Abstract
Purpose
To improve noninvasive diagnosis of HCC using a combination of CE US LI-RADS and alpha-fetoprotein (AFP).
Methods
757 solitary liver nodules from 757 patients at risk of HCC with CE US and serum AFP test were categorized as LR-1 to LR-5 through LR-M according to CE US LI-RADS version 2017. In LR-3, LR-4, and LR-M nodules, those with AFP > 200 ng/ml were reclassified as mLR-5. Nodules with LR-5 and mLR-5 were reclassified as definitely HCC to modify CE US LI-RADS. Diagnostic performance was assessed with specificity, sensitivity, and PPV.
Results
The sensitivity, specificity, and PPV of LR-5 as a predictor of HCC were 64.7%, 97.8%, and 98.9%, respectively. 32.1% patients with solitary liver nodule had AFP greater than 200 ng/ml, of which 98.8% were HCC (25.8%, 7.5%, 2.5% assigned to LR-M, LR-4, LR-3, respectively) and 1.2% were Combined Hepatocellular Cholangiocarcinoma. After modification, the sensitivity increased to 79.6% (P < 0.001), while specificity and PPV remained high (96.6% and 98.7%, P > 0.050).
Conclusion
The combination of CE US LI-RADS and AFP for diagnosing HCC improved diagnostic sensitivity significantly, while maintaining high PPV and specificity in patients with the solitary liver nodule.
Graphical abstract

Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death globally [1]. The noninvasive diagnostic criteria for HCC are improving. In the guideline published by the American Association for the Study of Liver Diseases (AASLD) in 2005, the criteria for diagnosing high-risk patients with HCC includes alpha-fetoprotein (AFP). When AFP is higher than 200 ng/ml, nodules larger than 2 cm with typical imaging findings suggest HCC [2,3,4]. In 2010, AFP is excluded from the noninvasive diagnostic criteria by AASLD since AFP also increases in intrahepatic cholangiocarcinoma (ICC), colon cancer, gastric cancer metastasis, and most small HCC (< 2 cm) do not have elevated AFP levels. Furthermore, it lacks adequate sensitivity and specificity for the diagnosis [5,6,7]. In the latest Practice Guidance updated by AASLD in 2018 [8], it suggests that Liver Imaging Reporting and Data System (LI-RADS) on multiphase imaging should be applied to enable noninvasive diagnosis of HCC in high-risk patients, but AFP is still not included.
LI-RADS, proposed by the American College of Radiology (ACR), standardizes the imaging diagnosis of HCC according to the specific characteristics. Two series including CT/MRI and Contrast-Enhanced US (CE US) are released [9, 10]. Like CT/MR, the CE US LI-RADS cannot achieve optimal sensitivity with high specificity for the noninvasive diagnosis of HCC [11, 12]. Several studies have shown that the specificity was up to 98%, while the sensitivity was only 60–75% [13,14,15,16]. This means that 25–40% of HCC cannot obtain a noninvasive diagnosis and requires an invasive biopsy. A large multicenter retrospective study of CE US LI-RADS showed that a risk range of HCC observed in LR-4 lesions was 85–90%, while 48% in LR-M and 47% in LR-3 [17]. To improve the noninvasive diagnostic algorithms, many studies have focused on the improvement of imaging characteristics or standards, but most studies ignored the non-imaging index of AFP.
Serum AFP levels are higher than normal in 60% to 80% of HCC and have great significance for screening and evaluation of treatment response as a simple, rapid, and easy-to-obtain test. Despite the comments of a systematic review that AFP with a cut-off value of 200 ng/ml had no adequate sensitivity and specificity [18, 19], the latest research showed that AFP combined with other serum markers has high sensitivity (91%) and specificity (83%) for the diagnosis of HCC [20]. Chan SL et al. indicated that the use of AFP with a cut-off value of 200 ng/ml may help clinicians to diagnose with high specificity and reasonable sensitivity and suggested that serum AFP should not be excluded from the noninvasive diagnostic criteria of HCC [21]. Coincidentally, these studies only focused on improving laboratory indicators and ignored imaging findings.
No study has concerned the value of combining AFP and LI-RADS for the noninvasive diagnosis of HCC. Therefore, we compared the diagnostic performance of CE US LI-RADS v2017 and the combination of CE US LI-RADS and AFP, trying to improve the noninvasive diagnostic criteria of HCC.
Materials and methods
Patients
This study was approved by the Institutional Review Board of our institution and informed consent was obtained from each patient. This study with patients collected prospectively and data analyzed retrospectively finally conducted about 757 patients between September 2016 and July 2018 with focal liver lesions (FLL). These data were selected based on the following criteria: (1) age 18 years or older, (2) visible solitary liver nodule at the US, (3) final diagnosis proved clinically or histopathologically, (4) availability of serum AFP levels concentration within 1 month of histological diagnosis and before any treatment for cancer, and (5) with high risk for HCC (cirrhosis or chronic hepatitis viral infection).
Those patients who were not FLL or previously treated, those with cirrhosis because of vascular disorder, those who had tumor in vein (TIV), and those with poor CE US image quality were ruled out.
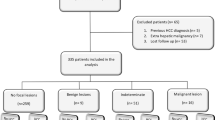
A flow diagram of our study is presented in Fig. 1.
CE US technique
US studies for scanning the entire liver were first performed by an experienced radiologist with the equipment as following: (1) Aixplorer Ultrasound system (SuperSonic Imagine, Aix-en-Provence, France) equipped with the SC6-1 convex probe with a frequency range of 1.0 to 6.0 MHz. (2) Acuson Sequoia 512 (Siemens Medical Solutions, Mountain View, CA, USA) with a 4V1 vector transducer with a frequency range of 1.0 to 4.0 MHz. (3) Aplio SSA-770 or Aplio 500 (Toshiba Medical Systems, Tokyo, Japan) with a 375BT convex transducer with a frequency range of 1.9 to 6.0 MHz. A low mechanical index (< 0.1) was selected. After identifying the target lesion on baseline US and storing images recorded size, location, and echogenicity, CE US examination was performed after administration of 2.4 ml of SonoVue (Bracco Imaging, Milan, Italy) within 1–2 s into the antecubital vein followed by a 5 ml normal saline flush. The target lesion was observed continuously for the arterial and beginning of portal venous phases (up to 60 s after the contrast injection) and intermittently after 1-min post-injection and recording the dynamic image of the process simultaneously. The examination needs to be continued for at least 5 min.
Reference standard
All nodules in this study were confirmed by histopathological or comprehensive clinical diagnosis.
Clinical diagnosis criteria: (1) lesion assigned to LR-5 by CT/MRI LI-RADS v2018 can be diagnosed as definite HCC; (2) lesion with typical features of hemangioma on CT/MR (peripheral discontinuous globular enhancement with progressive centripetal fill-in in the portal venous phase) and no change of size during a follow-up period of more than 1 year can be diagnosed as definite hemangioma [22]; (3) lesion with no enhancement in all phases and considered cyst by CT/MRI can be diagnosed as definite cyst; (4) lesion from which pus can be extracted by percutaneous aspiration or considered liver abscess by CT/MRI with size reduced in follow-up can be diagnosed as definite liver abscess; (5) lesion in patient with history of extrahepatobiliary tumor that grew after follow-up more than 6 months or revealed typical imaging features (halo sign/bull's eye sign) on two imaging modalities can be diagnosed as definite metastatic liver cancer (MLC) [23]; and (6) lesion with typical imaging features of regenerative nodule or focal fatty sparing on CT or MRI and no change in size during a follow-up period of more than 1 year can be diagnosed as definite regenerative nodule or focal fatty sparing [24].
Except for the lesions mentioned above, other malignant or benign lesions, such as ICC, combined hepatocellular cholangiocarcinoma (CHC), and focal nodular hyperplasia (FNH), only could be evaluated histologically for definitive diagnosis.
CE US LI-RADS categories
All images of baseline US and CE US procedure were independently analyzed by two experienced radiologists (not participating in original examinations) with more than 5 years of experience in hepatic imaging and CE US. If there is a disagreement between the two readers, a third more experienced radiologist (with 10 years of hepatic CE US imaging experience) reevaluated images to reach a consensus. All of them were blinded to the results of pathologic evaluation or any other diagnostic information.
All nodules were categorized as CE US LR-1 to LR-5 or LR-M according to CE US LI-RADS v2017. The enhancement and washout pattern can be studied during the process which was divided into arterial (0–30 s after contrast agent injection), portal venous (31–120 s), and late phases (121–300 s). Hyperenhancement is defined as entirely or partially hyperechoic compared with the surrounding liver parenchyma and the patterns of which were described as homogenous, heterogeneous, or rim enhancement. Washout is described as occurring of hypoechoic relative to liver beginning in or after the arterial phase. Early washout is defined as that occurring within 60 s after injection of the contrast agent, and marked washout is defined when punched out appearance (markedly hypoechoic emerging black) appears within 2 min. The typical enhancement pattern of HCC on CE US was arterial phase hyperenhancement (not rim and peripheral discontinuous globular) with portal venous phase mild and late (beginning time > 60 s) washout.
Modified CE US LI-RADS combined with AFP
To evaluate the diagnostic performance of CE US LI-RADS with or without the combination of AFP, we modified CE US LI-RADS algorithm as follows:
-
(1)
LR-3 nodules with AFP > 200 ng/ml were recategorized as mLR-5a,
-
(2)
LR-4 nodules with AFP > 200 ng/ml were recategorized as mLR-5b, and
-
(3)
LR-M nodules with AFP > 200 ng/ml were recategorized as mLR-5c,
then these nodules of mLR-5a/b/c together with LR-5 nodules were reclassified as definitely HCC (Fig. 2).
The diagnostic performance analysis of LR-5 and adding mLR-5a/b/c as well as AFP alone as the predictors of HCC were conducted, respectively.
Statistical analysis
Characteristics of the lesions were reported as proportion and absolute numbers. A comparison of categorical variables was done using the Paired Chi-square test to analyze the significance of the difference that was set at P < 0.05. The overall diagnostic performance of LI-RADS was assessed by sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with 95% confidence interval (CI). Statistical analysis was performed using Medcalc (version 11.2, Mariakerke, Belgium).
Results
The final study sample consisted of 757 solitary nodules from 757 patients (median age, 52 years ± 11 [standard deviation]; 603 men). Each patient had HBV infection and 6 patients had HBV and HCV infection. The etiology of liver cirrhosis in patients was chronic HBV or HCV hepatitis; there were no patients with liver cirrhosis caused by alcoholic hepatitis or nonalcoholic fatty liver disease. Of 757 lesions, 67.4% were proven pathologically, and in 578 HCC, 385 were pathologically confirmed and 193 were clinically diagnosed. The diagnoses of nodules are detailed in Table 1. 70.5% of benign nodules were confirmed by means of histologic evaluation and the rest by composite clinical and imaging reference standard. Basic characteristics of patients and nodules are summarized in Table 1. 32.1% of lesions had AFP greater than 200 ng/ml, of which 98.8% were HCC and 1.2% were CHC.
LI-RADS categories and diagnostic performance
Of 757 nodules, 18 cyst, 13 hemangioma, and 1 cholesteatoma were assigned to LR-1, 6 hemangioma, 1 FNH, 1 regenerative nodule, and 1 focal fatty sparing were assigned to LR-2, and there were 107 (14.1%) LR-3, 47 (6.2%) LR-4, 184 (24.3%) LR-M, and 378 (49.9%) LR-5 nodules. No malignancies were classified as LR-1 or LR-2. 18.7%, 83.0%, 98.9%, and 78.8% nodules of LR-3, LR-4, LR-5, and LR-M were HCC. Diagnostic classification of nodules in LR-3–LR-5 and LR-M are shown in Table 2.
The proportion of HCC was progressively higher in the LR-3 to LR-5 categories. However, only 374 (64.7%) of 578 HCC nodules were correctly classified as LR-5 and the remaining 39 (6.7%) were classified as LR-4; 20 (3.5%) as LR-3 and 145 (25.1%) as LR-M. The sensitivity, specificity, PPV, and NPV of LR-5 as a predictor of HCC were 64.7% (95% CI 61, 69), 97.8% (95% CI 94, 99), 98.9% (95% CI 97, 99), and 46.2% (95% CI 43, 49).
The sensitivity, specificity, and NPV of LR-M to correctly classify non-HCC malignancy were 87.5% (95% CI 73, 96), 79.2% (95% CI 76, 82), and 99.1% (95% CI 98, 100), respectively, whereas the PPV was only 19.0% (95% CI 16, 22).
Diagnostic performance of modified LI-RADS combined with AFP
41.5% HCC cases had AFP levels over 200 ng/ml. Only 3 CHC cases had AFP > 200 ng/ml in 40 non-HCC malignancies. Of 17 ICC cases, 8 cases had elevated AFP levels (> 20 ng/ml), but the values were all less than 100 ng/ml. In 15 cases of metastasis that had elevated AFP levels, the values were also less than 200 ng/ml. 34.8% LR-M nodules had AFP levels over 200 ng/ml, of which 96.9% were HCC and 3.1% were CHC. All the nodules classified as LR-3/4 with AFP > 200 ng/ml were HCC (Table 2).
The sensitivity, specificity, PPV, and NPV of AFP > 200 ng/ml as a predictor of HCC were 41.5% (95% CI 37, 46), 98.3% (95% CI 95, 99), 98.8% (95% CI 96, 99), and 34.2% (95% CI 33, 36).
The nodules classified to LR-3 mostly were less than 2 cm. There were only 2.2% of the nodules smaller than 2 cm with AFP > 200 ng/ml in LR-3, 27.3% in LR-4, and 36.3% in LR-5, while none of the nodules less than 2 cm had AFP > 200 ng/ml in LR-M (Table 3).
After the combination of CE US LI-RADS and AFP, 86 HCC which as classified as LR-3/4/M was accurately diagnosed as definitely HCC and the diagnostic accuracy increased by 14.9% (86/578), while only 2 non-HCC malignancies were misclassified as mLR-5 (Table 2), and about 90% of these nodules "upgraded" by AFP were larger than 2 cm. 62 HCC originally classified as LR-M were reclassified correctly to HCC (LR-5). The cases diagnosed as definitely HCC increased significantly (from 64.7 to 68.9–79.6%), up to 86 nodules of HCC have been reclassified correctly, and the number of misclassified HCC decreased from 204 to 118, which may reduce the necessity of biopsy (Table 2). After modification, the sensitivity of LR-5 + mLR-5(a + b + c) as a predictor of HCC significantly increased to 79.6% (95% CI 76, 83) (P < 0.001) compared with CE US LI-RADS or AFP alone, while specificity and PPV remained high (96.6% [95% CI 93, 99] (P > 0.050) and 98.7% [95% CI 97, 99] (P > 0.050), respectively). The sensitivity of LR-5 + mLR-5(b + c) or LR-5 + mLR-5c as a predictor of HCC also increased significantly (from 64.7 to 78.5%, P < 0.001), with a negligible drop in specificity (from 97.8 to 96.6%) and PPV (from 98.9 to 98.6%) (Table 4).
After reclassification based on the combination of CEUS LI-RADS and AFP, the specificity of LR-M to correctly classify non-HCC malignancies was significantly improved (from 79.2 to 87.9%, P < 0.001), while NPV was still 98.9%. The modified LI-RADS did not affect the classification accuracy of non-HCC malignancies by LR-M.
Discussion
In our study, the noninvasive diagnostic performance of CE US LI-RADS assisted with AFP for HCC diagnosis was analyzed. Our study demonstrated that the sensitivity of the combination of CE US LI-RADS and AFP was improved significantly than that of LI-RADS or AFP alone, while maintaining a high PPV in patients with the solitary liver nodule.
The improvement of noninvasive diagnostic criteria is of great significance to improve the accuracy of liver cancer diagnoses. Since the AASLD guideline has excluded AFP, imaging plays an essential role in the diagnosis of HCC due to the further perfect imaging technology. CE US LI-RADS has the same sensitivity and specificity as CT/MRI, and CE US can be used for diagnosis in all patients except those with definite contraindications (such as allergic reaction to contrast agent) or unsatisfied observation on lesion at the US. Most studies have shown that this system has high PPV and specificity for noninvasive diagnosis of HCC in Western cirrhosis patients or high-risk groups of HBV infection in Asia Pacific region, but without ideal sensitivity [11, 17, 24, 25]. About 30–50% of HCC may be assigned to other categories of LR-3, LR-4, and LR-M [17, 26,27,28]. Our data showed that CE US LI-RADS category LR-5 also had a high PPV (98.9%) for diagnosing HCC but not high sensitivity (64.7%), validating arterial phase hyperenhancement with late and mild washout as the diagnostic criteria for LR-5 nodules. This is consistent with previous reports [17].
However, current researches only focus on the analysis and improvement of imaging characteristics to modify the criterion of LI-RADS for increasing diagnostic accuracy [26]. Our study firstly combined CE US LI-RADS and AFP to explore an improved noninvasive diagnostic algorithm.
The reason for the exclusion of AFP from HCC diagnosis in guidelines version 2015 is that it lacks adequate sensitivity and specificity for the diagnosis and some literature mentioned that AFP has also increased in ICC and MLC, but most of them are case reports [6, 29, 30], and there is no large amount of data for statistical analysis of the correlation between AFP and non-HCC malignancy. Furthermore, there were researches which indicated that AFP has high specificity with good sensitivity when the threshold is 200 ng/ml [18, 19]. Some studies also believed that AFP to complement ultrasound may improve the detection of early HCC in clinical practice, although AFP was not optimal, and better methods for early HCC were necessary [31, 32].
According to our data, the non-HCC malignancy with AFP > 200 ng/ml was only CHC. In all of 17 ICC cases, no one had AFP levels higher than 200 ng/ml. No matter whether the LI-RADS category is modified or not, no ICC in our study is wrongly classified as LR-5, which proves that CE US is unlikely to misdiagnose ICC as HCC in high-risk background [33]. As for MLC, the incidence rate is quite low in high-risk patients, and the 15 cases of MLC had mild-elevated AFP levels less than 100 ng/ml. As a result, the combination of LI-RADS and AFP did not weaken the diagnostic accuracy. Our data have shown that the specificity and PPV of serum AFP > 200 ng/ml as a predictor of HCC was as high as 98.3% and 98.8%, which indicates AFP is useful for the diagnosis of HCC. The weakness of AFP in HCC diagnosis is the low sensitivity as only 41.5% cases of HCC had elevated AFP levels.
The sensitivity of LI-RADS or AFP alone as a predictor of HCC independently is not high, so investigation for refined diagnostic value of the combination of CE US LI-RADS and AFP would be performed. After reclassification, we found the sensitivity of modified LI-ARDS for HCC diagnosis was significantly higher than LI-RADS v2107 with keeping the same high PPV (98.7%), for an increased case of 14.9% of HCC (86 of 578) in LR-3/4/M was accurately diagnosed, which means these patients can get clinical confirmed diagnoses without biopsy or repeat imaging examination. After modification, the percentage of HCC which can be diagnosed without biopsy significantly improved from 64.7 to 79.6%. On the other hand, the percentage of HCC which need a biopsy or repeat imaging examination dropped dramatically from 35.3 to 20.4%. Since biopsy has disadvantages, including invasiveness, false-negative diagnosis due to the limited sample tissue, risk of needle track implantation metastasis, and bleeding [34, 35]. At the same time, it can avoid unnecessarily repeated examination or delaying the confirmative diagnosis, thus delaying the early and timely treatment of patients. There was a negligible drop in specificity in our study. The reason for the slight drop was that 2 cases of CHC in LR-M were recategorized as LR-5. However, due to the very low incidence rate of CHC, the impact on PPV for diagnosing HCC is negligible.
In our study, 78.8% of LR-M nodules were HCC, which is higher than other recent studies. It was also shown in a study by Huang et al. where 75% of LR-M were HCC and over 90% chronic hepatitis B in the study sample [36]. That may be due to high-HBV prevalence (all patients in our study had HBV infection) and the very high prevalence of HCC in China, which are reported to account for over 50% of cases of HCC worldwide [37, 38].
There are limitations to our study. First, there are fewer cases of benign lesions and non-HCC malignancies, which is related to the fact that most of the benign lesions or non-HCC malignancies cannot meet the strict diagnostic criteria of our study, and we screened based on the high-risk factors of HCC. Second, our study only included patients with solitary liver nodule and relatively single clinical condition. The former is because some liver nodules may be overestimated by LI-RADS combined with AFP in patients with multiple liver nodules, but the accurate diagnosis of HCC in these patients also need to pay attention. The latter is due to the national condition in our country that China is a high-HBV prevalence region, and chronic hepatitis B infection is the leading cause of HCC in China [37, 39]. Third, in our study, most small nodules (< 2 cm) had not AFP levels over 200 ng/ml, and 90% of nodules “upgraded” by AFP were larger than 2 cm, which means that our modified CE US LI-RADS may not have good diagnostic performance for small HCC, most of which had not elevated AFP. Fourth, we used data from a single-center experience of patients with FLL. Last, our study results need more tests for verification.
Conclusion
The combination of CEUS LI-RADS and AFP could significantly improve the sensitivity of the diagnostic algorithm, while maintaining quite high PPV, which could reduce the necessity of the invasive biopsy or repeat imaging examinations in patients with the solitary liver nodule. So CE US LI-RADS combined with AFP may be more suitable for the diagnosis of HCC in patients with the solitary liver nodule.
Abbreviations
- AFP:
-
Alpha-fetoprotein
- AML:
-
Angiomyolipoma
- APHE:
-
Arterial phase hyperenhancement
- CHC:
-
Combined hepatocellular cholangiocarcinoma
- CE:
-
Contrast enhanced
- CI:
-
Confidence interval
- FLL:
-
Focal liver lesions
- FNH:
-
Focal nodular hyperplasia
- HCC:
-
Hepatocellular carcinoma
- ICC:
-
Intrahepatic cholangiocarcinoma
- LI-RADS:
-
Liver Imaging Reporting and Data System
- MLC:
-
Metastatic liver cancer
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- TIV:
-
Tumor in vein
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394-424.
Bruix J, Sherman M (2005) Management of hepatocellular carcinoma. Hepatology 42(5):1208–36.
Levy I, Greig PD, Gallinger S, Langer B, Sherman M (2001) Resection of hepatocellular carcinoma without preoperative tumor biopsy. Ann Surg 234(2):206–9.
Torzilli G, Minagawa M, Takayama T et al (1999) Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology 30(4):889–93.
Adachi Y, Tsuchihashi J, Shiraishi N, Yasuda K, Etoh T, Kitano S (2003) AFP-producing gastric carcinoma: multivariate analysis of prognostic factors in 270 patients. Oncology 65(2):95–101.
Sato Y, Sekine T, Ohwada S (1994) Alpha-fetoprotein-producing rectal cancer: calculated tumor marker doubling time. J Surg Oncol 55(4):265–8.
Bruix J, Sherman M (2011) Management of hepatocellular carcinoma: an update. Hepatology 53(3):1020–2.
Marrero JA, Kulik LM, Sirlin CB et al (2018) Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 68(2):723-50.
Piscaglia F, Wilson SR, Lyshchik A et al (2017) American College of Radiology Contrast Enhanced Ultrasound Liver Imaging Reporting and Data System (CEUS LI-RADS) for the diagnosis of Hepatocellular Carcinoma: a pictorial essay. Ultraschall Med 38(3):320–324.
Kono Y, Lyshchik A, Cosgrove D et al (2017) Contrast Enhanced Ultrasound (CEUS) Liver Imaging Reporting and Data System (LI-RADS ®): the official version by the American College of Radiology (ACR). Ultraschall Med 38(1):85–86.
Jo PC, Jang H, Burns PN, Burak KW, Kim TK, Wilson SR (2017) Integration of Contrast-enhanced US into a Multimodality Approach to Imaging of Nodules in a Cirrhotic Liver: How I Do It. Radiology 282(2):317–31.
Kim TK, Noh SY, Wilson SR et al (2017) Contrast-enhanced ultrasound (CEUS) liver imaging reporting and data system (LI-RADS) 2017 – a review of important differences compared to the CT/MRI system. Clin Mol Hepatol 23(4):280-289.
Choi BI, Lee JM, Kim TK, Dioguardi BM, Vilgrain V (2015) Diagnosing Borderline Hepatic Nodules in Hepatocarcinogenesis: Imaging Performance. AJR Am J Roentgenol 205(1):10–21.
Boozari B, Soudah B, Rifai K et al (2011) Grading of hypervascular hepatocellular carcinoma using late phase of contrast enhanced sonography - a prospective study. Dig Liver Dis 43(6):484–90.
Mitchell DG, Bruix J, Sherman M, Sirlin CB (2015) LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 61(3):1056–65.
Tang A, Valasek MA, Sirlin CB (2015) Update on the Liver Imaging Reporting and Data System: What the Pathologist Needs to Know. Adv Anat Pathol 22(5):314–22.
Terzi E, Iavarone M, Pompili M, Veronese L, Cabibbo G, Fraquelli M (2018) Contrast ultrasound LI-RADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1,006 nodules. J Hepatol 68(3):485–492.
Tateishi R, Yoshida H, Matsuyama Y, Mine N, Kondo Y, Omata M (2008) Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int 2(1):17–30.
Zhang J, Chen G, Zhang P et al (2020) The threshold of alpha-fetoprotein (AFP) for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. PloS One 15(2):e228857.
Song PP, Xia JF, Inagaki Y et al (2016) Controversies regarding and perspectives on clinical utility of biomarkers in hepatocellular carcinoma. World J Gastroenterol 22(1):262–74.
Chan SL, Mo F, Johnson PJ et al (2014) Performance of serum alpha-fetoprotein levels in the diagnosis of hepatocellular carcinoma in patients with a hepatic mass. HPB (Oxford) 16(4):366–72.
Lv P, Lin XZ, Li J, Li W, Chen K (2011) Differentiation of small hepatic hemangioma from small hepatocellular carcinoma: recently introduced spectral CT method. Radiology 259(3):720–9.
Lee DH, Lee JM, Hur BY et al (2016) Colorectal Cancer Liver Metastases: Diagnostic Performance and Prognostic Value of PET/MR Imaging. Radiology 280(3):782–92.
Zheng W, Li Q, Zou XB et al (2020) Evaluation of Contrast-enhanced US LI-RADS version 2017: Application on 2020 Liver Nodules in Patients with Hepatitis B Infection. Radiology 294(2):299-307.
Bolondi L, Cillo U, Colombo M et al (2013) Position paper of the Italian Association for the Study of the Liver (AISF): The multidisciplinary clinical approach to hepatocellular carcinoma. Dig Liver Dis 45(9):712–23.
Schellhaas B, Gortz RS, Pfeifer L, Kielisch C, Neurath MF, Strobel D (2017) Diagnostic accuracy of contrast-enhanced ultrasound for the differential diagnosis of hepatocellular carcinoma: ESCULAP versus CEUS-LI-RADS. Eur J Gastroenterol Hepatol 29(9):1036–44.
Huang JY, Li JW, Lu Q et al (2020) Diagnostic Accuracy of CEUS LI-RADS for the Characterization of Liver Nodules 20 mm or Smaller in Patients at Risk for Hepatocellular Carcinoma. Radiology 294(2):329–39.
Schellhaas B, Hammon M, Strobel D et al (2018) Interobserver and intermodality agreement of standardized algorithms for non-invasive diagnosis of hepatocellular carcinoma in high-risk patients: CEUS-LI-RADS versus MRI-LI-RADS. Eur Radiol 28(10):4254–64.
Yoh T, Kato T, Hirohata Y, Nakamura Y, Nakayama H, Okamura R (2016) Cholangiolocellular carcinoma with rapid progression initially showing abnormally elevated serum alfa-fetoprotein. Clin J Gastroenterol 9(4):257–60.
Lin YX, Fu YY, Cui T, Jia QB (2018) Hepatobiliary and Pancreatic: Alpha fetoprotein producing distal cholangiocarcinoma metastasized to the ovary. J Gastroenterol Hepatol 33(6):1167.
Lok AS, Sterling RK, Everhart JE et al (2010) Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology 138(2):493–502.
Singal A, Volk ML, Waljee A et al (2009) Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 30(1):37–47.
Chen LD, Ruan SM, Liang JY et al (2019) Comparison between M-score and LR-M in the reporting system of contrast-enhanced ultrasound LI-RADS. Eur Radiol 29(8):4249–57.
David E. Kleiner BM (2018) Hepatocellular Carcinoma: Liver Biopsy in the Balance. Hepatology 68(1): 13–15.
Di Tommaso L, Spadaccini M, Donadon M et al (2019) Role of liver biopsy in hepatocellular carcinoma. World J Gastroenterol 25(40):6041–52.
Huang JY, Li JW, Lu Q, et al (2020) Diagnostic Accuracy of CEUS LI-RADS for the Characterization of Liver Nodules 20 mm or Smaller in Patients at Risk for Hepatocellular Carcinoma. Radiology 294(2):329–339.
Yu R, Fan R, Hou J (2014) Chronic hepatitis B virus infection: epidemiology, prevention, and treatment in China. Front Med 8(2):135–144.
McGlynn KA, Petrick JL, London WT (2015) Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis 19(2):223–238.
Chan HL, Sung JJ (2006) Hepatocellular carcinoma and hepatitis B virus. Semin Liver Dis 26(2):153–161.
Funding
This study was supported by the National Nature Science Foundation of China (Nos. 81971630, 82171960 and 82102078), Guangdong Natural Science Foundation (No. 2021B1515020054), and Guangzhou Science and Technology Project (No. 201904010187).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Ethical approval
Our study was approved by the institutional ethics committee of the first affiliated hospital of Sun Yat-sen University, and written informed consent was obtained from each patient for CEUS examination.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Cq., Huang, H., Ruan, Sm. et al. An assessment of liver lesions using a combination of CEUS LI-RADS and AFP. Abdom Radiol 47, 1311–1320 (2022). https://doi.org/10.1007/s00261-022-03428-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03428-1