Abstract
Purpose
Elastography was primarily used as an adjunctive method along with ultrasonography in differentiation between benign from malignant lesions. Occasionally, overlaps can occur which are caused by some rare invasive breast cancers. Our aim is to analyze the role of rare breast cancers in false negative strain elastography results and to assess the relation among false negative results and tumor size, lesion distance to skin, and tumor grade.
Methods
Patients with BI-RADS 5 category underwent strain elastography and core biopsy. All those with confirmed invasive breast cancer were included. For each rare breast cancer, four usual invasive breast cancer cases were taken as a control group. The cut-off value of strain ratio was considered as 2.3. The true positive and the false negative groups were compared in terms of histological type (rare carcinomas and the others) and the other parameters. Pearson Chi-square and Fisher’s exact test were used for statistical analyses. P values < 0.05 were considered statistically significant.
Results
One hundred-thirteen patients were defined as true positive (70.6%), and 47 patients were defined as false negative (29.4%). Strain ratio values of the rare breast cancers were significantly lower than those of the other breast cancers (p = 0.012). There was no statistically significant difference between the groups with respect to tumor size, distance to skin, and tumor grade (p > 0.05).
Conclusion
The rare breast cancers are an important cause of false negativity in elastographic evaluation of invasive breast cancers. The results should be interpreted in combination with grayscale US findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most common malignancy is breast cancer over the past three decades [1]. Ultrasonography (US) is widely used for completing mammographic screening in the breast imaging [2]. Addition of elastography to US has been shown to improve diagnostic accuracy [3, 4]. Both strain elastography (SE) and shear wave elastography (SWE) have been developed to evaluate tissue stiffness or elasticity. They can be performed during breast US in that it only adds a few minutes to each examination, and the results can be interpreted synchronously [5]. In SE method, qualitative (color map) and semi-quantitative (strain ratio = SR) data were obtained, whereas SWE provided quantitative data regarding tissue elasticity. Many studies have suggested that SR provides more objective result than color map and SR is superior to color scoring in differentiation of benign and malignant lesions [6,7,8].
Elastography was primarily used as an adjunctive method with US in differentiation of benign and malignant lesions [5]. Diagnosis by elastography alone could not replace a grayscale US examination, although specificity tended to be higher than for grayscale US [9]. Combination of elastography with the grayscale US usually demonstrates convincing results, as well [10, 11]. Malignant lesions are known to be more stiff than benign ones [2]. However, there is a limited overlap between benign and malignant lesions [5]. It has been reported that the reason for this overlap in elastography is rare breast cancers such as tubular carcinoma and mucinous carcinoma [12,13,14]. These cancers morphologically resemble benign lesions, and this means false negativity in grayscale US and elastography [1]. Also, it is controversial that the tumor size, depth, and grade may affect elastography [14,15,16,17,18,19,20].
In this study, it was aimed to investigate role of rare breast cancers in false negative results of SE and to evaluate possible factors such as tumor size, lesion distance to skin, and tumor grade, which may result with false negativity in SE.
Materials and methods
This study was planned retrospectively after the approval of the local ethics committee. Since the study was retrospective, informed consent by patients was not required. A total of consecutive 169 patients with Breast Imaging Reported and Data System (BI-RADS). Five lesions were reviewed between June 2016 and June 2018. Patients with ductal carcinoma in situ (n = 3), patients with sclerosing adenosis (n = 2), and patients with atypical ductal hyperplasia (n = 2) were excluded. Two cases were also excluded due to technical problems in color mapping. For each rare breast cancer, four usual invasive breast cancer cases were taken as a control group.
US, elastography, and percutaneous biopsy
B-mode US and SE examinations of all patients were performed by a radiologist with 10 years of experience in breast imaging using 13-MHz surface probe (Hitachi Ezu-MT28-S1 model, Hitachi Inc. Japan). Elastography was performed with minimal compression and decompression until 5–6 similar sinusoidal waves were obtained. In compression phase, region of interests (ROI) were placed to the target tissue and following the normal tissue at the same depth and diameter (approximately 2 mm).
The ROI was placed on the hard portion representing stiffness of the target lesion. Then, normal/target tissue elasticity ratios (SR) were automatically calculated. Three consecutive measurements were obtained for SR, and the highest SR value was taken into account.
In several studies, the cut-off values of SR were ranging between 2.3 and 4.8 in differentiation of benign and malignant lesions [14, 21,22,23]. In our study, we considered the cut-off value as 2.3. SRs below 2.3 were identified as false negative.
After SE examination, the patients underwent biopsy with 16 gauge full-automatic core biopsy needle (Bard Magnum, Covington, Georgia, USA). The biopsies were performed by the radiologist who performed the US and SE examinations.
Pathology
Histopathological examination and immunohistochemical analyses were performed by a pathologist with 10 years of experience in breast diseases. All assessments were performed according to results of core biopsy.
We compared the true positive and the false negative groups in terms of grade (low grade, high grade), tumor size (≤ 20, > 20 mm), the distances of the lesions to the skin (≤ 4, > 4 mm), and histological type (Table 1).
Tumors were categorized according to their histological type as rare (= special) carcinomas [glycogen-rich clear cell carcinoma (GRCC), apocrine carcinoma, mucinous carcinoma, and invasive cribriform carcinoma (ICC)] and the other carcinomas [No-special-type invasive ductal carcinoma (NST), invasive lobular carcinoma (ILC), invasive ductolobular carcinoma (IDLC)].
Statistical analysis
We used the independent sample T test to compare the age distribution between the true positive and the false negative groups. The frequency distributions of tumor size, histological type, grade, and the distances of the lesions to the skin in the two groups were examined using Pearson Chi-square analysis and Fisher’s exact test. P values < 0.05 were considered statistically significant.
Results
A total of 160 women with single lesion were included in the study. One hundred-thirteen patients were defined as true positive (70.6%), and 47 patients were defined as false negative (29.4%). The median was 57 years (mean 57.7 years; standard deviation (SD) ± 12.8 years; range 27–90 years). The numbers of NST, ILC, and IDLC were 114 (71.25%), 7 (4.4%), and 7 (4.4%), respectively (128/160, 80%). The rare breast cancers were seen in 32 cases (32/160, 20%) including GRCC (n = 12), apocrine (n = 10), mucinous (n = 6), ICCs (n = 4).
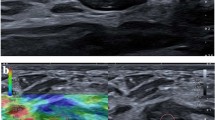
Mean SR of all cohort was 6.67 (standard error ± 0.72; range 0.24–41.76). Number of the patients with SR ≥ 2.3 was 113 (70.6%) and defined as true positive. Strain ratio was below 2.3 in 47 (29.4%) patients grouped as false negative. The findings of false negative and true positive groups are summarized in Table 2. Strain ratio values of the rare breast cancers were significantly lower (Figs. 1, 2) than the other breast cancers (p = 0.012) (Table 1). The mean age was 56.4 ± 11.83 years in true positive and 61.03 ± 14.61 years in false negative group. There was no significant age difference of the two groups (p = 0.115).
Tumor size ranged between 5 and 42 mm (mean 17.1 mm, SD ± 7 mm). The mean distance of the lesion from the skin was 7.33 ± 2.2 mm (between 2 and 13 mm). There was no statistically significant difference between the groups with respect to tumor size, distance of lesion to skin, and tumor grades (p > 0.05) (Table 1).
Discussion
Most of the breast cancers are (95%) epithelial carcinomas [24]. The remaining 5% are stromal tumors and metastases. Among epithelial carcinomas, 5% represent rare breast cancers such as mucinous, papillary, and medullary carcinomas (4.75% of total breast cancers) and 10% (9.5% of total breast cancers) are lobular carcinomas. The remaining 85% are NST (80.75% of total breast cancers). In our study, NST was majority of the study groups 71.25% and also, the majority of false negative cases (26/47, 55.3%) were NST. However, in total, 16.25% of NSTs (26/160) were in the false negative group.
The elastographic findings of GRCC, apocrine, and ICC cancers have not been studied in previous studies. It is very important that our study included elastographic findings of these rare cancers. Most of breast carcinomas tend to have typical features for malignancy, such as irregular shape, spiculated margin, antiparallel orientation, and posterior shadowing and hyperechogenic halo, while some carcinomas can exhibit as probably benign lesions (BI-RADS 3) on US [1]. These carcinomas are usually related to rare breast carcinomas, such as medullary, mucinous, and papillary carcinomas [24].
The approximate ratio of GRCC among breast cancer is less than 3% [25]. In our series, GRCC is found to be more frequent than expected, 7.5% (12/160). However, we have taken for each rare breast cancer four usual invasive breast cancer cases as a control group, in our study. So the calculated one was not a real incidence. The remarkable point is that 10 (83.33%) out of 12 GRCC cases were in the false negative group in this study. In GRCC, more than 90% of tumor cells contain clear cytoplasm-containing glycogen [25]. There are no data on how rich glycogen content affects this outcome yet. GRCC imaging findings are not characteristically defined in the literature. They can be oval, round, and microlobular as well as cystic, as in our study [26]. These findings are not typical signs of malignancy. Also, elastographic findings have not been reported [26]. The SE findings of GRCC needed to be analyzed with large series.
Apocrine carcinoma accounts for < 1% of all breast cancers in the literature [27], while apocrine carcinoma rate was in 6.25% of patients (10/160) in our study. Six of ten apocrine carcinomas (60%) were included in the false negative group. This finding was surprising, too. More than 90% of tumor cells are apocrine cells in invasive apocrine carcinoma. There are no data on how apocrine cells affect the lower SR values yet.
The incidence of ICC ranges between 0.3 and 6% in all breast cancers [28]. It was seen in 2.5% of our cases (4/160). A few cases showed benign characteristics, but most of them appeared with typical features of malignancy. We did not have the false negative result in this group. This result may be related to we have a few number of cases. The elastographic findings of ICC, GRCC, and apocrine carcinoma have not been defined yet.
The elastographic findings of mucinous cancers in the literature are contradictory. While Evans [12] reported that mucinous cancers were at least as stiff as others, Ganau [15] shared data that they were softer than others. In our study, two out of six mucinous cancers were in the false negative group. In some other studies, the high stiffness of mucinous cancers was associated with the small number of study groups [7, 29]. Due to the presence of mucin, these cancers are expected to be soft but may be stiff in relation to the tumor/stromal interface ratio or peritumoral stroma [30].
In a recent study, it was reported that the lesions smaller than 2 cm were associated with false negative results [16]. In the study of Ganau et al., the lesions larger than 2 cm were reported to be more stiff than the lesions smaller than 2 cm [15]. In our study, tumor size was not significantly related to false negativity. In this respect, our results were not consistent with the literature.
The distance of the lesion to the skin may influence SR. It has been reported that elastographic evaluation will be false or unsuccessful in lesions deeper than 10 mm by Thomas [31], 12 mm by Raza [17], and 15 mm by Chang [18]. Stachs suggested that lesions with a distance of ≤ 4 mm to the skin should not be evaluated with SE [14]. The distance to the skin of our deepest lesion was 13 mm, and there were 32 our lesions in ≤ 4 mm group. The distances to the skin of the lesions were not significantly related to false negativity.
There were several studies which demonstrated that stiffness increased when tumor grade increased [13, 19, 32,33,34]. Vinnicombe et al. underlined that low-grade tumors play a role in false negativity [13]. In contrast, Durhan [20] and Chamming [35] reported that high-grade cancers were softer due to high amount of necrosis. In our study, no significant relationship was between tumor grade and false negativity.
Glandular tissue adjacent to breast and/or subcutaneous adipose tissue can be used as reference in SR measurement [36, 37]. We placed the ROI for the reference tissue on the subcutaneous fat or on the normal breast tissue for the SR calculations in different cases.
Currently, in elastographic assessment of the breast lesions, there is no evaluation standard for values of ROI. In some studies, ROIs have received as 1–3 mm located in the stiffest portion of the tumor [10, 38], whereas in the other some studies, large ROIs have used that cover the entire lesion [39, 40]. It has been reported that small ROIs using at the stiffest area of the lesion have a better diagnostic performance than large ROIs including the entire lesion [41]. Therefore, we also received ROI values of approximately 2 mm in all lesions, so that they are not a variable at the same time.
The limitations of this study were the relatively small number of patients and a single-center study. Another limitation was the absence of some rare breast cancer groups such as papillary, tubular, metaplastic, medullary, and secretory carcinomas in our study. Even in the extensive series of 1137 cases of Evans et al.’s study, only some of the rare cancers such as tubular, mucinous, metaplastic, and papillary tumors are studied [12]. Our study is important and, to the best of our knowledge, is the first study mentioning elastographic findings of rare cancers such as GRCC, apocrine cancer, and ICC. In addition, the study was performed according to the results of the core biopsy. Core biopsy can represent a small sample size of a tumor [42]. However, the limitations are similar for all because all cases were evaluated through core biopsy.
We conducted our study with SE [37], which could be made cheaper, more widespread, faster, and easier to implementation, but our references also include studies with SWE. Strain elastography is known to be a relatively operator-dependent method [5]. There may also be relative technical differences in each device [5]. In our study, all elastographic examinations were performed by one radiologist with 10 years of experience in breast diseases and using one device. So we did not have any interobserver variability and technical differences problems. However, these are also limitations. It would be appropriate to confirm the results with multiple evaluators and with different devices.
Conclusion
In this study, we demonstrated that rare breast cancers play an important role in case of false negativity associated with SR values. The rare breast cancers were significantly softer than other invasive breast cancers. Contribution of SE to diagnosis is limited, even misleading, in rare cancers with grayscale US findings that may resemble relatively benign lesions. In these lesions, BI-RADS categories and grayscale findings should be taken into consideration and biopsy is a must. Histological grade, tumor size, and distance of lesion to skin do not affect the false negativity. However, these results should be validated with large and multicenter studies.
References
Grajo JR, Barr RG (2014) Strain elastography for prediction of breast cancer tumor grades. J Ultrasound Med 33(1):129–134
Mutala TM, Ndaiga P, Aywak A (2016) Comparison of qualitative and semiquantitative strain elastography in breast lesions for diagnostic accuracy. Cancer Imaging 16(1):12
Itoh A, Ueno E, Tohno E et al (2006) Breast disease: clinical application of US elastography for diagnosis. Radiology 239:341–350
Ophir J, Céspedes I, Ponnekanti H et al (1991) Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging 13:111–134
Barr RG (2019) Future of breast elastography. Ultrasonography 38(2):93–105
Waki K, Murayama N, Matsumura T et al (2007) Investigation of strain ratio using ultrasound elastography technique. Paper presented at: first international symposium on information and computer elements 2007, 12–14, Kitakyushu, Japan
Chang JM, Moon WK, Cho N et al (2011) Clinical application of shear wave elastography (SWE) in the diagnosis of benign and malignant breast diseases. Breast Cancer Res Treat 129(1):89–97
Zhi H, Ou B, Luo BM et al (2007) Comparison of ultrasound and elastography, mammography, and sonography in the diagnosis of solid beast lesions. J Ultrasound Med 26:807–815
Dória MT, Jales RM, Conz L et al (2019) Diagnostic accuracy of shear wave elastography—Virtual touchTM imaging quantification in the evaluation of breast masses: impact on ultrasonography’s specificity and its ultimate clinical benefit. Eur J Radiol 113:74–80
Berg WA, Cosgrove DO, Doré CJ et al (2012) Shear-wave elastography improves the specificity of breast US: the BE1 multinational study of 939 masses. Radiology 262:435–449
Chang JM, Won JK, Lee KB et al (2013) Comparison of shear-wave and strain ultrasound elastography in the differentiation of benign and malignant breast lesions. Am J Roentgenol 201:347–356
Evans A, Sim YT, Thomson K et al (2016) Shear wave elastography of breast cancer: sensitivity according to histological type in a large cohort. Breast 26:115–118
Vinnicombe SJ, Whelehan P, Thomson K et al (2014) What are the characteristics of breast cancers misclassified as benign by quantitative ultrasound shear wave elastography? Eur Radiol 24(4):921–926
Stachs A, Hartmann S, Stubert J et al (2013) Differentiating between malignant and benign breast masses: factors limiting sonoelastographic strain ratio. Ultraschall Med 34(2):131–136
Ganau S, Andreu FJ, Escribano F et al (2015) Shear-wave elastography and immunohistochemical profiles in invasive breast cancer: evaluation of maximum and mean elasticity values. Eur J Radiol 84(4):617–622
Choi HY, Seo M, Sohn YM et al (2019) Shear wave elastography for the diagnosis of small (≤ 2 cm) breast lesions: added value and factors associated with false results. Br J Radiol 92(1097):20180341
Raza S, Odulate A, Ong EM et al (2010) Using real-time tissue elastography for breast lesion evaluation: our initial experience. J Ultrasound Med 29(4):551–563
Chang JM, Moon WK, Cho N et al (2011) Breast mass evaluation: factors influencing the quality of US elastography. Radiology 259(1):59–64
Zhu YC, Zhang Y, Deng SH et al (2018) Correlation between histopathological grading and shear-wave elastography in evaluating invasive carcinoma of no rare type. Exp Ther Med 16(6):4700–4706
Durhan G, Öztekin PS, Ünverdi H et al (2017) Do histopathological features and microcalcification affect the elasticity of breast cancer? J Ultrasound Med 36(6):1101–1108
Alhabshi SM, Rahmat K, Abdul Halim N et al (2013) Semi-quantitative and qualitative assessment of breast ultrasound elastography in differentiating between malignant and benign lesions. Ultrasound Med Biol 39:568–578
Farrokh A, Wojcinski S, Degenhardt F (2011) Diagnostic value of strain ratio measurement in the differentiation of malignant and benign breast lesions. Ultraschall Med 32:400–405
Thomas A, Degenhardt F, Farrokh A et al (2010) Significant differentiation of focal breast lesions: calculation of strain ratio in breast sonoelastography. Acad Radiol 17:558–563
Fleury Ede F, Assunção-Queiros Mdo C, Roveda D Jr (2014) Breast carcinomas: variations in sonoelastographic appearance. Breast Cancer 6:135–143
Ma X, Han Y, Fan Y et al (2014) Clinicopathologic characteristics and prognosis of glycogen-rich clear cell carcinoma of the breast. Breast J 20(2):166–173
Eun NL, Cha YJ, Son EJ et al (2019) Clinical imaging of glycogen-rich clear cell carcinoma of the breast: a case series with literature review. Magn Reson Med Sci 18(3):238–242
D’Arcy C, Quinn CM (2019) Apocrine lesions of the breast: part 2 of a two-part review. Invasive apocrine carcinoma, the molecular apocrine signature and utility of immunohistochemistry in the diagnosis of apocrine lesions of the breast. J Clin Pathol 72(1):7–11
Balci P, Başara Akin I, Köremezli N et al (2017) Evaluation and comparison of radiologic-pathologic findings in invasive cribriform carcinoma of the breast. Turk J Med Sci 47(3):738–747
Mori M, Tsunoda H, Kawauchi N et al (2012) Elastographic evaluation of mucinous carcinoma of the breast. Breast Cancer 19(1):60–63
Wang PL, Zeng FY, Lu Q et al (2019) Imaging features of pure mucinous breast carcinomas: correlation with extracellular mucus content. Clin Radiol 74(7):569.e9–569.e17
Thomas A, Kümmel S, Fritzsche F et al (2006) Real-time sonoelastography performed in addition to B-mode ultrasound and mammography: improved differentiation of breast lesions? Acad Radiol 13:1496–1504
You Y, Song Y, Li S et al (2019) Quantitative and qualitative evaluation of breast cancer prognosis: a sonographic elastography study. Med Sci Monit 5(25):9272–9279
Gemici AA, Ozal ST, Hocaoglu E et al (2020) Relationship between shear wave elastography findings and histologic prognostic factors of invasive breast cancer. Ultrasound Q 36(1):79–83. https://doi.org/10.1097/RUQ.0000000000000471
Choi WJ, Kim HH, Cha JH et al (2014) Predicting prognostic factors of breast cancer using shear wave elastography. Ultrasound Med Biol 40(2):269–274
Chamming’s F, Latorre-Ossa H, Le Frère-Belda MA et al (2013) Shear wave elastography of tumour growth in a human breast cancer model with pathological correlation. Eur Radiol 23(8):2079–2086
Samani A, Zubovits J, Plewes D (2007) Elastic moduli of normal and pathological human breast tissues: an inversion-technique-based investigation of 169 samples. Phys Med Biol 52:1565–1576
Yerli H, Yilmaz T, Kaskati T et al (2011) Qualitative and semiquantitative evaluations of solid breast lesions by sonoelastography. J Ultrasound Med 30(2):179–186
Au FW, Ghai S, Lu FI et al (2015) Quantitative shear wave elastography: correlation with prognostic histologic features and immunohistochemical biomarkers of breast cancer. Acad Radiol 22(3):269–277
Zhou J, Zhan W, Chang C et al (2014) Breast lesions: evaluation with shear wave elastography, with special emphasis on the ‘‘stiff rim’’ sign. Radiology 272:63–72
Wang ZL, Li JL, Li M et al (2013) Study of quantitative elastography with supersonic shear imaging in the diagnosis of breast tumours. Radiol Med 118:583–590
Moon JH, Hwang JY, Park JS et al (2018) Impact of region of interest (ROI) size on the diagnostic performance of shear wave elastography in differentiating solid breast lesions. Acta Radiol 59(6):657–663
Dhaliwal CA, Graham C, Loane J (2014) Grading of breast cancer on needle 435 core biopsy: does a reduction in mitotic count threshold improve 436 agreements with grade on excised specimens? J Clin Pathol 67:1106–1108
Funding
The authors declared that this study has received no financial support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by GS, MC, MA, and AA. The first draft of the manuscript was written by GS, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Izmir Katip Celebi University Non-Interventional Clinical Studies Institutional Review Board IRB Number: 169.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sezgin, G., Coskun, M., Apaydin, M. et al. The role of rare breast cancers in the false negative strain elastography results. Radiol med 126, 349–355 (2021). https://doi.org/10.1007/s11547-020-01270-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-020-01270-3