Abstract
Purpose
The aim of this study was to examine the correlation between ventricular function and the extracellular volume fraction (ECV) in patients with non-ischemic dilated cardiomyopathy (NIDCM) using 3.0 T magnetic resonance imaging (MRI). We also hypothesized that native T1 and ECV values would be increased in patients with NIDCM, independent of the left ventricular ejection fraction (LVEF). The findings of our study could lead to further studies of the follow-up protocols.
Materials and methods
In total, 53 consecutive dilated cardiomyopathy patients who had undergone cardiac MRI were functionally evaluated and underwent tissue characterization.
Results
The mean native T1 value was 1235 ± 10 ms, and the mean ECV value was 35.4 ± 2.7% in the myocardia. The LVEF values ranged from 29 to 44%. No significant correlations were observed between functional analysis measurements and native T1 or ECV values.
Conclusions
Our results showed that myocardial fibrosis is unrelated to cardiac functional findings in NIDCM patients. Therefore, we propose that these patients should be evaluated using MRI and tissue characterization techniques, in addition to cardiac functional analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-ischemic dilated cardiomyopathy (NIDCM) can cause heart failure and sudden cardiac death (SCD) and is clinically defined as left ventricular (LV) or biventricular enlargement with decreased systolic function [1–3]. This clinical entity is responsible for a significant proportion of newly diagnosed heart failure cases and is the most frequent reason for heart transplantation in adults and children [1–3]. NIDCM predisposes individuals to ventricular arrhythmias and consequent SCD [4–6]. Moreover, although a reduced left ventricular ejection fraction (LVEF) is a criterion for inserting an implantable cardioverter-defibrillator (ICD) to prevent SCD [7–9], ICD candidates should not be chosen solely on the basis of LVEF [6]. Myocardial fibrosis (MF) has been sufficiently defined as the main cause of arrhythmogenesis, which is a strong indicator of undesirable cardiac outcomes [5, 6, 10, 11]. MF changes and disrupts the myocardial structure, which results in mechanical and electrophysiological failure of the heart [12–15]. Therefore, assessment of MF has become important for the follow-up and recovery of patients with NIDCM.
Cardiac magnetic resonance (CMR) imaging is a powerful visualization method that provides the unique capability to not only detect the presence and position of MF but also quantitatively characterize examined tissue [16–22]. In contrast, although late gadolinium enhancement (LGE) imaging can successfully identify localized myocardial scar tissue, diffuse fibrosis may be overlooked, and quantitative assessment is not possible [23, 24]. In addition, McCrohon et al. showed that LGE is apparent in only 41% of NIDCM patients [25]. The native T1 mapping technique of CMR imaging, which does not require gadolinium enhancement, permits the detection of overall changes in myocardial tissue, such as fibrosis, edema, and the deposition of iron, lipids or proteins, that predict myocardial pathologies involving myocytes and interstitial spaces [26–28]. Furthermore, the extracellular volume fraction (ECV) technique of CMR imaging is a novel method that can differentiate the cellular and interstitial parts of the myocardium by measuring the extent of the extracellular space after administration of a contrast agent, thereby delineating MF. In addition, no approved treatment exists for reducing the duration of hospitalization or the mortality rate in heart failure patients who have sufficient systolic function. In these patients, reduced LVEF alone is not a sufficient assessment for predicting the prognosis of NIDCM [26, 29, 30].
The aim of this study was to examine the correlation between LV function and ECV values in NIDCM patients (with LVEF <45%) using 3.0-T CMR imaging. Our hypothesis was that native T1 and ECV values are increased in patients with NIDCM regardless of the LVEF. The findings of our study could lead to further studies of the follow-up protocols.
Methods
Study population
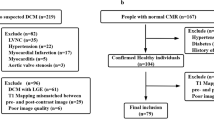
Based on clinical and echocardiographic findings, 53 patients with dilated cardiomyopathy (DCM) who underwent CMR imaging in our radiology department were retrospectively evaluated for NIDCM between March 2015 and February 2016. Patients were excluded if they exhibited the presence of ischemic DCM, an LVEF value >45%, a focal myocardial scar that was apparent via LGE imaging, and/or myocardial edema that could be observed via T2 mapping.
Scanning protocol
Cardiac magnetic resonance imaging examinations were performed with a 3.0 T clinical scanner (Magnetom Skyra, Version E11, Siemens Healthcare, Erlangen, Germany) with an 18-channel cardiac coil and electrocardiography gating. Images were acquired according to a routine clinical protocol for volumes, tissue characterization, and LGE. For functional evaluation of the LV and assessment of the heart chambers, steady-state free-precession cine images in the short axis-view and 3 long-axis views (2, 3, and 4-chamber views) were obtained using the following parameters: TR, 41 ms; TE, 1.2 ms; flip angle, 47°. In all patients, the native and post-contrast T1 mapping data were acquired in a single mid-ventricular short-axis slice using the modified look-locker inversion recovery (MOLLI) sequence. The native T1 mapping parameters consisted of TR, 272.1 ms; TE, 1.2 ms; flip angle, 35°. T2 mapping was obtained in the same mid-ventricular short-axis level used for T1 mapping with the following imaging parameters: TR, 207.4 ms; TE, 1.32 ms; flip angle, 12°. LGE images were acquired 15 min after administration of a dose of 0.2 mmol/kg gadolinium using a phase-sensitive inversion recovery sequence (TR, 775 ms; TE, 2.67 ms; flip angle, 25°). The imaging procedure was concluded with post-contrast T1 mapping using the following parameters: TR, 361.6 ms; TE, 1.12 ms; flip angle, 35°. All data were stored in DICOM format. The scanning time for the entire CMR protocol with the T1 mapping technique was approximately 40 min for each patient.
Image analysis
The acquired CMR images were analyzed on a Syngo Via workstation (Software Version VA30A, Siemens AG, Germany) by the same radiologist (CAG) who had 3 years of experience in cardiac imaging and was blinded to the patient age, patient gender, and echocardiographic results. T1 mapping images and findings from functional evaluations of the LV were analyzed in different readout sessions at three-day intervals to obtain objective and unbiased results. Endocardial and epicardial contours were semi-automatically traced at the end-diastolic phase and the end-systolic phase. The papillary muscles were included as part of the LV myocardial mass. Using the delineated endocardial and epicardial contours, the LVEF, left ventricular end-diastolic volume (LV-EDV), and left ventricular end-systolic volume (LV-ESV) were measured and normalized to the body surface area (BSA).
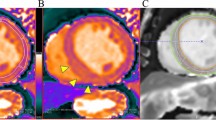
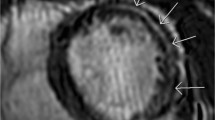
Focal scar tissue was identified by myocardial LGE imaging and qualitatively characterized by myocardial post-contrast signal intensity. Pre- and post-contrast pixelwise T1 relaxation times were measured by manual region of interest (ROI) tracing to detect the LV myocardial circumference at the mid-ventricular level in short-axis images. Pre- and post-contrast blood T1 values were also calculated in a ROI traced in the blood pool (Fig. 1). In addition, pixelwise images of myocardial T2 mapping were analyzed for qualitative and quantitative assessments of the myocardium with the same technique used to map the T1 ROI.
ECV values were calculated according to the following formula: ECV = (1 − hematocrit) × (1/T1 myocardium post-contrast − 1/T1 myocardium pre-contrast)/(1/T1 blood post-contrast − 1/T1 blood pre-contrast) [29, 31].
The total time required to analyze CMR images was approximately 30 min for each patient.
Statistical analysis
The data were summarized as means ± standard deviations (SDs) and as frequencies with percentages. Descriptive statistics were calculated for the variables. The correlations between the LVEF, LV-EDV, LV-ESV, native T1, and ECV variables were estimated using Pearson correlation coefficients. Intra-observer intraclass correlation coefficients (ICCs) were calculated to identify repeatability in the quantitative analyses, and the 95% confidence intervals (CIs) were also computed. For all quantitative values, measurements were obtained with intervals of at least 1 week between evaluation sessions for the ICC calculations. Values of P < 0.05 were considered statistically significant. IBM SPSS Statistics 23.0 (IBM Corp., Armok, NY, USA) and MedCalc 15.2 (MedCalc Software, Mariakerke, Belgium) were used for all statistical analyses.
Results
Of the 53 patients, 14 patients indicated focal contrast enhancement after LGE imaging. Two of these 53 patients had increased T2 mapping values and were excluded from the study, resulting in a total of 37 NIDCM patients included in the analyses. The average age of the patients was 43.5 ± 10 years (range 29–64 years); 54% (20/37) of the patients were women, and 46% (17/37) were men. The mean native T1 time was 1235 ms, and the mean ECV value in the myocardium was 35.4 ± 2.7%. The LVEF values ranged from 29 to 44% (mean 38 ± 4.4%). The mean LV-EDV normalized to the BSA was 90.1 ± 12 ml/m2, and the mean LV-ESV was 60.1 ± 13.5 ml/m2. The CMR imaging findings are summarized in Table 1.
Statistically significant negative correlations were observed between the LVEF and LV-EDV values and between the LVEF and LV-ESV values (Pearson correlation coefficient = −0.959, P < 0.001; Pearson correlation coefficient = −0.971, P < 0.001, respectively). A statistically significant positive correlation was also found between native T1 values and ECV values (Pearson correlation coefficient = 0.932, P < 0.001). However, no significant correlations were observed between LVEF, LV-EDV, LV-ESV, and native T1 values or ECV values (Fig. 2).
The ICC for the LVEF calculations was 0.989 (95% CI 0.979–0.994), indicating excellent agreement. The ICC for the LV-EDV and LV-ESV measurements showed nearly perfect agreement (0.991, 95% CI 0.983–0.995; 0.996, 95% CI 0.993–0.998, respectively). In addition, the native T1 mapping values (ICC = 0.911, 95% CI 0.828–0.954) and ECV measurements (ICC = 0.997, 95% CI 0.995–0.998) indicated very good agreement. Therefore, we concluded that these data were reliable and reproducible.
Discussion
In this study, to obtain a homogeneous cohort of NIDCM patients, we excluded patients who had edema on T2 mapping or positive LGE imaging findings. We observed several important results. First, we showed that the mean native T1 mapping values and ECV measurements in NIDCM patients could be obtained using 3.0 T magnetic resonance imaging (MRI); a limited number of previous studies in this area have been reported in the literature. More importantly, our study demonstrated that no significant correlations existed between metrics measuring LV function (LVEF, LV-EDV, and LV-ESV) and myocardial tissue characterization values (native T1 mapping and ECV) in NIDCM patients.
CMR imaging has rapidly improved and is the gold standard for evaluating cardiac function due to its high spatial resolution. Rapid improvements in CMR imaging have allowed for the quantitative assessment of myocardial tissue. Moreover, relative to prior scanners, 3.0 T MRI scanners have higher magnetic field strength and provide better signal-to-noise ratio (SNR) and T1 imaging of the myocardium; thus, the use of such scanners has advantages in the context of cardiac perfusion, myocardial mapping and tagging [27, 32–36]. In addition, T1 mapping and ECV techniques have been demonstrated as effective methods for evaluating diffuse MF in NIDCM patients [37–39]. Dass et al. [17] indicated that native T1 values in 18 DCM patients (mean T1 value = 1225 ± 42 ms) were significantly higher than in controls. A study of 27 DCM patients by Puntmann et al. [39] similarly showed increased T1 values (mean T1 value = 1239 ± 457 ms). Consistent with those studies, we showed high native T1 times (mean T1 value = 1235 ± 10 ms). In addition, Mordi et al. [40] (using 1.5 T MRI) and Puntmann et al. [39] (using 3.0 T MRI) found increased ECV values. Our study showed similar increased ECV values in NIDCM patients (mean ECV = 35.4 ± 2.7%).
Several studies have shown that determining the ECV value is a useful technique for characterizing interstitial fibrosis and its consequential relationship with mortality [41–43] Furthermore, Dass et al. [17] showed that native T1 mapping could be a useful biomarker for the initial diagnosis and prognostic evaluation of cardiomyopathies. These findings suggest that high native T1 values or an increase in the ECV value could be helpful for risk stratification of patients with cardiomyopathies. In addition, because no proven treatment option exists for decreasing the mortality or the hospitalization time for NIDCM patients who have sufficient LVEF [29, 30], the identification of MF has become important. In this study, we have demonstrated for the first time that no relationship exists between metrics of LV function and myocardial tissue characterization in NIDCM patients. Our study showed that T1 mapping and ECV values could have high levels despite the fact that LVEF was relatively unchanged. Thus, we suggest that MF could be an independent predictor of risk factors, in addition to the ventricular functional findings. The use of T1 mapping and ECV methods are as important as cardiac functional assessments for evaluation of the prognostic stratification of NIDCM patients. Further investigations are needed to determine the cut-off values for ECV and T1 mapping to prevent SCD, arrhythmogenesis, and heart failure.
There are several limitations to our study. First, although to the best of our knowledge, this study examined the largest group of NIDCM patients assessed using the ECV technique, our study cohort was relatively small. Therefore, a larger cohort is needed to generalize the findings. Second, we did not correlate our CMR imaging findings with the results of histological methods. However, a previous study of the detection of MF using CMR imaging showed that ECV images were closely correlated with fibrosis that had been histologically verified [43].
In conclusion, the evaluation of cardiac function alone is inadequate for prognostic stratification. Based on this premise, we demonstrated that for NIDCM patients, MF is unrelated to cardiac functional findings obtained using 3.0 T MRI. Therefore, we propose that these patients should be evaluated with CMR imaging using tissue characterization techniques, in addition to cardiac functional analysis. Further research is needed to confirm our findings and evaluate MF in NIDCM patients.
References
Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE (2006) Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA 296:1867–1876. doi:10.1001/jama.296.15.1867
Sanbe A (2013) Dilated cardiomyopathy: a disease of the myocardium. Biol Pharm Bull 36:18–22. doi:10.1248/bpb.b212023
Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P (1996) Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation 93:841–842. doi:10.1161/01.CIR.93.5.841
Meinertz T, Hofmann T, Kasper W, Treese N, Bechtold H, Stienen U, Pop T, Leitner ER, Andresen D, Meyer J (1984) Significance of ventricular arrhythmias in idiopathic dilated cardiomyopathy. Am J Cardiol 53:902–907. doi:10.1016/0002-9149(84)90522-8
de Leeuw N, Ruiter DJ, Balk AH, de Jonge N, Melchers WJ, Galama JM (2001) Histopathologic findings in explanted heart tissue from patients with end-stage idiopathic dilated cardiomyopathy. Transpl Int 14:299–306. doi:10.1007/s001470100339
Koutalas E, Kanoupakis E, Vardas P (2013) Sudden cardiac death in non-ischemic dilated cardiomyopathy: a critical appraisal of existing and potential risk stratification tools. Int J Cardiol 167:335–341. doi:10.1016/j.ijcard.2012.07.014
Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW (2008) ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. J Am Coll Cardiol 51:e1–62. doi:10.1016/j.jacc.2008.02.032
Packer DL, Prutkin JM, Hellkamp AS, Mitchell LB, Bernstein RC, Wood F, Boehmer JP, Carlson MD, Frantz RP, McNulty SE, Rogers JG, Anderson J, Johnson GW, Walsh MN, Poole JE, Mark DB, Lee KL, Bardy GH (2009) Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: analysis from the sudden cardiac death in heart failure trial. Circulation 120:2170–2176. doi:10.1161/CIRCULATIONAHA.109.853689
Gold MR, Linde C, Abraham WT, Gardiwal A, Daubert JC (2011) The impact of cardiac resynchronization therapy on the incidence of ventricular arrhythmias in mild heart failure. Heart Rhythm 8:679–684. doi:10.1016/j.hrthm.2010.12.031
Gunja-Smith Z, Morales AR, Romanelli R, Woessner JF Jr (1996) Remodeling of human myocardial collagen in idiopathic dilated cardiomyopathy. Role of metalloproteinases and pyridinoline cross-links. Am J Pathol 148:1639–1648
Nakayama Y, Shimizu G, Hirota Y, Saito T, Kino M, Kitaura Y, Kawamura K (1987) Functional and histopathologic correlation in patients with dilated cardiomyopathy: an integrated evaluation by multivariate analysis. J Am Coll Cardiol 10:186–192. doi:10.1016/S0735-1097(87)80178-X
Díez J, Querejeta R, López B, González A, Larman M, Martínez Ubago JL (2012) Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation 105:2512–2517. doi:10.1161/01.CIR.0000017264.66561.3D
Izawa H, Murohara T, Nagata K, Isobe S, Asano H, Amano T, Ichihara S, Kato T, Ohshima S, Murase Y, Iino S, Obata K, Noda A, Okumura K, Yokota M (2005) Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: a pilot study. Circulation 112:2940–2945. doi:10.1161/CIRCULATIONAHA.105.571653
Tamarappoo BK, John BT, Reinier K, Teodorescu C, Uy-Evanado A, Gunson K, Jui J, Chugh SS (2012) Vulnerable myocardial interstitium in patients with isolated left ventricular hypertrophy and sudden cardiac death: a postmortem histological evaluation. J Am Heart Assoc 1:e001511. doi:10.1161/JAHA.112.001511
Schelbert EB, Piehler KM, Zareba KM, Moon JC, Ugander M, Messroghli DR, Valeti US, Chang CC, Shroff SG, Diez J, Miller CA, Schmitt M, Kellman P, Butler J, Gheorghiade M, Wong TC (2015) Myocardial fibrosis quantified by extracellular volume is associated with subsequent hospitalization for heart failure, death, or both across the spectrum of ejection fraction and heart failure stage. J Am Heart Assoc 4:e002613. doi:10.1161/JAHA.115.002613
Ordovas KG, Higgins CB (2011) Delayed contrast enhancement on MR images of myocardium: past, present, future. Radiology 261:358–374. doi:10.1148/radiol.11091882
Dass S, Suttie JJ, Piechnik SK, Ferreira VM, Holloway CJ, Banerjee R, Mahmod M, Cochlin L, Karamitsos TD, Robson MD, Watkins H, Neubauer S (2012) Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging 5:726–733. doi:10.1161/CIRCIMAGING.112.976738
Leong DP, Chakrabarty A, Shipp N, Molaee P, Madsen PL, Joerg L, Sullivan T, Worthley SG, De Pasquale CG, Sanders P, Selvanayagam JB (2012) Effects of myocardial fibrosis and ventricular dyssynchrony on response to therapy in new-presentation idiopathic dilated cardiomyopathy: insights from cardiovascular magnetic resonance and echocardiography. Eur Heart J 33:640–648. doi:10.1093/eurheartj/ehr391
Giesbrandt KJ, Bolan CW, Shapiro BP, Edwards WD, Mergo PJ (2013) Diffuse diseases of the myocardium: MRI-pathologic review of cardiomyopathies with dilatation. AJR Am J Roentgenol 200:W274–W282. doi:10.2214/AJR.12.9634
Holmström M, Kivistö S, Heliö T, Jurkko R, Kaartinen M, Antila M, Reissell E, Kuusisto J, Kärkkäinen S, Peuhkurinen K, Koikkalainen J, Lötjönen J, Lauerma K (2011) Late gadolinium enhanced cardiovascular magnetic resonance of lamin A/C gene mutation related dilated cardiomyopathy. J Cardiovasc Magn Reson 13:30. doi:10.1186/1532-429X-13-30
Masci PG, Andreini D, Francone M, Bertella E, De Luca L, Coceani M, Mushtaq S, Mariani M, Carbone I, Pontone G, Agati L, Bogaert J, Lombardi M (2013) Prodromal angina is associated with myocardial salvage in acute ST-segment elevation myocardial infarction. Eur Heart J Cardiovasc Imaging 14:1041–1048. doi:10.1093/ehjci/jet063
Francone M (2014) Role of cardiac magnetic resonance in the evaluation of dilated cardiomyopathy: diagnostic contribution and prognostic significance. ISRN Radiol 2014:365404. doi:10.1155/2014/365404
Rogers T, Dabir D, Mahmoud I, Voigt T, Schaeffter T, Nagel E, Puntmann VO (2013) Standardization of T1 measurements with MOLLI in differentiation between health and disease—the ConSept study. J Cardiovasc Magn Reson 15:78. doi:10.1186/1532-429X-15-78
Pastor A, Voigt T, Schaeffter T, Nagel E, Puntmann VO (2012) Usefulness of cardiac magnetic resonance in early assessment of cardiomyopathies: myocardial fibrosis is a common denominator. Curr Cardiovasc Imaging Rep 5:77–82. doi:10.1007/s12410-012-9125-9
McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ (2003) Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation 108:54–59. doi:10.1161/01.CIR.0000078641.19365.4C
Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz-Menger J, Schelbert EB (2013) Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson 15:92. doi:10.1186/1532-429X-15-92
Roller FC, Harth S, Schneider C, Krombach GA (2015) T1, T2 mapping and extracellular volume fraction (ECV): application, value and further perspectives in myocardial inflammation and cardiomyopathies. Rofo 187:760–770. doi:10.1055/s-0034-1399546
Görmeli CA, Görmeli G, Yağmur J, Özdemir ZM, Kahraman AS, Çolak C, Özdemir R (2016) Assessment of myocardial changes in athletes with native T1 mapping and cardiac functional evaluation using 3 T MRI. Int J Cardiovas Imaging 32:975–981. doi:10.1007/s10554-016-0866-4
Arheden H, Saeed M, Higgins CB, Gao DW, Bremerich J, Wyttenbach R, Dae MW, Wendland MF (1999) Measurement of the distribution volume of gadopentetate dimeglumine at echo-planar MR imaging to quantify myocardial infarction: comparison with 99mTc-DTPA autoradiography in rats. Radiology 211:698–708. doi:10.1148/radiology.211.3.r99jn41698
Jerosch-Herold M, Sheridan DC, Kushner JD, Nauman D, Burgess D, Dutton D, Alharethi R, Li D, Hershberger RE (2008) Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 295:H1234–H1242. doi:10.1152/ajpheart.00429.2008
Kellman P, Wilson JR, Xue H, Ugander M, Arai AE (2012) Extracellular volume fraction mapping in the myocardium, part 1: evaluation of an automated method. J Cardiovasc Magn Reson 14:63. doi:10.1186/1532-429X-14-63
Yoon YE, Hong YJ, Kim HK, Kim JA, Na JO, Yang DH, Kim YJ, Choi EY, Korean Society of Cardiology and the Korean Society of Radiology (2014) 2014 Korean guidelines for appropriate utilization of cardiovascular magnetic resonance imaging: a joint report of the Korean Society of Cardiology and the Korean Society of Radiology. Korean J Radiol 15:659–688. doi:10.3348/kjr.2014.15.6.659
Hendel RC, Patel MR, Kramer CM, Poon M, Hendel RC, Carr JC, Gerstad NA, Gillam LD, Hodgson JM, Kim RJ, Kramer CM, Lesser JR, Martin ET, Messer JV, Redberg RF, Rubin GD, Rumsfeld JS, Taylor AJ, Weigold WG, Woodard PK, Brindis RG, Hendel RC, Douglas PS, Peterson ED, Wolk MJ, Allen JM, Patel MR; American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group; American College of Radiology; Society of Cardiovascular Computed Tomography; Society for Cardiovascular Magnetic Resonance; American Society of Nuclear Cardiology; North American Society for Cardiac Imaging; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology (2006) ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol 48:1475–1497
Di Cesare E, Cademartiri F, Carbone I, Carriero A, Centonze M, De Cobelli F, De Rosa R, Di Renzi P, Esposito A, Faletti R, Fattori R, Francone M, Giovagnoni A, La Grutta L, Ligabue G, Lovato L, Marano R, Midiri M, Romagnoli A, Russo V, Sardanelli F, Natale L, Bogaert J, De Roos A (2013) Clinical indications for the use of cardiac MRI. By the SIRM Study Group on Cardiac Imaging. Radiol Med 118:752–798. doi:10.1007/s11547-012-0899-2
Hays AG, Schs M, Kelle S (2009) Clinical applications for cardiovascular magnetic resonance imaging at 3 tesla. Curr Cardiol Rev 5:237–242. doi:10.2174/157340309788970351
Oshinski JN, Delfino JG, Sharma P, Gharib AM, Pettigrew RI (2010) Cardiovascular magnetic resonance at 3.0 T: current state of the art. J Cardiovasc Magn Reson 12:55. doi:10.1186/1532-429X-12-55
Kramer CM, Chandrashekhar Y, Narula J (2013) T1 mapping by CMR in cardiomyopathy: a noninvasive myocardial biopsy? JACC Cardiovasc Imaging 6:532–534. doi:10.1016/j.jcmg.2013.02.002
Parsai C, O’Hanlon R, Prasad SK, Mohiaddin RH (2012) Diagnostic and prognostic value of cardiovascular magnetic resonance in non-ischaemic cardiomyopathies. J Cardiovasc Magn Reson 14:54. doi:10.1186/1532-429X-14-54
Puntmann VO, Voigt T, Chen Z, Mayr M, Karim R, Rhode K, Pastor A, Carr-White G, Razavi R, Schaeffter T, Nagel E (2013) Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging 6:475–484. doi:10.1016/j.jcmg.2012.08.019
Mordi I, Carrick D, Bezerra H, Tzemos N (2015) T1 and T2 mapping for early diagnosis of dilated non-ischaemic cardiomyopathy in middle-aged patients and differentiation from normal physiological adaptation. Eur Heart J Cardiovasc Imaging 17:797–803. doi:10.1093/ehjci/jev216
Mascherbauer J, Marzluf BA, Tufaro C, Pfaffenberger S, Graf A, Wexberg P, Panzenböck A, Jakowitsch J, Bangert C, Laimer D, Schreiber C, Karakus G, Hülsmann M, Pacher R, Lang IM, Maurer G, Bonderman D (2013) Cardiac magnetic resonance postcontrast T1 time is associated with outcome in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging 6:1056–1065. doi:10.1161/CIRCIMAGING.113.000633
Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS, Mulukutla SR, Simon MA, Schelbert EB (2012) Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation 126:1206–1216. doi:10.1161/CIRCULATIONAHA.111.089409
de Meester de Ravenstein C, Bouzin C, Lazam S, Boulif J, Amzulescu M, Melchior J, Pasquet A, Vancraeynest D, Pouleur AC, Vanoverschelde JL, Gerber BL (2015) Histological validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified Look-Locker Imaging (MOLLI) T1 mapping at 3 T. J Cardiovasc Magn Reson 17:48. doi:10.1186/s12968-015-0150-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
This study was performed at a single tertiary center and was approved by the local medical ethics committee.
Informed consent
Informed consent was obtained from each participant included in this study.
Rights and permissions
About this article
Cite this article
Görmeli, C.A., Özdemir, Z.M., Kahraman, A.S. et al. The evaluation of non-ischemic dilated cardiomyopathy with T1 mapping and ECV methods using 3T cardiac MRI. Radiol med 122, 106–112 (2017). https://doi.org/10.1007/s11547-016-0701-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-016-0701-y