LHBT, long head of biceps tendon; SD, standard deviation. Humeral epicondylar tendons includes radial/ulnar epicondylar tendons
Shear-wave velocity (m/s)
Overall, eight studies assessed this outcome with seven including 316 pathologic tendons and 306 contralateral/healthy tendons providing useful outcome data. Meta-analysis showed substantial heterogeneity (I2 = 93%) (Fig. 3).
Meta-analysis on shear-wave velocity (m/s). Legend: APL, abductor pollicis longus; CET, common extensor tendon; CFT, common flexor tendon; CI, confidence interval; EPB, extensor pollicis brevis; LHBT, long head of biceps tendon; SD, standard deviation. Humeral epicondylar tendons includes radial/ulnar epicondylar tendons
Sensitivity analysis: shear-wave velocity (m/s)
According to a sensitivity analysis, excluding two studies [31, 34] with poor methodological quality, we pooled data from five studies including 114 pathologic tendons and 160 healthy tendons. Overall, patients with tendinopathy had a lower shear wave velocity, with a MD of − 1.69 m/s (95% CI − 1.85; − 1.52; I2 = 50%) compared to healthy tendons (Fig. 4).
Certainty of evidence
The certainty of evidence was not assessed for the primary analysis due to very substantial heterogeneity that prevent us to show overall quantitative results. For sensitivity analysis on Shear-wave velocity (m/s), the certainty of evidence was very low, due to serious imprecision ( − 1 level due to optimal information size not met, less than 400 participants). GRADE summary of findings is in supplementary Additional file 3, Table S1.
Publication bias
Funnel plots (Fig. 5) and Egger’s test (coefficient − 4.01; 95% CI − 7.64; − 0.38; p = 0.03) indicated there was presence of publication bias in Shear modulus (kPa). Publication bias for Shear-wave velocity (m/s) was not assessed (less then 10 studies).
Reproducibility
Among the selected studies, just four papers assessed the reproducibility of SWE values reporting highly heterogeneous in modalities of reporting results. Specifically, regarding intra-observer reproducibility of measurements done in different settings, Aubry et al. [35] reported ICC = 0.46 for Achilles tendon evaluation, Breda et al. [36] reported ICC = 0.95 for patellar tendon, Zhu et al. [34] reported ICC = 0.986 for common extensor tendon (CET), and Hackett et al. [37] reported ICC = 0.96 for supraspinatus tendon. Concerning inter-observer reproducibility, Breda et al. [36] reported ICC = 0.79 for patellar tendon, Zhu et al. [34] reported ICC = 0.993 for common extensor tendon, and Hackett et al. [37] reported ICC = 0.45 for supraspinatus tendon.
Discussion
The main result of our analysis is that pathologic tendons present lower SWE velocity than healthy tendons, but the effects are very uncertain due to serious inconsistency of findings (i.e., high heterogeneity, sample size less the 400 tendons) that may limit clinical application of SWE.
SWE has been introduced as a more reliable quantitative imaging modality with respect to strain elastography, given that the compressions of tissues are produced directly by the ultrasound beam provided by the probe [38]. This has been proven in other organs, but in tendons, according to our data, there is still no evidence to support such statement. Despite many papers have investigated the role of SWE for evaluating tendon, the reproducibility of SWE values still needs to be proven [39]. In fact, only four out of the 16 included studies assessed intra- and/or intra-observer reproducibility, reporting highly heterogenous findings. For this reason, data could not be meta-analyzed. To be more conservative, we preferred not pooling together data on stiffness and velocity. In fact, the equation to convert SWE velocity to the Young’s modulus is based on tissue density, but this parameter is not constant, given that tendon structure can be altered due to pathology and anisotropy making this conversion unreliable [40, 41]. For this reason, the evaluation of SWE velocity should be preferred in future studies.
Several drawbacks of SWE may partly justify the heterogeneity of published data on tendons. The elastogram is affected by tensile loads, which are related to joint angle, but also to rest, concentric-eccentric contraction and stretching. In this regard, for instance, it is recommended to evaluate Achilles tendons in relaxed or plantar flexion to not stretch the tendon, thereby avoiding a false increase of SWE velocity [42, 43]. Further, non-negligible differences in SWE velocity measurements have been reported when comparing different machines and when changing the depth of the investigated structure [44]. In this context, significant statistical differences have been noted in the quantification of elasticity based on the vendor system and the measurement depth when using SWE. Indeed, potential dissimilarities in the settings may potentially elucidate the scanner disparities. Additionally, variations might arise due to differences in the speckle tracking algorithms employed by different scanners. Nonetheless, there remains a necessity for studies that examine the reproducibility across different systems to ascertain the reliability of SWE measurements. Moreover, other factors may impact on SWE results, like the probe pressure and tilt, the interposition of a spacer, or the orientation of the probe that may affect the shear modulus [16]. Another point is where the tendon should be evaluated for estimating SWE velocity, specifically, how many and how large the regions of interest should be, and which portion of the tendon must be examined. Looking at previous papers, an extreme heterogeneity of methods can be observed, with some studies having focused on the whole thickness of the tendon, while others having used several regions of interest of different sizes. It has been shown that significantly different SWE results can be obtained applying regions of interest of different dimensions and with different time of acquisition [45]. As a matter of fact, the lack of consensus on a standard protocol to apply when using SWE in clinical and research practice limits the reliability and accuracy of this tool. Future studies and evidence-based recommendations are needed to standardize these important technical issues. Now, in clinical studies it should be essential to report detailed information about region of interest placement (location, number and dimensions), in order to apply the same methods to all subjects and to make the methods reproducible in further studies [46].
Another issue to consider when dealing with SWE is that all studies attempted to use it as a standalone method to detect tendon abnormalities, while none proposed it as an adjunct tool to complement B-mode US. Once resolved reproducibility and standardization issues, SWE might be used to supplement the B-mode examination, especially when negative but patient is still symptomatic [47].
Some limitations can be acknowledged at review level as well as study level. For the review we limited our search to English records. Then, we included different study design in order to achieve more literature however this choice has incorporated some methodological heterogeneity. For example, 5 studies (31.3% of the whole sample) used a contralateral limb as control group explaining part of heterogeneity by the sensitivity strategy. Further, we planned ICC in our protocol, but we found poor reporting of this outcome. This can be related to missing evidence as already known in literature. In addition, we did not search grey literature, and studies with negative findings may have not been retrieved as showed by both funnel plot and Egger’s test. At study level most studies have been focused just on Achilles, patellar and supraspinatus tendons, while not so many works have focused on the performance of SWE in different tissues. Hence, further studies should be carried on understanding how to apply SWE in other tendons, since each presents specific biomechanical and structural properties.
In conclusion, our meta-analysis underlines that pathological tendons may have reduced SWE velocity compared to controls, but the evidence is very uncertain. We have highlighted the low number and limited quality of studies focused on this topic, due to non-negligible differences of methods in the application of SWE and estimation of results of velocity and stiffness. SWE velocity still remains an interesting quantitative imaging tool for tendinopathies that could be helpful for diagnosis, prognosis, and assessment of treatment response, but future robust high-quality longitudinal studies and clear technical indications on the use of this tool are warranted to understand its actual role in tendon imaging.
Abbreviations
- APL:
-
Abductor pollicis longus
- CET:
-
Common extensor tendon
- CFT:
-
Common flexor tendon
- CIs:
-
Confidence intervals
- CoE:
-
Certainty of evidence
- EPB:
-
Extensor pollicis brevis
- GRADE:
-
Grades of Recommendation, Assessment, Development and Evaluation
- ICC:
-
Intraclass correlation coefficient
- LHBT:
-
Long head of biceps tendon
- MD:
-
Mean difference
- MINORS:
-
Methodological index for non-randomized studies
- MOOSE:
-
Meta-analysis of Observational Studies in Epidemiology
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RevMan:
-
Review manager
- SWE:
-
Shear-wave elastography
- US:
-
Ultrasound
References
Millar NL, Silbernagel KG, Thorborg K et al (2021) Tendinopathy. Nat Rev Dis Primers. https://doi.org/10.1038/s41572-020-00234-1
Hopkins C, Fu SC, Chua E et al (2016) Critical review on the socio-economic impact of tendinopathy. Asia Pac J Sports Med Arthrosc Rehabil Technol 4:9–20
Hubbard MJ, Hildebrand BA, Battafarano MM, Battafarano DF (2018) Common soft tissue musculoskeletal pain disorders. Prim Care Clin Off Pract 45:289–303
Sconfienza LM, Albano D, Allen G et al (2018) Clinical indications for musculoskeletal ultrasound updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) consensus. Eur Radiol 28:5338–5351. https://doi.org/10.1007/s00330-018-5474-3
Albano D, Bonifacini C, Zannoni S et al (2021) Plantar forefoot pain: ultrasound findings before and after treatment with custom-made foot orthoses. Radiol Med 126:963–970. https://doi.org/10.1007/s11547-021-01354-8
Albano D, Coppola A, Gitto S et al (2021) Imaging of calcific tendinopathy around the shoulder: usual and unusual presentations and common pitfalls. Radiol Med 126:608–619. https://doi.org/10.1007/s11547-020-01300-0
Gitto S, Messina C, Chianca V et al (2020) Superb microvascular imaging (SMI) in the evaluation of musculoskeletal disorders: a systematic review. Radiol Med 125:481–490. https://doi.org/10.1007/s11547-020-01141-x
Docking SI, Ooi CC, Connell D (2015) Tendinopathy: Is imaging telling us the entire story? J Orthop Sports Phys Ther 45:842–852
Albano D, Martinelli N, Bianchi A et al (2018) Posterior tibial tendon dysfunction: clinical and magnetic resonance imaging findings having histology as reference standard. Eur J Radiol 99:55–61. https://doi.org/10.1016/j.ejrad.2017.12.005
Albano D, Messina C, Usuelli FG et al (2017) Magnetic resonance and ultrasound in achilles tendinopathy: predictive role and response assessment to platelet-rich plasma and adipose-derived stromal vascular fraction injection. Eur J Radiol 95:130–135. https://doi.org/10.1016/j.ejrad.2017.08.006
Zappia M, Albano D, Aliprandi A et al (2023) Glenoid bone loss in anterior shoulder dislocation: a multicentric study to assess the most reliable imaging method. Radiol Med 128:93–102. https://doi.org/10.1007/s11547-022-01577-3
Albano D, Cortese MC, Duarte A et al (2020) Predictive role of ankle MRI for tendon graft choice and surgical reconstruction. Radiol Med 125:763–769. https://doi.org/10.1007/s11547-020-01177-z
Bellelli A, Silvestri E, Barile A et al (2019) Position paper on magnetic resonance imaging protocols in the musculoskeletal system (excluding the spine) by the Italian College of Musculoskeletal Radiology. Radiol Med 124:522–538. https://doi.org/10.1007/s11547-019-00992-3
Sconfienza LM, Albano D, Messina C et al (2018) How, when, why in magnetic resonance arthrography: an international survey by the European Society of Musculoskeletal Radiology (ESSR). Eur Radiol 28:2356–2368. https://doi.org/10.1007/s00330-017-5208-y
Gitto S, Messina C, Vitale N et al (2020) Quantitative musculoskeletal ultrasound. Semin Musculoskelet Radiol 24:367–374. https://doi.org/10.1055/s-0040-1709720
Albano D, Messina C, Gitto S et al (2023) Shear-wave elastography of the plantar fascia: a systematic review and meta-analysis. J Ultrasound 26:59–64. https://doi.org/10.1007/s40477-022-00770-4
Bartolotta TV, Orlando AAM, Dimarco M et al (2022) Diagnostic performance of 2D-shear wave elastography in the diagnosis of breast cancer: a clinical appraisal of cutoff values. Radiol Med. https://doi.org/10.1007/s11547-022-01546-w
Argalia G, Ventura C, Tosi N et al (2022) Comparison of point shear wave elastography and transient elastography in the evaluation of patients with NAFLD. Radiol Med. https://doi.org/10.1007/s11547-022-01475-8
Ruan S, Huang H, Cheng M et al (2023) Shear-wave elastography combined with contrast-enhanced ultrasound algorithm for noninvasive characterization of focal liver lesions. Radiol Med. https://doi.org/10.1007/s11547-022-01575-5
Ventura C, Baldassarre S, Cerimele F et al (2022) 2D shear wave elastography in evaluation of prognostic factors in breast cancer. Radiol Med. https://doi.org/10.1007/s11547-022-01559-5
Finnamore E, Waugh C, Solomons L et al (2019) Transverse tendon stiffness is reduced in people with Achilles tendinopathy: a cross-sectional study. PLoS ONE. https://doi.org/10.1371/journal.pone.0211863
Liberati A, Altman DG, Tetzlaff J, et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 62:e1–34. https://doi.org/10.1016/j.jclinepi.2009.06.006
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting—Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group B. JAMA Neurol 283:2008–2012
Slim K, Nini E, Forestier D et al (2003) Methodological index for non-randomized studies (Minors): development and validation of a new instrument. ANZ J Surg. https://doi.org/10.1046/j.1445-2197.2003.02748.x
Deeks JJ, Higgins JP, Altman DG (2019) Chapter 10: analysing data and undertaking meta‐analyses. In: Cochrane handbook for systematic reviews of interventions. vol 2
Riley RD, Higgins JPT, Deeks JJ (2011) Interpretation of random effects meta-analyses. BMJ. https://doi.org/10.1136/bmj.d549
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Br Med J 327:557–560
Schünemann H, Brożek J, Guyatt G, Oxman A (eds) GRADE Handbook. Available from: https://gdt.gradepro.org/app/handbook/handbook.html. Accessed 15 Oct 2023
Higgins JPT, Thomas J, Chandler J, et al (2021) Cochrane handbook for systematic reviews of interventions version 6.2 [updated February 2021]
Rendina-Gobioff G (2006) Detecting publication bias in random effects meta-analysis: an empirical comparison of statistical methods. USF Tampa Graduate Theses and Dissertations. https://digitalcommons.usf.edu/etd/2671
Dirrichs T, Quack V, Gatz M et al (2016) Shear wave elastography (SWE) for the evaluation of patients with tendinopathies. Acad Radiol 23:1204–1213. https://doi.org/10.1016/j.acra.2016.05.012
Dirrichs T, Quack V, Gatz M et al (2018) Shear wave elastography (SWE) for monitoring of treatment of tendinopathies: a double-blinded, longitudinal clinical study. Acad Radiol 25:265–272. https://doi.org/10.1016/j.acra.2017.09.011
Saha D, Prakash M, Sinha A et al (2022) Role of shear-wave elastography in Achilles tendon in psoriatic arthritis and its correlation with disease severity score, psoriasis area and severity index. Indian J Radiol Imaging 32:159–165. https://doi.org/10.1055/s-0042-1743116
Zhu B, You Y, Xiang X et al (2020) Assessment of common extensor tendon elasticity in patients with lateral epicondylitis using shear wave elastography. Quant Imaging Med Surg 10:211–219. https://doi.org/10.21037/qims.2019.10.07
Aubry S, Nueffer JP, Tanter M et al (2015) Viscoelasticity in Achilles tendonopathy: quantitative assessment by using real-time shear-wave elastography. Radiology. https://doi.org/10.1148/radiol.14140434
Breda SJ, van der Vlist A, de Vos RJ et al (2020) The association between patellar tendon stiffness measured with shear-wave elastography and patellar tendinopathy—a case-control study. Eur Radiol. https://doi.org/10.1007/s00330-020-06952-0
Hackett L, Aveledo R, Lam PH, Murrell GAC (2020) Reliability of shear wave elastography ultrasound to assess the supraspinatus tendon: an intra and inter-rater in vivo study. Shoulder Elbow 12:18–23. https://doi.org/10.1177/1758573218819828
Taljanovic MS, Gimber LH, Becker GW et al (2017) Shear-wave elastography: basic physics and musculoskeletal applications. Radiographics. https://doi.org/10.1148/rg.2017160116
Albano D, Martinelli N, Bianchi A et al (2017) Evaluation of reproducibility of the MOCART score in patients with osteochondral lesions of the talus repaired using the autologous matrix-induced chondrogenesis technique. Radiol Med 122:909–917. https://doi.org/10.1007/s11547-017-0794-y
Chen XM, Cui LG, He P et al (2013) Shear wave elastographic characterization of normal and torn Achilles tendons: a pilot study. J Ultrasound Med. https://doi.org/10.7863/jum.2013.32.3.449
Turner J, Malliaras P, Goulis J, Auliffe SM (2020) “it’s disappointing and it’s pretty frustrating, because it feels like it’s something that will never go away.” A qualitative study exploring individuals’ beliefs and experiences of Achilles tendinopathy. PLoS ONE. https://doi.org/10.1371/journal.pone.0233459
Aubry S, Risson JR, Kastler A et al (2013) Biomechanical properties of the calcaneal tendon in vivo assessed by transient shear wave elastography. Skelet Radiol. https://doi.org/10.1007/s00256-013-1649-9
Cao W, Sun Y, Liu L et al (2019) A multicenter large-sample shear wave ultrasound elastographic study of the Achilles tendon in Chinese adults. J Ultrasound Med 38:1191–1200. https://doi.org/10.1002/jum.14797
Ryu JA, Jeong WK (2017) Current status of musculoskeletal application of shear wave elastography. Ultrasonography 36:185
Kot BCW, Zhang ZJ, Lee AWC et al (2012) Elastic modulus of muscle and tendon with shear wave ultrasound elastography: variations with different technical settings. PLoS ONE. https://doi.org/10.1371/journal.pone.0044348
Cipriano KJ, Wickstrom J, Glicksman M et al (2022) A scoping review of methods used in musculoskeletal soft tissue and nerve shear wave elastography studies. Clin Neurophysiol 140:181–195
Sconfienza LM, Silvestri E, Orlandi D et al (2013) Real-time sonoelastography of the plantar fascia: comparison between patients with plantar fasciitis and healthy control subjects. Radiology 267:195–200. https://doi.org/10.1148/radiol.12120969
Bang JY, Hahn S, Yi J et al (2021) Clinical applicability of shear wave elastography for the evaluation of medial epicondylitis. Eur Radiol 31:6726–6735. https://doi.org/10.1007/s00330-021-07791-3
Baumer TG, Dischler J, Davis L et al (2018) Effects of age and pathology on shear wave speed of the human rotator cuff. J Orthop Res 36:282–288. https://doi.org/10.1002/jor.23641
Corrigan P, Cortes DH, Grävare Silbernagel K (2019) Immediate effect of photobiomodulation therapy on Achilles tendon morphology and mechanical properties: an exploratory study. Transl Sports Med. https://doi.org/10.1002/tsm2.78
Gatz M, Betsch M, Bode D et al (2020) Intra individual comparison of unilateral Achilles tendinopathy using B-mode, power doppler, ultrasound tissue characterization and shear wave elastography. J Sports Med Phys Fit. https://doi.org/10.23736/S0022-4707.20.11031-4
Hong S, Hahn S, Yi J et al (2023) Comparing the clinical application of strain elastography and shear wave elastography for the evaluation of lateral epicondylitis. J Clin Ultrasound 51:123–130. https://doi.org/10.1002/jcu.23356
Hou SW, Merkle AN, Babb JS et al (2017) Shear wave ultrasound elastographic evaluation of the rotator cuff tendon. J Ultrasound Med 36:95–106
Şahan MH, Inal M, Burulday V, Kültür T (2018) Evaluation of tendinosis of the long head of the biceps tendon by strain and shear wave elastography. Med Ultrason 20:192–198. https://doi.org/10.11152/mu-1323
Turkay R, Inci E, Aydeniz B, Vural M (2017) Shear wave elastography findings of de Quervain tenosynovitis. Eur J Radiol 95:192–196. https://doi.org/10.1016/j.ejrad.2017.08.011
Yoo SJ, Lee S, Song Y et al (2020) Elasticity of torn supraspinatus tendons measured by shear wave elastography: a potential surrogate marker of chronicity? Ultrasonography 39:144–151. https://doi.org/10.14366/usg.19035
Funding
The authors declare no funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by DA, MB, SG, SB and GC. The first draft of the manuscript was written by DA and MB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to disclose.
Consent to participate
Written informed consent was not required for this study because no patient data was used for this study.
Consent to publish
Written informed consent for publication was not required for this study because no patient data was used for this study.
Ethical approval
Institutional Review Board approval was not required because no patient data was used for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Albano, D., Basile, M., Gitto, S. et al. Shear-wave elastography for the evaluation of tendinopathies: a systematic review and meta-analysis. Radiol med 129, 107–117 (2024). https://doi.org/10.1007/s11547-023-01732-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-023-01732-4








