Abstract
Purpose
To evaluate and compare the diagnostic performance of revised contrast-enhanced ultrasound (CEUS) Liver Imaging Reporting and Data System version by combining LR-M category and serum alpha-fetoprotein (AFP) under different cut-off values.
Material and methods
This retrospective study enrolled 152 high-risk patients with 152 histology-proven nodules. For revised LI-RADS, nodules in LR-M with different elevated AFP thresholds have been reclassified as the LR-5 category. The diagnostic performances of original and revised CEUS LI-RADS were evaluated and compared.
Results
To compare with the original version, the sensitivity of revised LR-5 (adjusted with AFP value > 200 ng/ml or 400 ng/ml) for the diagnosis of hepatocellular carcinoma (HCC) improved from 52.5 to 69.2% or 65.0%, respectively (both p < 0.001) without compromising specificity (87.5% vs. 71.9% or 78.1%, respectively, both p > 0.05). For the diagnosis of non-HCC malignancy, the specificity of the LR-M after reclassification was improved (69.6% vs. 84.4% or 80.7%, respectively, both p < 0.001) with a non-significant sensitivity reduction (100.0 vs. 70.6% or 82.4%, respectively, both p > 0.05). After modification, the sensitivity of LR-5 also increased to 69.1% or 64.9% (both p < 0.001), while the specificity and PPV did not change (both p > 0.05) for larger nodules (> 20 mm).
Conclusion
The diagnostic performance of CEUS LI-RADS can be further improved by reclassifying LR-M nodules with elevated AFP thresholds to LR-5.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) constitutes the most common primary liver malignancy, accounting for about approximately 80% [1]. Accurate differentiation of hepatic lesions has significant importance in clinical management [2,3,4]. With the development of contrast-enhanced media, imaging modalities can replace invasive biopsy as the confirmative diagnostic approach in high-risk populations of HCC. Taking advantage of microbubbles within the intravascular space, contrast-enhanced ultrasound (CEUS) is recommended as the first-line diagnostic technology in the assessment of focal liver lesions (FLL) according to several international guidelines and recommendations [5,6,7,8]. Moreover, some western guidelines restricted the use of biopsy in cases of imaging uncertainty after examination with contrast-enhanced media (computed tomography, magnetic resonance imaging, CEUS) [9, 10]. Therefore, CEUS must be integrated with other methods.
The Liver Imaging Reporting and Data System (LI-RADS) version 2017 was developed by the American College of Radiology (ACR) to standardize terminology, reduce variance and errors in imaging interpretation, enhance communication between radiologists and clinicians, and create a structured report for the diagnosis of HCC [11, 12]. CEUS LI-RADS could assign the increasing probability of HCC from LR-1 to LR-5 and provide an LR-M class that encompasses probably or definitely malignancies but is not HCC-specific [11]. Previous studies demonstrated that CEUS LI-RADS category 5 had high specificity in predicting HCC, ensuring nearly 100% certainty [13, 14]. However, a significant drawback in CEUS LI-RADS is based on the fact that a high proportion of HCCs were still misdiagnosed as LR-M category, leading to a relatively lower sensitivity (44.8–64.2%) and accuracy (60.4–70.8%) of LR-5 [15]. Therefore, LR-M criteria must be revised to improve CEUS LI-RADS diagnostic performance.
Convenient and cost-effective serum alpha-fetoprotein (AFP) detection is frequently utilized for HCC diagnosis and surveillance [16]. However, different thresholds for AFP were adopted in the diagnosis of HCC. A study in the United States compared different cut-off values (20 ng/mL, 50 ng/mL, 100 ng/mL, 400 ng/mL) and found that when the threshold of AFP was 20 ng/ml, the sensitivity was approximately 60% with the specificity of 90%, while when the threshold was 400 ng/ml, the sensitivity was only 17%, but the specificity increased to 99.4% [16]. Moreover, an elevation of AFP also occurred in some patients involving chronic liver disease with non-cancerous causes [17]. Most guidelines discouraged the use of AFP alone due to its limited performance in diagnosing HCC, especially in patients with viral hepatitis [18, 19]. On the other hand, some experts have successfully incorporated the use of serum biomarkers in conjunction with CEUS characteristics to get an accurate diagnosis of FLLs [20, 21]. It should be noted that few studies focused on the role of serum AFP level in improving the diagnostic ability of CEUS LR-5 and LR-M.
Therefore, our objective was to combine different serum AFP cut-off values with the LR-M category to improve the current CEUS LI-RADS diagnostic performance.
Materials and methods
The retrospective study was approved by the Institutional Review Board, which waived the requirement of written informed consent.
Patient selection
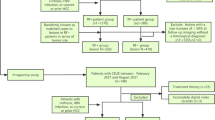
The inclusion criteria were (a) high-risk factors (cirrhosis or chronic hepatitis B viral infection or current or prior HCC) for HCC according to ACR CEUS LI-RADS (v2017) guidelines [11], (b) visible FLL in the B-mode ultrasound (US), (c) pathological proven FLL, and (d) availability of serum AFP levels concentration within two weeks before any treatments for FLL. The exclusion criteria were (a) previously treated nodules or recurrent HCCs and (b) image degradation or missing CEUS data. Finally, 152 patients with 152 FLLs were eventually included in this study. A detailed flowchart of patient selection is shown in Fig. 1.
Standard reference
In this study, pathological results were used as the gold standard. Histological results were confirmed by ultrasound (US)-guided biopsy or surgical pathology within half a month after CEUS examination. If patients underwent both US-guided biopsy and surgery, surgical pathology results were used.
B-mode US and CEUS examination
This study was performed by ultrasound machines including Aplio 900 (Toshiba Medical Systems, Tokyo, Japan) and Aixplorer Ultrasound system (SuperSonic Imagine, AixenProvence, France). Sonographers with more than six years of relevant clinical experience conducted the CEUS examination. Under the conventional B-mode US prior to CEUS, we collected the size, location, nodule echogenicity, and surrounding liver parenchyma. Then, all FLLs were examined using low mechanical index (MI) imaging and a dual-screen format after an injection of 2.4 ml SonoVue bolus followed by a 5 mL saline flush through the median cubital vein. The sonographer started the timer and saved the dynamic images after injecting the bolus of contrast agent. During the initial 60 s, we continually scanned the target nodule and the liver parenchyma surrounding it. To avoid continuous destruction of microbubbles, we swept the nodule at 15 s intervals and recorded for 4–6 min until the bolus cleared from circulation. The SonoVue could be reinjected 15 min after the first injection if the CEUS result was unclear. All imaging data were exported to a removable hard drive for later evaluation. The CEUS process was classified into arterial (10–20 s to 30–45 s), portal venous (30–45 s to 120 s) and late phase (120–300 s).
Image interpretation
Two highly skilled ultrasound physicians with at least five years of CEUS experience independently reviewed the CEUS images. The nodules were classified according to CEUS LI- RADS (v2017). If no consensus was reached, the final categorization was achieved by another expert ultrasound physician with 10 years of experience in CEUS. Medical information was not disclosed to all readers.
Serum AFP examination
In the fasting state in the early morning, 5 ml of venous blood from the patients was detected by electrochemiluminescence immunoassay. In this study, we selected conventional AFP thresholds based on previous studies (20 ng/ml [22], 50 ng/ml [23], 100 ng/ml [16], 200 ng/ml [24], and 400 ng/ml [25]) and optimal thresholds derived from receiver operating characteristic (ROC) analysis according to our data.
Diagnostic criteria for modified CEUS LI-RADS
Criteria 1: The original CEUS LI-RADS v2017.
Criteria 2: LR-5 and LR-M with AFP > optimal threshold according to our data as a predictor of HCC, LR-M with AFP ≤ optimal threshold according to our data as a predictor of non-HCC malignancy.
Criteria 3: LR-5 and LR-M with AFP > 20 ng/ml as a predictor of HCC, LR-M with AFP ≤ 20 ng/ml as a predictor of non-HCC malignancy.
Criteria 4: LR-5 and LR-M with AFP > 50 ng/ml as a predictor of HCC, LR-M with AFP ≤ 50 ng/ml as a predictor of non-HCC malignancy.
Criteria 5: LR-5 and LR-M with AFP > 100 ng/ml as a predictor of HCC, LR-M with AFP ≤ 100 ng/ml l as a predictor of non-HCC malignancy.
Criteria 6: LR-5 and LR-M with AFP > 200 ng/ml as a predictor of HCC, LR-M with AFP ≤ 200 ng/ml as a predictor of non-HCC malignancy.
Criteria 7: LR-5 and LR-M with AFP > 400 ng/ml as a predictor of HCC, LR-M with AFP ≤ 400 ng/ml as a predictor of non-HCC malignancy.
Statistical analysis
The SPSS software package version 23.0 and GraphPad Prism version 9.0 were used to perform the statistical analysis. Quantitative data were presented as medians ± standard deviation, while categorical data were identified as numbers and percentages. ROC was used to determine the optimal threshold for serum AFP level for HCC diagnosis. Pairwise comparisons of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated by McNemar test or chi-square test if applicable. Diagnostic performance in diagnosing HCC were assessed by ROC curve analysis, sensitivity, specificity, PPV and NPV. A two-sided p-value less than 0.05 was established to be a significant difference.
Results
Patients and nodule characteristics
Based on inclusion criteria, the study population consisted of 152 patients with 152 FLLs (mean age, 56 years; range 27–78 years). Among all the patients, the mean maximum diameter of liver nodules was 42.5 mm (range 9.0–146.2 mm). The detailed clinical characteristics, including age, gender, nodule size, etiology of liver disease, serum AFP value, and pathological analysis, are presented in Table 1.
Imaging characteristics and CEUS LI-RADS categories
The CEUS imaging features and CEUS LI-RADS categories of nodules are shown in Table 2. Of the 152 nodules, there were 11 LR-3, 16 LR-4, 67 LR-5, and 58 LR-M nodules. Similar to expectations, the incidence of HCC from LR-3 to LR-5 category was increased gradually (45.5%, 81.3% and 94.0%, respectively). Although all non-HCC malignancies were classified as LR-M category, 39 HCCs were also classified as LR-M due to early washout (< 60 s).
Diagnostic performance of AFP alone to predict HCC
ROC analysis was used to determine the optimal cut-off value as 2.5 ng/ml. When using AFP alone to predict HCC, the diagnostic performances of different thresholds are shown in Online Resource 1. Under all thresholds, the AUC was from 0.53 to 0.63, and the accuracy was from 34.2 to 78.9%. Furthermore, different AFP values varied in sensitivity from 20.8 to 53.3% and specificity from 34.4 to 84.4%.
Diagnostic performance of the revised CEUS LI-RADS by combining with serum AFP
The AFP values of LR-M category with different pathologic types are shown in Fig. 2. FLLs in LR-M category with elevated AFP level were reclassified as LR-5 (Fig. 3). In contrast, LR-M FLLs with normal AFP did not change the classification (Fig. 4). The sensitivity and specificity of Criteria 1 to diagnose HCC were 52.5% and 87.5%, respectively. Criteria 2, Criteria 3, Criteria 4, and Criteria 5 improved the sensitivity of HCC diagnosis obviously (all p < 0.001). But the specificity of Criteria 2, Criteria 3, Criteria 4, and Criteria 5 dropped significantly (all p < 0.05). Moreover, both Criteria 6 and Criteria 7 elevated the sensitivity (69.2% vs. 52.5% and 65.0% vs. 52.5%, respectively) (both p < 0.001) without significantly reducing the specificity (71.9% vs. 87.5% and 78.1% vs. 87.5%, respectively) (p > 0.05). When it came to the diagnostic performance to identify non-HCC malignancy, Criteria 2, Criteria 3, Criteria 4, and Criteria 5 could elevate the specificity of LR-M (all p < 0.001), accompanied by a significant decrease in sensitivity (all p < 0.05). Criteria 6 and Criteria 7 could meanwhile increase the specificity of LR-M (84.4% vs. 69.6% and 80.7% vs. 69.6%, respectively) (both p < 0.001) without significant loss of sensitivity (70.6% vs. 100.0% and 82.4% vs. 100.0%, respectively) (both p > 0.05) (Table 3).
HCC nodule with elevated serum AFP value (301.32 ng/mL). a A 46-mm hypoechoic lesion in a 66-year-old male patient with HBV; b The nodule showed APHE 21 s after SonoVue injection, and c early washout was observed at 55 s. d A mild washout was presented at 151 s. The nodule would be classified as CEUS LR-M (criteria 1 and 7) or reidentified as CEUS LR-5 (criteria 2, 3, 4, 5, 6)
ICC nodule with normal serum AFP value (1.83 ng/mL). a A 40-mm hypoechoic nodule in a 72-year-old female patient with HBV; b The nodule showed APHE 12 s after SonoVue injection, and c early washout was observed at 45 s. d A mild washout was presented at 117 s. The nodule would be classified as CEUS LR-M (criteria 1, 2, 3, 4, 5, 6, 7)
Diagnostic performance of the revised CEUS LI-RADS for FLLs size > 20 mm
In subgroup of liver nodules larger than 20 mm, the sensitivity and specificity of LR-5 in the diagnosis of HCC were 48.9% and 92.6%, respectively. Besides Criteria 6 and Criteria 7, Criteria 5 could also increase the sensitivity (69.1% vs. 48.9%, p < 0.001) without significant loss of specificity (74.1% vs. 92.6%, p = 0.063) (Table 4).
Discussion
In this retrospective study, we performed multiple comparative studies of 152 nodules based on combining CEUS LI-RADS and different serum AFP cut-off values in the diagnosis of HCC. Eventually, at thresholds of 200 ng/ml and 400 ng/ml, the sensitivity improved effectively (p < 0.001) without significant loss of specificity (p = 0.063 and 0.250).
In this study, the incidences of HCC in LR-3, LR-4, LR-5 category nodules were 45.5%, 81.3% and 94.0% respectively, which corresponded to a higher probability of HCC in CEUS LI-RADS [26]. Meanwhile, our research also demonstrated an excellent specificity of 87.5% and PPV of 94.0%, consistent with previous research ranging 78.5–96.0% and 92.7–98.0% [27,28,29]. Thus, CEUS LR-5 could accurately identify HCCs and avoid the use of percutaneous biopsy which may cause bleeding or implantation metastasis [30]. However, another study showed that 68.5% of HCCs were in the LR-M category, which caused a relatively poor sensitivity of 65.5% [31]. It was similar to our study of 67.2% and 52.5%, respectively. In order to improve the sensitivity of HCC detection and to reduce missed diagnoses, it is necessary for us to reclassify HCCs in the LR-M category. Several studies focused only on the modification of imaging characteristics, such as adjusting the time to define early washout [31] or combining the time and degree of early washout [27, 28], but few attention was paid to the role of serum biomarkers.
Serum AFP evaluation is a simple, rapid, and inexpensive means of HCC surveillance and it is also of great significance for evaluating treatment response [32]. However, the role of AFP alone is limited in the diagnosis of HCC [33]. One probable reason is that increased AFP level could reflect the severity of hepatic destruction and subsequent regeneration, which could be presented in patients with active hepatitis or cirrhosis even without HCC [16]. Elevated AFP (> 20 ng/mL) was observed in about 40.6% of high-risk patients without HCC, according to our data. Another explanation might be that AFP levels are normal (≤ 20 ng/ml) in about 30% to 40% of patients with HCC, which is consistent with our findings [32]. Consequently, combining imaging features and AFP seems promising in distinguishing FLLs. To improve the efficacy of differentiating the diagnosis of FLLs, Yang et al. tried to associate CEUS LI-RADS with serum biomarkers in diagnosing non-HCC malignancy [20]. Serum biomarkers were also associated with CT/MRI LI-RADS for the differentiation of non-HCC malignancies [34]. By combining serum AFP and LR-M criteria, we revised CEUS LI-RADS. A recent CEUS LI-RADS study illustrated that CEUS LR-3, 4, 5, M category combined with elevated level of AFP (AFP > 200 ng/ml) could significantly increase the sensitivity (79.6%) for the diagnosis of HCC while specificity remained high (96.6%) [35]. However, only 67.2% of FLLs in that study were confirmed by histological examination, which may hinder the validity of the gold standard. Furthermore, Li et al. did not evaluate the role of different AFP cut-off values in the CEUS LI-RADS workup. In contrast, our study found that besides 200 ng/ml, incorporating AFP level higher than 400 ng/ml and LR-M category could also satisfy increasing sensitivity without compromising specificity for the diagnosis of HCC.
According to our data, at the AFP cut-off value of 200 ng/ml, there were still 2 CHCs, 2 ICCs and 1 metastasis erroneously reclassified to the LR-5 category. Some non-HCC malignancies with elevated AFP could be observed, which was consistent with previous findings [16]. Furthermore, another research showed that the usefulness of AFP for the diagnosis of HCC in small HCCs was limited [18]. In our subgroup analysis (nodules size > 20 mm), adjustment with AFP level > 100 ng/ml maintained a relatively higher specificity with improved sensitivity, just as other revised CEUS LR-5 did (combining AFP above 200 ng/ml or 400 ng/ml).
There were still several limitations in our study. Firstly, enrolling patients who originated from this single-center, retrospective study with histopathological confirmation may inevitably lead to a small sample size (152 nodules) and selection bias. Prospective validation of multi-center cohorts with large samples is required. Secondly, based on indications of CEUS LI-RADS v2017 [11], patients at high risk for HCC were enrolled in this study, which may be the main reason why there were relatively low incidences of other malignancies than HCC (11.2% vs.78.9%) in this paper, which was similar to previous research (8.7% vs. 84.5%) [31]. Thirdly, since we enrolled high-risk patients strictly following ACR recommendations, the overwhelmingly high rate of hepatitis B virus infection (85%) might limit the application of our findings in the western population. Moreover, the AFP presents several issues in patients with viral hepatitis (physiological increase without HCC), which may influence cut-offs and the applicability of our results. Fourth, the US-guided biopsy can cause false-negative results, which cannot eliminate the possibility of HCC [7]. Fifth, the different US machines adopted in this study may limit the interpretation of images by physicians. However, both machines used in this study were high-end instruments operated by experienced sonographers. In addition, all CEUS images were scanned and stored under the same protocol.
In conclusion, CEUS LR-5 demonstrated a high specificity but a low sensitivity in diagnosing HCC. A combination of LR-M category and serum AFP level at a cut-off value of 200 ng/ml or 400 ng/ml significantly improved CEUS LR-5's ability to diagnose HCCs and LR-M's ability to diagnose non-HCC malignancies.
Abbreviations
- ACR:
-
American college of radiology
- AFP:
-
Alpha-fetoprotein
- AUC:
-
Area under the ROC curve
- CEUS:
-
Contrast-enhanced ultrasound
- CHC:
-
Combined hepatocellular cholangiocarcinoma
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- ICC:
-
Intrahepatic cholangiocarcinoma
- LI-RADS:
-
Liver imaging reporting and data system
- MLC:
-
Metastasis liver carcinoma
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- ROC:
-
Receiver operating characteristic
- US:
-
Ultrasound
References
McGlynn KA, Petrick JL, London WT (2015) Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis 19(2):223–238. https://doi.org/10.1016/j.cld.2015.01.001
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR (2019) A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 16(10):589–604. https://doi.org/10.1038/s41575-019-0186-y
Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ (2014) Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 60(6):1268–1289. https://doi.org/10.1016/j.jhep.2014.01.021
Leoni S, Sansone V, Lorenzo S, Ielasi L, Tovoli F, Renzulli M, Golfieri R, Spinelli D, Piscaglia F (2020) Treatment of combined hepatocellular and cholangiocarcinoma. Cancers (Basel). https://doi.org/10.3390/cancers12040794
Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsoe CP, Piscaglia F, Wilson SR, Barr RG, Chammas MC, Chaubal NG, Chen MH, Clevert DA, Correas JM, Ding H, Forsberg F, Fowlkes JB, Gibson RN, Goldberg BB, Lassau N, Leen EL, Mattrey RF, Moriyasu F, Solbiati L, Weskott HP, Xu HX (2013) Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver–update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM. FLAUS and ICUS Ultraschall Med 34(1):11–29. https://doi.org/10.1055/s-0032-1325499
Schellhaas B, Bernatik T, Dirks K, Jesper D, Mauch M, Potthoff A, Zimmermann P, Strobel D (2021) Contrast-enhanced ultrasound patterns for the non-invasive diagnosis of hepatocellular carcinoma: a prospective multicenter study in histologically proven liver lesions in a real-life setting demonstrating the benefit of extended late phase observation. Ultrasound Med Biol 47(11):3170–3180. https://doi.org/10.1016/j.ultrasmedbio.2021.07.010
Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, Han GH, Wang MQ, Liu RB, Lu LG, Ren ZG, Chen MS, Zeng ZC, Liang P, Liang CH, Chen M, Yan FH, Wang WP, Ji Y, Cheng WW, Dai CL, Jia WD, Li YM, Li YX, Liang J, Liu TS, Lv GY, Mao YL, Ren WX, Shi HC, Wang WT, Wang XY, Xing BC, Xu JM, Yang JY, Yang YF, Ye SL, Yin ZY, Zhang BH, Zhang SJ, Zhou WP, Zhu JY, Liu R, Shi YH, Xiao YS, Dai Z, Teng GJ, Cai JQ, Wang WL, Dong JH, Li Q, Shen F, Qin SK, Fan J (2018) Guidelines for diagnosis and treatment of primary liver cancer in China (2017 Edition). Liver Cancer 7(3):235–260. https://doi.org/10.1159/000488035
Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, Hiraoka A, Ikeda M, Ogasawara S, Yamashita T, Minami T, Yamakado K, Liver Cancer Study Group of J (2014) JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer 3(3–4):458–468. https://doi.org/10.1159/000343875
Ayuso C, Rimola J, Vilana R, Burrel M, Darnell A, García-Criado Á, Bianchi L, Belmonte E, Caparroz C, Barrufet M, Bruix J, Brú C (2018) Diagnosis and staging of hepatocellular carcinoma (HCC): current guidelines. Eur J Radiol 101:72–81. https://doi.org/10.1016/j.ejrad.2018.01.025
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA (2018) AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67(1):358–380. https://doi.org/10.1002/hep.29086
ACR American College of Radiology (2017) Liver Imaging Reporting and Data System. American College of Radiology. https://www.acr.org/Quality-Safety/Resources/LIRADS. Accessed 12 Sep 2017
Bartolotta TV, Terranova MC, Gagliardo C, Taibbi A (2020) CEUS LI-RADS: a pictorial review. Insights Imaging 11(1):9. https://doi.org/10.1186/s13244-019-0819-2
Huang JY, Li JW, Lu Q, Luo Y, Lin L, Shi YJ, Li T, Liu JB, Lyshchik A (2020) Diagnostic accuracy of CEUS LI-RADS for the characterization of liver nodules 20 mm or smaller in patients at risk for hepatocellular carcinoma. Radiology 294(2):329–339. https://doi.org/10.1148/radiol.2019191086
Terzi E, Iavarone M, Pompili M, Veronese L, Cabibbo G, Fraquelli M, Riccardi L, De Bonis L, Sangiovanni A, Leoni S, Zocco MA, Rossi S, Alessi N, Wilson SR, Piscaglia F, collaborators CL-RIsg (2018) Contrast ultrasound LI-RADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1,006 nodules. J Hepatol 68(3):485–492. https://doi.org/10.1016/j.jhep.2017.11.007
Zhou H, Zhang C, Du L, Jiang J, Zhao Q, Sun J, Li Q, Wan M, Wang X, Hou X, Wen Q, Liu Y, Zhou X, Huang P (2022) Contrast-enhanced ultrasound liver imaging reporting and data system in diagnosing hepatocellular carcinoma: diagnostic performance and interobserver agreement. Ultraschall Med 43(1):64–71. https://doi.org/10.1055/a-1168-6321
Hanif H, Ali MJ, Susheela AT, Khan IW, Luna-Cuadros MA, Khan MM, Lau DT (2022) Update on the applications and limitations of alpha-fetoprotein for hepatocellular carcinoma. World J Gastroenterol 28(2):216–229. https://doi.org/10.3748/wjg.v28.i2.216
Johnson P, Zhou Q, Dao DY, Lo YMD (2022) Circulating biomarkers in the diagnosis and management of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. https://doi.org/10.1038/s41575-022-00620-y
Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK (2017) Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 11(4):317–370. https://doi.org/10.1007/s12072-017-9799-9
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69(1):182–236. https://doi.org/10.1016/j.jhep.2018.03.019
Yang J, Zhang YH, Li JW, Shi YY, Huang JY, Luo Y, Liu JB, Lu Q (2020) Contrast-enhanced ultrasound in association with serum biomarkers for differentiating combined hepatocellular-cholangiocarcinoma from hepatocellular carcinoma and intrahepatic cholangiocarcinoma. World J Gastroenterol 26(46):7325–7337. https://doi.org/10.3748/wjg.v26.i46.7325
Zhang HC, Zhu T, Hu RF, Wu L (2020) Contrast-enhanced ultrasound imaging features and clinical characteristics of combined hepatocellular cholangiocarcinoma: comparison with hepatocellular carcinoma and cholangiocarcinoma. Ultrasonography 39(4):356–366. https://doi.org/10.14366/usg.19093
Tayob N, Kanwal F, Alsarraj A, Hernaez R, El-Serag HB (2022) The performance of AFP, AFP-3, DCP as biomarkers for detection of hepatocellular carcinoma (HCC). A phase 3 Biomarker study in the United States. Clin Gastroenterol Hepatol. https://doi.org/10.1016/j.cgh.2022.01.047
Gambarin-Gelwan M, Wolf DC, Shapiro R, Schwartz ME, Min AD (2000) Sensitivity of commonly available screening tests in detecting hepatocellular carcinoma in cirrhotic patients undergoing liver transplantation. Am J Gastroenterol 95(6):1535–1538. https://doi.org/10.1111/j.1572-0241.2000.02091.x
Chan SL, Mo F, Johnson PJ, Siu DY, Chan MH, Lau WY, Lai PB, Lam CW, Yeo W, Yu SC (2014) Performance of serum alpha-fetoprotein levels in the diagnosis of hepatocellular carcinoma in patients with a hepatic mass. HPB (Oxford) 16(4):366–372. https://doi.org/10.1111/hpb.12146
Trevisani F, D’Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S, Roda E, Bernardi M (2001) Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol 34(4):570–575. https://doi.org/10.1016/s0168-8278(00)00053-2
Schellhaas B, Wildner D, Pfeifer L, Goertz RS, Hagel A, Neurath MF, Strobel D (2016) LI-RADS-CEUS - proposal for a contrast-enhanced ultrasound algorithm for the diagnosis of hepatocellular carcinoma in high-risk populations. Ultraschall Med 37(6):627–634. https://doi.org/10.1055/s-0042-112221
Zeng D, Xu M, Liang JY, Cheng MQ, Huang H, Pan JM, Huang Y, Tong WJ, Xie XY, Lu MD, Kuang M, Chen LD, Hu HT, Wang W (2022) Using new criteria to improve the differentiation between HCC and non-HCC malignancies: clinical practice and discussion in CEUS LI-RADS 2017. Radiol Med 127(1):1–10. https://doi.org/10.1007/s11547-021-01417-w
Zheng W, Li Q, Zou XB, Wang JW, Han F, Li F, Huang LS, Li AH, Zhou JH (2020) Evaluation of contrast-enhanced US LI-RADS version 2017: application on 2020 liver nodules in patients with hepatitis B infection. Radiology 294(2):299–307. https://doi.org/10.1148/radiol.2019190878
Li W, Li L, Zhuang BW, Ruan SM, Hu HT, Huang Y, Lin MX, Xie XY, Kuang M, Lu MD, Chen LD, Wang W (2021) Inter-reader agreement of CEUS LI-RADS among radiologists with different levels of experience. Eur Radiol 31(9):6758–6767. https://doi.org/10.1007/s00330-021-07777-1
Kleiner DE (2018) Hepatocellular carcinoma: liver biopsy in the balance. Hepatology 68(1):13–15. https://doi.org/10.1002/hep.29831
Ding J, Qin Z, Zhou Y, Zhou H, Zhang Q, Wang Y, Jing X, Wang F (2021) Impact of revision of the LR-M criteria on the diagnostic performance of contrast-enhanced ultrasound LI-RADS. Ultrasound Med Biol 47(12):3403–3410. https://doi.org/10.1016/j.ultrasmedbio.2021.08.007
Galle PR, Foerster F, Kudo M, Chan SL, Llovet JM, Qin S, Schelman WR, Chintharlapalli S, Abada PB, Sherman M, Zhu AX (2019) Biology and significance of alpha-fetoprotein in hepatocellular carcinoma. Liver Int 39(12):2214–2229. https://doi.org/10.1111/liv.14223
Wen N, Cai Y, Li F, Ye H, Tang W, Song P, Cheng N (2022) The clinical management of hepatocellular carcinoma worldwide: a concise review and comparison of current guidelines: 2022 update. Biosci Trends 16(1):20–30. https://doi.org/10.5582/bst.2022.01061
Zhou Y, Yin S, Zhao L, Zhang X, Li M, Ding J, Yan K, Jing X (2022) CEUS and CT/MRI LI-RADS in association with serum biomarkers for differentiation of combined hepatocellular-cholangiocarcinoma from hepatocellular carcinoma. Front Oncol 12:897090. https://doi.org/10.3389/fonc.2022.897090
Li CQ, Huang H, Ruan SM, Hu HT, Xian MF, Xie XY, Lu MD, Kuang M, Wang Y, Chen LD (2022) An assessment of liver lesions using a combination of CEUS LI-RADS and AFP. Abdom Radiol (NY). https://doi.org/10.1007/s00261-022-03428-1
Funding
This work was supported by National Natural Science Foundation of China for Youth Scholars (Grant No. 82202241); Heilongjiang Postdoctoral Science Foundation (Grant No. LBH-Z21022); Innovative Research Project of Harbin Medical University (Grant No. 31041210025).
Author information
Authors and Affiliations
Contributions
Conceptualization: JW; Data curation: WG, RM; Formal analysis: HW, RM; Funding acquisition: HZ; Investigation: JW, CW; Methodology: MW, WX; Project administration: HZ, XZ; Resources: JW, HW; Software: ZJ, WX; Supervision: HZ, XZ; Validation: MW, CW; Visualization: ZJ; Writing-original draft: WG, HZ; Writing-review and editing: WG, JW, HZ; all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee the Second Affiliated Hospital of Harbin Medical University.
Ethcial statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Written informed consent was waived by the Institutional Review Board of the Second Affiliated Hospital of Harbin Medical University.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in Figs. 3 and 4.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gong, W., Wu, J., Wei, H. et al. Combining serum AFP and CEUS LI-RADS for better diagnostic performance in Chinese high-risk patients. Radiol med 128, 393–401 (2023). https://doi.org/10.1007/s11547-023-01614-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-023-01614-9