Abstract
Objective
The purpose of this study is to assess the elastic and anisotropic properties of normal calcaneal tendon in vivo by transient shear wave elastography (SWE).
Materials and methods
This study was approved by our institutional ethics committee. Eighty healthy subjects over 18 years of age were prospectively included. Data on the patients’ height, weight, sporting activities, and take-off foot were assessed. The thickness, width, and cross-sectional area of the calcaneal tendons were measured. The shear wave propagation velocity (Vmean) was measured by three radiologists on axial and sagittal SWE images at four different degrees of ankle flexion, enabling to calculate elasticity modulus (Emean), and relative anisotropy coefficient (A) values.
Results
In complete plantar flexion, Vmean was 6.8 ± 1.4 m.s−1 and 5.1 ± 0.8 m.s−1, respectively, on the sagittal and axial SWE image, resulting in an elastographic anisotropy A of 0.24 ± 0.16. The best interobserver correlation coefficient of Emean and Vmean was 0.43 and 0.46, respectively, in the sagittal SWE for complete plantar flexion. Vmean and Emean significantly increase when the tendon is stretched by ankle dorsiflexion. The maximal values in sagittal SWE were Vmean = 16.1 ± 0.7 m.s−1, Emean = 779.5 ± 57.1kPa and A = 0.63 ± 0.07.
Conclusions
SWE allows the elastic properties of the calcaneal tendon to be evaluated quantitatively in vivo, but interobserver reproducibility is questionable. It confirms the tendinous elastographic anisotropy and stiffness augmentation of stretched tendon.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tendons adapt dynamically to mechanical stress, increasing their resistance to rupture when in use [1]. Conversely, a change in their elastic properties is found in immobilization and in aging [2]. Tendinopathies occur frequently, and have increased over the last 30 years due to the rise in sporting activity [3, 4]. The calcaneal tendon (or Achilles tendon) is subjected to the greatest mechanical stress and is the most frequently injured tendon: calcaneal tendon injury rate is estimated at 12 in 100,000 individuals [5]. This presents a diagnostic and therapeutic challenge in which imaging plays a crucial role. The current gold-standard imaging techniques for tendon injury assessment are ultrasound (US) and magnetic resonance imaging (MRI). They allow precise morphological analysis of normal and damaged calcaneal tendons, but do not quantitatively evaluate biomechanical properties such as viscoelasticity and anisotropy.
Tissue elasticity is generally measured by a physical constant, Young’s modulus, which is defined as the ratio of the uniaxial stress applied to an isotropic elastic material over the uniaxial strain. Most studies focusing on tendon elasticity are carried out in vitro with longitudinal stretching tests that provide stress-elongation and stress-deformation curves, making it possible to extract the main elasticity parameters. However, further analysis of published results shows considerable disparity in the values obtained. This is explained by numerous difficulties encountered in the experiment [2, 6, 7]. Tendon elongation has also been measured in vivo using ultrasound or MRI with a force-measuring device using a dynamometer [8–11]. Transient shear wave elastography (SWE) is a recent non-invasive ultrasonographic imaging technique introduced in 2002 [12], which can evaluate soft tissue stiffness [12–14]. SWE has the advantage of being able to measure the speed of shear stress wave propagation in tissue, allowing Young’s modulus to be calculated [14, 15]. The first applications for SWE were the characterization of mammary nodules [16] and the evaluation of hepatic fibrosis [12, 17]. To our knowledge, only two studies of normal calcaneal tendon in SWE have been published: the first was carried out using a prototype with an upper elasticity modulus measurement limit of 600 kPa on 30 healthy volunteers [18]. The second studied the calcaneal tendon in only one position [19].
The main objective of our study was to quantify the elastic properties of normal calcaneal tendon with SWE in vivo and to estimate anisotropy. The secondary objectives were to study the interobserver reproducibility of the measurements and to look for factors in the variation of shear modulus such as age, sex, body mass index, sporting activity, and dominant leg.
Materials and methods
Study population
Institutional review board and written informed consent from all volunteers were obtained. Eighty healthy volunteers were included in this prospective study from January 2011 to September 2011. The inclusion criterion was healthy volunteers over 18 years of age. The exclusion criteria were pregnant women, history of calcaneal tendinopathy or calcaneal tendon surgery, pain in the calcaneal tendon, and morphological anomaly on ultrasound (B mode and power Doppler). Volunteers with a history of systemic, metabolic, or endocrine diseases, psoriasis, or those treated with corticosteroids, estrogens, quinolones, and cholesterol drugs were also excluded due to the association between these factors and tendinopathies [20, 21].
The following data were collected by the main investigator: inclusion date, date of birth, weight (kg), height (m), sex, number of hours spent exercising per week, and dominant leg. Patients’ age was calculated using the difference between their inclusion date and date of birth. The body mass index (BMI) of each volunteer was calculated using the formula BMI = weight (kg)/size (m)2. The volunteers were identified as “sporty” if they claimed to spend 1 h or more exercising per week. Otherwise they were considered “non-sporty”.
Procedure
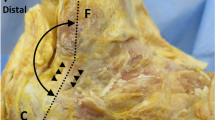
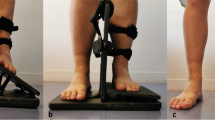
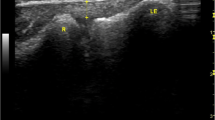
All ultrasounds were bilateral according to a standardized protocol using the Aixplorer machine (SuperSonic Imagine, Aix-En-Provence, France) equipped with a 12-MHz superficial linear transducer. Patients were placed in a prone position, with legs extended. The examination began with grayscale B mode and power Doppler ultrasound to eliminate any signs of tendinopathy. The width (mm) and thickness (mm) of each tendon were measured with axial (i.e., perpendicular to the direction of the fibers) grayscale ultrasound 5 cm from the end of the tendon. The cross-sectional area (mm2) of each tendon was calculated using the following formula: cross-sectional area = π × width/2 × thickness/2. Measurements in elastographic mode were made successively for four passively mobilized ankle positions: position no. 1: maximal plantar flexion; position no. 2: 45° plantar flexion; position no. 3: 0° flexion; position no. 4: 45° dorsiflexion or maximal dorsiflexion for patients who did not sustain 45° dorsiflexion (Fig. 1). The mean shear wave velocity (Vmean, m.s−1) was measured in elastographic mode (SWE) by placing a region of interest (ROI) with the following requirements: constant round size (3 mm in diameter) situated in the center of the tendon 5 cm from its end, both in sagittal (i.e., parallel to the direction of the fibers) and in axial SWE (Fig. 2). Young’s modulus Emean (kPa) was calculated from Emean = 3 ρVmean 2[13] where density ρ is assumed to be constant (1,000 kg.m−3), under the assumption of a purely elastic model. Standard deviation of the ROI was used as an estimate of the quality of the measurement: standard deviation of shear wave velocity (VSD) and the standard deviation of Young’s modulus (ESD) within the ROI were also measured. The relative anisotropy coefficient (A) was calculated using the following formula: A = (sagittal Vmean – axial Vmean)/sagittal Vmean.
Calcaneal tendon was examined at 5 cm from the end of the tendon consecutively for four degrees of ankle flexion. Position no. 1: maximal plantar flexion; position no. 2: 45° plantar flexion; position no. 3: 0° flexion; position no. 4: 45° dorsiflexion. As it may have increased tissue stiffness, pre-compression was avoided by interposition of a gel pad between the probe and the skin. The position was maintained and controlled by fastening feet on an articulated board
In accordance with the protocol, the examinations of the 80 healthy volunteers were performed by the main investigator (S.A., with more than 10 years of experience in musculoskeletal ultrasound). However, in 30 of these cases, volunteers also underwent examinations of two other investigators (J.R.R. and B.B.B., both with more than 6 years of experience in musculoskeletal ultrasound). These 30 subjects were examined independently by the three investigators, such that the ankle positioning and imaging were repeated three times.
Statistical analysis
Continuous variables are expressed as mean ± SD. A mixed model was used to evaluate the elastic and anisotropic variations for the four ankle positions.
An overall p value of less than 0.05 was considered statistically significant.
Univariate analysis was performed to show whether the following variables influenced elastic and anisotropic tendon properties: age, sex, physical exercise, dominant leg, weight, height, BMI, as well as the thickness, width, and cross-sectional area of the calcaneal tendon.
Interobserver reproducibility was evaluated using the intraclass correlation coefficient (ICC). An ICC between 0.8 and 1 was considered to be very reproducible, between 0.6 to 0.79 was considered moderately reproducible, and less than 0.6 was considered not very reproducible. Statistical analysis was performed using SAS for Windows version 9.1.3 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
Forty-three women and 37 men with a mean age of 45.4 (range, 20–83) were included. Their mean weight was 68.6 ± 12.6 kg, mean height was 168.6 ± 9.1 cm, and mean BMI was 24.1 ± 3.8. The mean exercise time per week was 1.7 ± 2.4 h. Forty-three volunteers were considered “sporty” (53.7 %, 43/80) and 37 “non-sporty” (46.3 %, 37/80). In 66.2 % of the cases (53/80), the dominant leg was the right leg.
Ultrasound and elastographic variables
Data of the right and left tendons were grouped together so that 160 tendons were studied. In the axial ultrasound, the thickness of the calcaneal tendon was 4.4 ± 0.6 mm, the width was 15.1 ± 1.9 mm, and the cross-sectional area was 53.1 ± 11.7 mm2. The results of the elastographic analysis are shown in Table 1. In the axial SWE, there was a significant increase in the Vmean and Emean between positions no. 1 and no. 2 (p < 0.001), and between no. 3 and no. 4 (p < 0.001), but there was no significant difference in these variables between positions no. 2 and no. 3 (Vmean p = 0.62 and Emean p = 0.7, respectively). In the sagittal SWE, there was a significant increase in Vmean and Emean between positions no. 1 and no. 2, no. 2 and no. 3, and between no. 3 and no. 4 (p < 0.001). The relative anisotropy ratio A was 0.24 ± 0.16 in position no. 1. It increased significantly between positions no. 1 and no. 2 (p < 0.001), and between positions no. 2 and no. 3 (p < 0.001). However, there was a statistically significant drop between positions no. 3 and no. 4 (p < 0.001). In the sagittal SWE, there was a significant increase in VSD and ESD between position no. 1 and no. 2 (p < 0.001), and between no. 2 and no. 3 (p < 0.001), but there was a significant reduction in these two variables between positions no. 3 and no. 4 (p < 0.001). In the axial SWE, there was no significant difference in VSD and ESD between positions no. 1 and no. 2 (VSD p = 0.1 and ESD p = 0.6), but they both increased from positions no. 2 to no. 3 and from no. 3 to no. 4 (p < 0.001).
The univariate analysis found that Vmean was significantly reduced with age on the axial SWE acquisitions in position no. 1 (p < 0.001) and no. 2 (p = 0.0015), and in the sagittal SWE in position no. 2 (p = 0.004). Vmean was significantly higher in men in axial acquisitions in position no. 1 (p = 0.005) and in sagittal acquisitions in position no. 1 (p = 0.03), no. 2 (p < 0.001), and no. 3 (p = 0.017). However, exercise, dominant leg, weight, height, BMI, and the thickness, width, and cross-sectional area of the calcaneal tendon did not have a significant influence on Vmean regardless of the orientation of the transducer and the position of the ankle.
Interobserver reproducibility
The different intraclass correlation coefficients are shown in Table 2.
Discussion
Biomechanical properties of tendons are difficult to assess because tendons are highly complex and are made of dense fibrous connective tissue that transmits the mechanical force of muscle contraction to the bones. Although the role of viscoelasticity in tendon biomechanics is well recognized, methods of measuring viscoelasticity in vivo are currently limited. The recent development of a new quantitative elastography technique has made it possible to explore elastic properties of the tendon depending on whether it is stretched. The Aixplorer system generates push waves, which are akin to the pulses used in color Doppler, to vibrate the tissue using the acoustic radiation force impulse effect. These minute vibrations set up shear waves, transverse waves that travel out at right angles to push pulses. The displacements produced by the movement of the shear waves are measured with ultrafast plane wave imaging. Our study confirms that SWE enables us to evaluate the elastic properties of the calcaneal tendon in vivo. At rest in full extension, the values obtained (sagittal Emean 147 ± 62 kPa, axial Emean 78 ± 26≤kPa) (Table 1) are similar to those of the earliest studies published in the literature (Fig. 3): the first results (sagittal Emean 104 ± 46 kPa, axial Emean 64 ± 22 kPa [18]) were obtained using a prototype. Those published by Arda (sagittal Emean 74 ± 45 kPa, axial Emean 54 ± 25 kPa [19]) are of the same order of magnitude. The four studies on the elastic properties of the calcaneal tendon published previously [22–25] were all carried out static elastography. Compression was applied by the user, induced deformation was calculated by the ultrasound system, and then a color map reflecting tissue stiffness was displayed [26, 27]. Because an unknown amount of stress was applied, Young’s modulus could not be calculated. As is the case in our study, tendon stiffness seemed to be greater in the sagittal acquisitions than in the axial acquisitions [24], but the static elastography technique does not provide quantitative data on tendon anisotropy.
The calcaneal tendon has tendinous fibers that lie parallel to each other. There is therefore an axis of symmetry along the fibers that relates to a hexagonal system (transverse isotropy) according to the viscoelasticity theory [28]; This enables to define two main axes: one parallel and one perpendicular to the fibers. The small difference in the mean values of Young’s modulus along the direction perpendicular to the fiber axis (E11 and E22, Fig. 3) measured by Kuo [29] seems to confirm the transverse isotropy model. In a study of muscle elastography, Gennisson [30] showed that shear wave velocity is a function of the angle between the transducer and the main axis of the muscle fibers. Maximum velocity is reached when the transducer is parallel to the fibers and minimum velocity when the transducer is perpendicular to the fibers. Our results on the calcaneal tendon are similar, and we estimate the relative anisotropy coefficient A of the calcaneal tendon at rest at 0.24 ± 0.16. This confirms that, much like muscle, normal tendon is anisotropic, and that the orientation of the transducer axis in relation to the tendon fiber axis must be known in order to accurately interpret the quantitative values provided by the device. Anisotropy is almost three times higher for a stretched tendon (position no. 3) than at rest (position no. 1). This result is consistent with previous work on muscle anisotropy [30].
Our results also confirm the increase in Emean, Vmean, and A when the calcaneal tendon was stretched, regardless of whether the measurements were made in the sagittal SWE (Fig. 3a) or in the axial SWE (Fig. 3b). Arda [19] did not carry out a dynamic study. In vivo, the princeps study was carried out on a smaller number of volunteers (n = 30), with only three different ankle positions [18]. The upper limit for measuring Young’s modulus (600 kPa) was lower than that of our device (800 kPa). Therefore, the values published in the sagittal SWE at 0° and in complete dorsiflexion were under-estimated [18]. These values are probably still under-estimated because the upper limit (800 kPa) is often reached for these two positions in the sagittal SWE. Consequently, it is unrealistic to try to reproduce usual movement and position with extra-weight (or supporting the weight of the patient) because the current limit of the device (800 kPa; 16 m.s−1) would also be exceeded.
In vitro studies based on methods other than elastography (uniaxial contraction test) report variable Young’s modulus values for the tendons studied, between 1 and 2 GPa [31]. The differences in values found seem to be linked mainly to the experimental conditions (changes in the tendon on its support system) but also to the methods used for preserving tendon samples (fixation) [32]. We should therefore be cautious when extrapolating in vivo experimental data. In 2001, Kuo [29] estimated the elastic properties of calcaneal tendons of cattle in vitro using a process similar to elastography. Shear wave velocity was measured parallel and perpendicular to the direction of tendon, with different levels of stress by modifying the stretch percentage compared to the initial length. The measurements were made at rest (0 %), and then in the elastic stress-deformation curve (an intermediate position at a +4.7 % stretch compared to the initial length) and lastly in the highest position (+9.5 %). The results of various quantitative studies [18, 19, 29] are compared in Fig. 3. These studies are consistent in terms of the increase of the absolute values of Emean when the tendon is stretched. The differences are, however, relatively large when stress is applied, particularly for the highest position. Because stress is considered to be non-existent at rest, quantitative comparison with our results is possible. On the other hand, comparison with intermediate and highest positions in which degree of stress is unknown must remain qualitative. The stress in vitro, which is near the breaking point, is probably higher than that in our experimental conditions.
Of the factors that may influence the elastic properties of the calcaneal tendon, we found significantly higher Emean and Vmean values in position no. 1 in men compared with women, similarly to Arda [19]. In position no. 1 and no. 2, we showed a significant reduction in Emean and Vmean according to age. Although they have not been found before [18, 19], this association suggests tendon senescence and should be confirmed in further studies. Unlike a previous study [18], we found that physical activity made no difference to the elastic properties of the calcaneal tendon. These inconsistent results should be regarded with caution and should be compared to the results of future studies on higher numbers of subjects.
In position 1, the mean values for ESD and VSD are low compared to the values for Emean and Vmean (Table 1). This shows that there is a high consistency in values for ROI used for the measurement. In the axial SWE, ESD and VSD significantly increase when the calcaneal tendon is stretched progressively to position 4, which may be secondary to the tendon’s increase in heterogeneity or noise of elastographic imaging. In the sagittal SWE, ESD and VSD increase up to position 3. However, ESD and VSD significantly decrease in position 4 due to the upper limit of ROI measurements being reached.
In the sagittal SWE and at rest (no. 1), there is a statistically significant interobserver correlation for Emean and Vmean measurements. Such reproducibility is questionable (Emean ICC = 0.43, Vmean ICC = 0.46) but better than in the axial SWE (Emean ICC = 0.16, Vmean ICC = 0.15). In position 2, the interobserver reproducibility of Emean and Vmean measurements is poor. The interobserver reproducibility in positions no. 3 and no. 4, although questionable, is better in axial SWE than in sagittal SWE, perhaps because the upper limits of the measurement device are reached in the sagittal SWE (Table 2). Due to this poor reproducibility of Emean and Vmean, interobserver reproducibility of A is also questionable regardless of the position. Quantitative values measured in positions other than at rest should be interpreted with great caution.
Tendons are made of collagen and extracellular matrix whose proportions seem to be variable and are linked to their elastic properties. It has been shown that the increase in the amount of collagen is associated with an increase in the elastic modulus of the tendon [33]. In 1980, Woo et al. [34] demonstrated on a porcine model the relationship between physical activity and increase in the stiffness of tendons associated with an increase in their collagen content. Several studies have proven that excessive strain stimulates the production of metalloproteases, causing deterioration in the collagen network and tendon degeneration [6]. This histological degeneration seems greater in symptomatic subjects, and reaches the maximum level after a tendon rupture [35]. Conversely, the low level of tenocyte stimulation, such as prolonged immobilization, may lead to a reduction in metalloprotease expression [6]. The pathogenesis of tendinopathies is a complex biomechanical and biochemical change, which might be better understood through elastography. The first studies to have included symptomatic patients are inconsistent, and were carried out with static elastography. One showed stiffer tendons in symptomatic subjects [25] while the other showed softer tendons [23]. According to Khoury’s study in tennis elbow [36], “tenomalacia” could be a new sign of tendinopathy. This might be better described as tendon-softening (or tenosoftening) and should be confirmed by additional studies with a higher number of tendinopathies. It would also be interesting to see whether elastographic anisotropy is modified in calcaneal tendinopathy. Because shear waves do not propagate in liquids, tendon tears may appear with no signal on Young’s modulus map (Fig. 4). However, because of moderate to low inter-observer reproducibility of the measures of tendon elasticity, any further study on this topic should be performed taking into account several biases; first, a bias related to the possible contraction of the subjects’ calf. It may also be difficult to reproduce precisely different grades of dorsiflexion in patients with local pain; second, measurement bias related to positioning or direction of the transducer or positioning of the ROI. As the tendon is highly anisotropic, a difference of a few degrees in the positioning of the transducer can be responsible for significant differences in measurements. To overcome this bias, the US beam and tendon fibers should always be strictly parallel or perpendicular, which can be difficult to achieve in practice. One can imagine that in the future manufacturers develop probes with 3D SWE acquisition in different directions to achieve SWE tendon tractography, by analogy with MRI diffusion tensor imaging; finally, a possible measurement bias may be due to the device itself: its frame per second is low (approx. 10 Hz), and its intrinsic accuracy is not known.
Despite these limitations, transient shear wave elastography enables quantifying the elastic properties of normal calcaneal tendon in vivo and following the variations in these properties when the tendon is stretched. Further studies are necessary in order to validate the benefit of this technique in tendon pathologies, especially to verify if tendinosis is softer than normal tendons and to check if tendon tears appear as SWE signal void areas.
References
Reeves ND. Adaptation of the tendon to mechanical usage. J Musculoskelet Neuronal Interact. 2006;6(2):174–80.
Narici MV, Maganaris CN. Adaptability of elderly human muscles and tendons to increased loading. J Anat. 2006;208(4):433–43.
Jarvinen TA, Kannus P, Paavola M, Jarvinen TL, Jozsa L, Jarvinen M. Achilles tendon injuries. Curr Opin Rheumatol. 2001;13(2):150–5.
Jarvinen TA, Kannus P, Maffulli N, Khan KM. Achilles tendon disorders: etiology and epidemiology. Foot Ankle Clin. 2005;10(2):255–66.
Hess GW. Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec. 2010;3(1):29–32.
Jung HJ, Fisher MB, Woo SL. Role of biomechanics in the understanding of normal, injured, and healing ligaments and tendons. Sports Med Arthrosc Rehabil Ther Technol. 2009;1(1):9.
Hashemi J, Chandrashekar N, Slauterbeck J. The mechanical properties of the human patellar tendon are correlated to its mass density and are independent of sex. Clin Biomech (Bristol, Avon). 2005;20(6):645–52.
Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol. 2003;548(Pt 3):971–81.
Maganaris CN, Paul JP. In vivo human tendon mechanical properties. J Physiol. 1999;521(Pt 1):307–13.
Maganaris CN. Tensile properties of in vivo human tendinous tissue. J Biomech. 2002;35(8):1019–27.
Kubo K, Kanehisa H, Fukunaga T. Effects of different duration isometric contractions on tendon elasticity in human quadriceps muscles. J Physiol. 2001;536(Pt 2):649–55.
Sandrin L, Tanter M, Catheline S, Fink M. Shear modulus imaging with 2-D transient elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2002;49(4):426–35.
Bercoff J, Tanter M, Fink M. Supersonic shear imaging: a new technique for soft tissue elasticity mapping. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51(4):396–409.
Gennisson JL, Renier M, Catheline S, Barriere C, Bercoff J, Tanter M, et al. Acoustoelasticity in soft solids: assessment of the nonlinear shear modulus with the acoustic radiation force. J Acoust Soc Am. 2007;122(6):3211–9.
Boisserie-Lacroix M. Elastography: an old concept for a new tool. J Radiol. 2007;88(5 Pt 1):625–6.
Athanasiou A, Tardivon A, Tanter M, Sigal-Zafrani B, Bercoff J, Deffieux T, et al. Breast lesions: quantitative elastography with supersonic shear imaging–preliminary results. Radiology. 2010;256(1):297–303.
Bavu E, Gennisson JL, Couade M, Bercoff J, Mallet V, Fink M, et al. Noninvasive in vivo liver fibrosis evaluation using supersonic shear imaging: a clinical study on 113 hepatitis C virus patients. Ultrasound Med Biol. 2011;37(9):1361–73.
Aubry S, Risson JR, Barbier-Brion B, Tatu L, Vidal C, Kastler B. Transient elastography of calcaneal tendon: preliminary results and future prospects. J Radiol. 2011;92(5):421–7.
Arda K, Ciledag N, Aktas E, Aribas BK, Kose K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. AJR Am J Roentgenol. 2011;197(3):532–6.
Abate M, Gravare Silbernagel K, Siljeholm C, Di Iorio A, De Amicis D, Salini V, et al. Pathogenesis of tendinopathies: inflammation or degeneration? Arthritis Res Ther. 2009;11(3):235.
Holmes GB, Lin J. Etiologic factors associated with symptomatic Achilles tendinopathy. Foot Ankle Int. 2006;27(11):952–9.
De Zordo T, Fink C, Feuchtner GM, Smekal V, Reindl M, Klauser AS. Real-time sonoelastography findings in healthy Achilles tendons. AJR Am J Roentgenol. 2009;193(2):W134–138.
De Zordo T, Chhem R, Smekal V, Feuchtner G, Reindl M. Fink C, et al. Real-time sonoelastography: findings in patients with symptomatic Achilles tendons and comparison to healthy volunteers. Ultraschall Med; 2009.
Drakonaki EE, Allen GM, Wilson DJ. Real-time ultrasound elastography of the normal Achilles tendon: reproducibility and pattern description. Clin Radiol. 2009;64(12):1196–202.
Sconfienza LM, Silvestri E, Cimmino MA. Sonoelastography in the evaluation of painful Achilles tendon in amateur athletes. Clin Exp Rheumatol. 2010;28(3):373–8.
Hall TJ, Zhu Y, Spalding CS. In vivo real-time freehand palpation imaging. Ultrasound Med Biol. 2003;29(3):427–35.
Ophir J, Cespedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13(2):111–34.
Zimmer JE, Cost JR. Determination of the elastic constants of an unidirectional fiber composite using ultrasonic velocity measurements. J Acoust Soc Am. 1970;47:795–803.
Kuo PL, Li PC, Li ML. Elastic properties of tendon measured by two different approaches. Ultrasound Med Biol. 2001;27(9):1275–84.
Gennisson JL, Deffieux T, Mace E, Montaldo G, Fink M, Tanter M. Viscoelastic and anisotropic mechanical properties of in vivo muscle tissue assessed by supersonic shear imaging. Ultrasound Med Biol. 2010;36(5):789–801.
Maganaris CN, Narici MV, Maffulli N. Biomechanics of the Achilles tendon. Disabil Rehabil. 2008;30(20–22):1542–7.
Smith CW, Young IS, Kearney JN. Mechanical properties of tendons: changes with sterilization and preservation. J Biomech Eng. 1996;118(1):56–61.
Haut RC, Lancaster RL, DeCamp CE. Mechanical properties of the canine patellar tendon: some correlations with age and the content of collagen. J Biomech. 1992;25(2):163–73.
Woo SL, Ritter MA, Amiel D, Sanders TM, Gomez MA, Kuei SC, et al. The biomechanical and biochemical properties of swine tendons–long term effects of exercise on the digital extensors. Connect Tissue Res. 1980;7(3):177–83.
Tallon C, Maffulli N, Ewen SW. Ruptured Achilles tendons are significantly more degenerated than tendinopathic tendons. Med Sci Sports Exerc. 2001;33(12):1983–90.
Khoury V, Cardinal E. "Tenomalacia": a new sonographic sign of tendinopathy? Eur Radiol. 2009;19(1):144–6.
Acknowledgments
We thank Frances Sheppard (Clinical Investigation Center of Besançon, Inserm CIT 808) for translating the manuscript into English, and Philippe Manzoni (University Hospital of Besançon) for technical support.
Conflict of interest declaration
The authors declare that they have no conflicts of interest.
Acknowledgments of funding and grants
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aubry, S., Risson, JR., Kastler, A. et al. Biomechanical properties of the calcaneal tendon in vivo assessed by transient shear wave elastography. Skeletal Radiol 42, 1143–1150 (2013). https://doi.org/10.1007/s00256-013-1649-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-013-1649-9