Abstract
Potato common scab caused by bacterial pathogen Streptomyces scabies (Thaxt.) Waksman & Henrici is one of the most important diseases of potato (Solanum tuberosum L.) worldwide. Currently, the most effective and desirable method to control common scab is through the use of resistant cultivars. In order to decipher the genetic control of resistance to common scab disease in Canadian potato germplasm, an association panel of 143 clones including advanced breeding clones and commercial cultivars was genotyped with 12K SolCAP SNPs and phenotyped for potato common scab resistances in multiple years. By conducting a genome-wide association analysis (GWAS) using GWASpoly R package, three resistance QTLs were identified on potato chromosomes 2, 4, and 12, respectively. The phenotypic variation explained by these QTLs was 21%, 19%, and 26%, respectively. The QTL on chromosome 2 was simplex-dominant whereas duplex-dominant QTLs were identified on chromosomes 4 and 12. These findings will be useful to design marker-assisted selection and breeding strategies to improve resistance to common scab in new potato cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potato common scab caused by soilborne gram-positive bacteria in the genus Streptomyces is one of the most commercially devastating potato diseases (Loria 2001; Hill and Lazarovits 2005; Hao et al. 2009). Among several hundred species of Streptomyces, S. scabies (Thaxter) Waksman & Henrici was one of the few species that is pathogenic on potato plants (Waksman and Henrici 1948; Lambert and Loria 1989; Braun et al. 2017a, b). Many more pathogenic Streptomyces including S. turgidiscabies and S. acidiscabies also cause scab diseases in potato worldwide (Miyajima et al. 1998; Kim et al. 1999; Kreuze et al. 1999; Lehtonen et al. 2004; St-Onge et al. 2008; Zhao et al. 2008; Thwaites et al. 2010; Dees et al. 2013). Potato common scab produces pitted corky lesions on the periderm of potato tubers which result in downgrading or rejection of affected tubers. The incidence and severity of common scab can vary depending on the distribution of the pathogen, environmental conditions, and host-pathogen interaction (Bukhalid et al. 1998; Hill and Lazarovits 2005; Lazarovits et al. 2007). Structural properties of potato skin and the sensitivity to the toxin, thaxtomin, produced by S. scabies are considered to be factors contributing to the cultivar susceptibility. The management of potato scab disease can be complicated by environmental factors including soil pH, moisture, and microbial components of the soil (Peters et al. 2004; Sturz et al. 2004; Weinert et al. 2011; Dees and Wanner 2012). Control measures often fail because little is known about the pathogenesis and contrasting requirements of favourable conditions for different diseases (Wang and Lazarovits 2005; Wilson et al. 2009).
Currently, the most effective and desirable method to combat the soilborne disease is to develop resistant cultivars. Potato resistance to common scab has been investigated in a number of studies (Goth et al. 1993; Murphy et al. 1995; Haynes et al. 2010). In most cases, the resistance appeared to be quantitatively inherited with continuous distribution (Driscoll et al. 2009; Haynes et al. 2010). Alam (1972) suggested that resistance was controlled by two independent loci. The resistance appears to require the presence of a single dominant allele at the first locus, and the presence of two homozygous recessive alleles at the second locus. This model was supported by Murphy et al. (1995), who successfully demonstrated the transmission of scab resistance from the diploid to the tetraploid background. However, unlike the simple genetic control of most loci in diploid populations, the inheritance of multiple alleles at a locus is complicated in commercial cultivars or tetraploid germplasm, due to tetrasomic segregation (Wricke and Weber 1986) and the resistance to common scab appears as a quantitatively inherited trait (Haynes et al. 2009). Furthermore, the allele dosage may affect the level of gene expression and result in different phenotypes (Guo et al. 1996).
Genome-wide association studies (GWAS) are a powerful tool to identify marker variants that are significantly associated with complex traits (Pritchard et al. 2000; Kang et al. 2008, 2010; Zhang et al. 2010). Unlike traditional mapping studies using biparental populations, GWAS usually use a germplasm panel taking the marker-estimated population structure and marker-estimated kinship as covariates (denoted Q and K, respectively). Rosyara et al. (2016) developed an R package, GWASpoly to conduct GWAS in autopolyploid populations using Q + K linear mixed model with bi-allelic SNPs. The detection power of the platform was also validated in a tetraploid potato diversity panel, which was genotyped using 8303 SNPs as part of the USDA-NIFA Solanaceae Coordinated Agricultural Project (SolCAP) (Hamilton et al. 2011; Felcher et al. 2012; Hirsch et al. 2013). The objective of this study was to identify loci significantly associated with common scab disease resistance in a Canadian potato germplasm panel using the GWASpoly software.
Materials and Methods
Plant Material
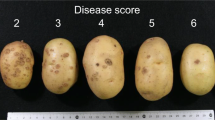
The study was conducted at the Fredericton Research and Development Centre of Agriculture Agri-Food Canada (AAFC, Fredericton, New Brunswick). An association panel of 143 clones including 20 commercial cultivars and 123 advanced breeding clones was used in the analysis. The advanced breeding clones were mainly developed for the processing and fresh markets. They were chosen based on their breeding potential or agronomic merits following many years of field selection at the Benton Ridge Breeding substation of Agriculture Agri-Food Canada. Commercial cultivars in this panel represented a range of materials that are commercially grown in North America, and some were used as parental lines in the AAFC potato breeding program. Plant materials were planted in a scab nursery maintained at AAFC’s Experimental Farm (Fredericton, New Brunswick, Canada). The field was repeatedly used to grow cultivated potato over past 10 years in order to promote high disease pressure of potato common scab (Streptomyces scabies (Thaxt.) Waksman & Henrici). The experiment was carried out using a randomized block design with three replications. Each block consisted of 16 rows, each 102.4 m long. Individual plots consisted of 10 hills planted in 4.3-m-long rows. In these field trials, every 4 rows were bordered by a check row of a susceptible variety, Green Mountain. Data used in the study were obtained from evaluations conducted in 2012, 2014, and 2016 at Fredericton. Tubers were rated at harvest for pustule type (1–5 scale) and surface area covered. Raw data were converted to a relative percentage of the susceptible check. The percentage values were then indexed on a 1–9 scale of increasing susceptibility. Usually, Green Mountain (susceptible check) has a score of 8, whereas Hindenburg (resistant check) has a score of 2 on this scale.
DNA Extraction and Genotyping
Potato genomic DNA was extracted from young leaf tissue following the standard CTAB (cetyltrimethyl ammonium bromide) method (Hoisington et al. 1994). The association panel was genotyped using the Illumina 12K SolCAP BeadChip (Illumina, San Diego, USA) by NeoGen Inc. (Lansing, MI, USA). The Theta values of samples were obtained using Illumina’s GenomeStudio software (Illumina, San Diego, CA, v2011.1) following the company’s protocol. The quality of each SNP was visually inspected using Excel sorting function. The SNPs without assigned physical positions on potato genome were mapped to physical position in genome using the BLAST tool in Spud DB (http://solanaceae.plantbiology.msu.edu/integrated_searches.shtml). Non-polymorphic SNPs (minor allele frequency < 5%) were discarded from the dataset. R-script (fitTetra) was used to convert the Theta value from GenomeStudio into five types of potato genotypes, nulliplex (AAAA), simplex (AAAB), duplex (AABB), triplex (ABBB), and quadriplex (BBBB) states (Voorrips et al. 2011). These bi-allelic SNP genotypes in autotetraploids were then converted into the dosage of the minor alleles, 0, 1, 2, 3, and 4 for nulliplex, simplex, duplex, triplex, and quandriplex, respectively.
Statistical Analysis and Heritability
Neighbour-joining (NJ) trees were generated to visually decipher the population stratification by a pairwise distance matrix derived from the Nei’s genetic distance for all polymorphic SNPs using PowerMarker version 3.25 (Liu and Muse 2005) and displayed on Figtree version 1.4.3 (Rambaut 2016). Both linear regression and analysis of variance (ANOVA) in R (www.r-project.org) were used to conduct the analysis of the phenotypic traits. A principal component analysis (PCA) was conducted for phenotypic traits used as selection criteria as a complementary approach to estimate population structure in the association panel (Joliffe 2002). The detailed definition of field selection traits and PCA were described in our previous study (Yuan et al. 2016). The broad-sense heritability (h2) for common scab resistance was estimated on a phenotypic mean basis using R according to Wricke and Weber’s algorithm (Wricke and Weber 1986).
Genome-Wide Association Mapping
The R package GWASpoly for tetrapolyploids (Rosyara et al. 2016) was used to conduct the GWAS analysis on the panel. Principal component analysis was used to estimate the population stratification using prcomp algorithm (www.r-project.org). The top four principal components were used to build up the P matrix for population structure correction. Kinship matrices were calculated with the algorithm built in GWASpoly package. The Q + K linear mixed model under P3D was used to test the associations between the SNPs and phenotypic variations (Rosyara et al. 2016). In addition to the general model, four different genetic models (additive, simplex dominant, duplex dominant, and diploidized additive) were tested. In the additive models, the SNP effect is proportional to allele dosage. For dominant models, tests were done considering whether reference or alternative allele was dominant. The quantile-quantile (QQ) plots were generated by plotting the expected log p value against the observed values to test appropriateness of model to avoid false discovery caused by population stratification. The 1000 permutation method was used for establishing a p value detection threshold for statistical significance. The Manhattan plots were produced based on the vignette of GWASpoly with minor modification (Rosyara et al. 2016).
Results and Discussion
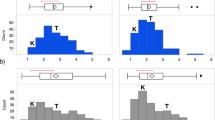
The phenotypic distribution of potato resistance to common scab in the study panel is presented in Fig. 1. The phenotypic distribution is continuous and slightly positively skewed. The estimated broad-sense heritability (h2) for common scab resistance based on the data collected in 2012, 2014, and 2016 was 0.81. A total of 10,392 polymorphic SNPs from 12K SolCAP remained after filtering minor allele frequency (MAF < 0.05). These SNPs uniformly distributed across the 12 potato chromosomes were used in the GWAS.
Distribution of common scab ratings in the study panel. The disease was scored on a 1–9 scale where 1 is the disease free on the surface of tubers and 9 has the worst disease symptom on tubers. The estimated broad-sense heritability (h2) for common scab resistance based on the field evaluation in 2012, 2014, and 2016 was 0.81
Population Structure
The phylogenetic tree analysis using neighbour-joining method demonstrated the non-random distribution of genotypes within the association panel including the advanced breeding clones and commercial cultivars. The panel displayed both significant population structure and familial relationships and clustered into 5 distinct subpopulations (Fig. 2). The fixation index (FST), an assessment of population differentiation due to genetic structure, was around 0.16 suggesting a moderate differentiation of genetic structure within the association panel of clonal propagated crop. Among these five subpopulations, potato clones for processing were grouped into group I, II, III, and V while clones for fresh market were mostly clustered into group IV. Therefore, the diverged association panel in the NJ tree derived from potato genotypes indicated the pedigree closeness among these clones. Furthermore, these subpopulations also shared similar clusters based on the market types suggesting that the genetic loci of these clones might be wedged by potato breeding effort, especially selection for the quality traits such as specific gravity, sugar, and starch content. Results of population structure analysis partially agree with Hirsch et al. (2013) and Deperi et al. (2018) who reported diversification of market classes resulting from long-term breeding process and divergent allele frequencies for SNPs related to processing traits such as carbohydrate metabolism.
Neighbour-joining tree of potato clones in the association panel. A total of five subpopulations were identified using PowerMarker (Liu and Muse 2005)
GWAS Analysis
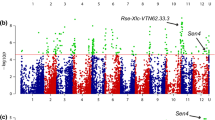
The association mapping analysis identified a total number of 211, 209, and 290 polymorphic SNP markers on chromosomes 2, 4, and 12, respectively, among 143 clones of the panel. Three QTLs defined by the significant SNPs for the common scab resistance were identified on chromosome 2, 4, and 12, respectively (Table 1; Fig. 3a). Three SNP markers (solcap_snp_c2_17864 (Chr2:36550070), solcap_snp_ c2_17867 (Chr2:36548178), and solcap_snp_c1_5844 (Chr2:36769707) underlying a QTL on chromosome 2 were identified using with the simplex-dominant-alternate allele (1-dom-alt) model. The proportions of phenotypic variation for potato resistance to common scab explained by those markers were 21%, 8%, and 12%, respectively (Table 1). The second QTL defined by two significant SNPs characterized by both general and duplex-dominant-alternate allele models was detected on chromosome 4 underlying solcap_snp_c1_16291 (Ch4: 1259748) and solcap_snp_c1_54791 (Ch4: 1162713). These markers explained 19% and 6.6% of the phenotypic variation, respectively. The third QTL was defined by three SNPs ((solcap_snp_c2_3251, Chr2:9554964), (solcap_snp_c2_3189, Chr2:9518026), and (solcap_snp_c2_3247, Chr2:9564388)) on chromosome 12 with the alternate allele of duplex dominant model gene action (Table 1). The solcap_snp_c1_16291 SNP on chromosome 4 was detected by two different (general and duplex-dominant-alternate) models. The solcap_snp_c2_3251SNP marker of chromosome 12 explained the highest (26%) phenotypic variation of the resistance. Our results appear to confirm previous report by Alam (1972) that resistance to common scab requires the presence of a dominant allele at one locus and the presence of two homozygous recessive alleles at a second locus. In addition, we identified the location of the two recessive alleles at separate loci on chromosomes 4 and 12 as indicated by duplex-dominant-alternate allele models. However, not all SNPs passed the genome-wide significance threshold by GWASpoly software even though they explained a meaningful amount of phenotypic variation (Fig. 3b). Bradshaw et al. (2008) have previously identified two QTLs on chromosome 2 and 6 using AFLP and SSR markers via interval mapping with 227 clones derived from a cross of 12601ab1 and Stirling. The significant SNP marker mapped on the chromosome 2 in our study shared a similar location of the QTL with that of Bradshaw et al. (2008). Braun et al. (2017a, b) showed the presence of QTLs for common scab reaction expressed as the percentage of surface area covered with lesions and the lesion type, respectively, in a diploid population in the same location on chromosome 11 and these explained a large portion of phenotypical variation. None of the Braun et al. (2017a, b) QTLs was overlapping with those found in this study.
QQ plots (a) and Manhattan plots of GWAS results (b–d) in the potato association panel. a QQ plots were generated under three models “general”, “1-dom-alt”, and 2-dom-alt”, respectively, in the association panel. b–d Manhattan plots were generated using the − log10p values from a genome-wide scan against the position on each of the 12 chromosomes in the association panel under these three models. The genome-wide significance threshold was marked by the horizontal line
The QQ (quantile-quantile) plots of the genome-wide distribution of results (Fig. 3a) showed lower inflation of departure from the null hypothesis which indicates proper control of inflation caused by population structure or kinship. As displayed in the QQ plots of the expected distribution of the test statistics (X-axis) across the SNPs compared with the observed values (Y-axis) based on six mapping models, the nominal p values consistently show a solid line matching X = Y until it sharply curves at the end against the distribution of observed − log10p values from the mapping models.
The mechanism of potato resistance to the infestation of S. scabies is still not clearly understood (Braun et al. 2017a, b). It may also depend on the differences in pathogen isolates, inoculation method, cultivars, and location of the research plots (Loria et al. 2006; Koyama et al. 2006; Qu et al. 2008). In our study, potato cultivars showed almost a normal distribution (Fig. 1). Three genomic regions significantly associated with common scab resistance were identified. It is possible that these regions harbour other important traits. An association of scab reaction with fry colour was also reported by Bradshaw et al. (2008), indicating possible linkage or pleiotropic effects. Using principal component analysis, scab was found to be correlated with specific gravity (a major market class differentiation trait), and both traits were found to have negative projection on the first principal component and positive projection on the second principal component (Table 2). The first two PCs were visualized in a biplot using ggbiplot package (Fig. 4). Graphical representation revealed that scab and specific gravity were grouped into similar direction, indicating a relationship of these traits as found in the PCA. This study was conducted on single-SNP association analysis. A number of studies have shown that combining single SNP markers into haplotypes can provide greater QTL detection power and mapping accuracy than single SNP markers in genome-wide association, in addition to capturing epistatic interaction (He et al. 2011; N’Diaye et al. 2017).
Conclusions
Significant SNPs for potato common scab resistance were identified using GWAS on potato chromosomes 2, 4, and 12 in an association panel composed of advanced potato clones and commercial cultivars. These findings showcase the importance of high-resolution genome-wide association mapping to decipher complex quantitative disease-resistant traits in potato. These findings will be useful to design marker-assisted selection and breeding strategies to improve resistance to common scab and overall performance in new potato cultivars.
References
Alam Z (1972) Inheritance of scab resistance in 24-chromosome potatoes. Ph.D. Thesis, University of Wisconsin-Madison, pp 58
Bradshaw JE, Hackett CA, Pande B, Waugh R, Bryan GJ (2008) QTL mapping of yield, agronomic and quality traits in tetraploid potato (Solanum tuberosum subsp. tuberosum). Theor Appl Genet 116:193–211. https://doi.org/10.1007/s00122-007-0659-1
Braun S, Endelman J, Haynes K, Jansky S (2017a) Quantitative trait loci for resistance to common scab and cold-induced sweetening in diploid potato. Plant Genome 10:1–9. https://doi.org/10.3835/plantgenome2016.10.0110
Braun S, Gevens A, Charkowski A, Allen C, Jansky S (2017b) Potato common scab: a review of the causal pathogens, management practices, varietal resistance screening methods, and host resistance. Am J Potato Res 94:283–296. https://doi.org/10.1007/s12230-017-9575-3
Bukhalid R, Chung S, Loria R (1998) Nec1, a gene conferring a necrogenic phenotype, is conserved in plant-pathogenic Streptomyces spp. and linked to a transposase pseudogene. Mol Plant Microbe Interact 11:960–967
Dees M, Wanner LLA (2012) In search of better management of potato common scab. Potato Res 55:249–268
Dees M, Sletten A, Hermansen A (2013) Isolation and characterization of Streptomyces species from potato common scab lesions in Norway. Plant Pathol 62:217–225
Deperi SI, Tagliotti ME, Bedogni MC, Manrique-Carpintero NC, Coombs J, Zhang R (2018) Discriminant analysis of principal components and pedigree assessment of genetic diversity and population structure in a tetraploid potato panel using SNPs. PLoS One 13(3):e0194398. https://doi.org/10.1371/journal.pone.0194398
Driscoll J, Coombs J, Hammerschmidt R, Kirk W, Wanner L, Douches D (2009) Greenhouse and field nursery evaluation for potato common scab tolerance in a tetraploid population. Am J Potato Res 86:96–101
Felcher KJ, Coombs J, Massa A, Hansey C, Hamilton J, Veilleux RE, Buell CR, Douches DS (2012) Integration of two diploid potato linkage maps with the potato genome sequence. PLoS One 7:e36347
Goth R, Haynes KG, Wilson D (1993) Evaluation and characterization of advanced potato breeding clones for resistance to scab by cluster analysis. Plant Dis 77:911–914
Guo M, Davis D, Birchler JA (1996) Dosage effects on gene expression in a maize ploidy series. Genetics 142:1349–1355
Hamilton J, Hansey C, Whitty B, Stoffel K, Massa A, Van Deynze A, De Jong W, Douches D, Buell CR (2011) Single nucleotide polymorphism discovery in elite North American potato germplasm. BMC Genomics 12:302–313
Hao J, Meng Q, Yin J, Kirk W (2009) Characterization of new Streptomyces strain, SD3024, that causes potato common scab. Plant Dis 93:1329–1334
Haynes KG, Christ BJ, Burkhart CR, Vinyard BT (2009) Heritability of resistance to common scab in diploid potatoes. Am J Potato Res 86:165–170
Haynes KG, Wanner LA, Thill CA, Bradeen JM, Miller J, Novy RG, Whitworth JL, Corsini DL, Vinyard BT (2010) Common scab trials of potato varieties and advanced selections at three U.S. locations. Am J Potato Res 87:261–276
He Y, Li C, Amos CI, Xiong M, Ling H (2011) Accelerating haplotype-based genome-wide association study using perfect phylogeny and phase- known reference data. PLoS One 6(7):e22097. https://doi.org/10.1371/journal.pone.0022097
Hill J, Lazarovits G (2005) A mail survey of growers to estimate potato common scab prevalence and economic loss. Can J Plant Pathol 27:46–52
Hirsch C, Hirsch C, Felcher K, Coombs J, Zarka D, Van Deynze A, De Jong W, Veilleux RE, Jansky S, Bethke P, Douches DS, Buell CR (2013) Retrospective view of North American potato (Solanum tuberosum L.) breeding in the 20th and 21st centuries. G3 (Bethesda) 3:1003–1013
Hoisington DA, Khairallah MM, Gonzales-de-Leon D (1994) Laboratory protocols. CIMMYT Applied Molecular Genetics Laboratory. CIMMYT Hisfoa
Jolliffe IT (2002) Principal component analysis, 2nd edn. Springer series in statistics. Springer- Verlag, New York
Kang HM, Zaitlen N, Wade C, Kirby A, Heckerman D, Daly M, Eskin E (2008) Efficient control of population structure in model organism association mapping. Genetics 178:1709–1723
Kang HM, Sul J, Service S, Zaitlen N, Kong S, Freimer N, Sabatti C, Eskin E (2010) Variance component model to account for sample structure in genome-wide association studies. Nat Genet 42:348–354
Kim Y, Cho J, Park D, Lee H, Kim J, Seo S, Shin K, Hur J, Lim C (1999) Production of thaxtomin A by Korean isolates of Streptomyces turgidiscabies and their involvement in pathogenicity. Plant Pathol 15:168–171
Koyama O, Manome A, Kurata S, Yokomaku T, Tanaka H (2006) Correlation between nec1 gene copy number detected in soils by quantitative competitive quenching probe PCR and incidence of potato common scab disease. Microbes Environ 21:185–188
Kreuze J, Suomalainen S, Paulin L, Valkonen J (1999) Phylogenetic analysis of 16S rRNA genes and PCR analysis of the nec1 gene from Streptomyces spp. causing common scab, pitted scab, and netted scab in Finland. Phytopathology 89:462–469
Lambert DH, Loria R (1989) Streptomyces scabies sp nov, nom rev. Int J Syst Bacteriol 39(4):387–392
Lazarovits G, Hill J, Patterson G, Conn KL, Crump NS (2007) Edaphic soil levels of mineral nutrients, pH, organic matter, and cationic exchange capacity in the geocaulosphere associated with potato common scab. Phytopathology 97(9):1071–1082
Lehtonen M, Rantala H, Kreuze J, Bang H, Kuisma L, Koski P, Virtanen E, Vihlman K, Valkonen J (2004) Occurrence and survival of potato scab pathogens (Streptomyces species) on tuber lesions: quick diagnosis based on a PCR-based assay. Plant Pathol 53:280–287
Liu K, Muse S (2005) PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics 21:2128–2129
Loria R (2001) Common scab. In: Stevenson WR, Loria R, Franc GD, Weingartner DP (eds) Compendium of potato diseases, 2nd edn. The American Phytopathological Society, St. Paul, pp 14–15
Loria R, Kers J, Joshi M (2006) Evolution of plant pathogenicity in Streptomyces. Annu Rev Phytopathol 44:469–487
Miyajima K, Tanaka F, Takeuchi T, Kuninaga S (1998) Streptomyces turgidiscabies sp. nov. Int J Syst Bacteriol 48:495–502
Murphy AM, De Jong H, Tai GCC (1995) Transmission of resistance to common scab from the diploid to the tetraploid level via 4x-2x crosses in potatoes. Euphytica 82:227–233
N’Diaye A, Haile JK, Cory AT, Clarke FR, Clarke JM, Knox RE et al (2017) Single marker and haplotype-based association analysis of semolina and pasta colour in elite durum wheat breeding lines using a high-density consensus map. PLoS One 12(1):e0170941. https://doi.org/10.1371/journal.pone.0170941
Peters RD, Carter MR, Sanderson JB, Av S (2004) Influence of crop rotation and conservation tillage practices on the severity of soil-borne potato diseases in temperate humid agriculture. Can J Soil Sci 84:397–402
Pritchard JK, Stephens P, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Qu XS, Wanner LA, Christ BJ (2008) Using the txtAB operon to quantify pathogenic Streptomyces in potato tubers and soil. Phytopathology 98:405–412
Rambaut A (2016) http://tree.bio.ed.ac.uk/software/figtree/
Rosyara U, De Jong W, Douches D, Endelman J (2016) Software for genome-wide association studies in autopolyploids and its application to potato. Plant Genome 9:1–10
St-Onge R, Goyer C, Coffin R, Filion M (2008) Genetic diversity of Streptomyces spp. causing common scab of potato in eastern Canada. Syst Appl Microbiol 31:474–484
Sturz AV, Ryan DAJ, Coffin AD, Matheson BG, Arsenault WJ, Kimpinski J, Christie BR (2004) Stimulating disease suppression in soils: sulphate fertilizers can increase biodiversity and antibiosis ability of root zone bacteria against Streptomyces scabies. Soil Biol Biochem 36(2):343–352
Thwaites R, Wale S, Nelson D, Munday D, Elphinston J, Elphinstone J (2010) Streptomyces turgidiscabies and S. acidiscabies: two new casual agents of common scab of potato (Solanum tuberosum) in the UK. Plant Pathol 59:804
Voorrips RE, Gort G, Vosman B (2011) Genotype calling in tetraploid species from bi-allelic marker data using mixture models. BMC Bioinformatics 12:172
Waksman SA, Henrici AT (1948) Family II. Actinomycetaceae Buchanan and family Streptomycetaeeae Waksman and Henrici. In: Breed RS, Murray EGD, Hitchens AP (eds) Bergey’s manual of determinative microbiology, VI edn. Williams & Wilkins, Baltimore, pp 892–980
Wang A, Lazarovits G (2005) Role of seed tubers in the spread of plant pathogenic Streptomyces and initiating potato common scab disease. Am J Potato Res 82:221–230
Weinert N, Piceno Y, Ding GC, Meincke R, Heuer H, Berg G, Schloter M, Andersen G, Smalla K (2011) PhyloChip hybridization uncovered an enormous bacterial diversity in the rhizosphere of different potato cultivars: many common and few cultivar dependent taxa. FEMS Microbiol Ecol 75:497–506
Wilson CR, Luckman GA, Tegg RS, Yuan ZQ, Wilson AJ, Eyles A, Conner AJ (2009) Enhanced resistance to common scab of potato through somatic cell selection in cv. Iwa with the phytotoxin thaxtomin A. Plant Pathol 58:137–144
Wricke G, Weber EW (1986) Quantitative genetics and selection in plant breeding. Walter de Gruyter, New York, p 405
Yuan J, Murphy A, De Koeyer D, Lague M, Bizimungu B (2016) Effectiveness of the field selection parameters on potato yield in Atlantic Canada. Can J Plant Sci 96:701–710
Zhang Z, Ersoz E, Lai C, Todhunter R, Tiwari H, Gore M, Bradbury P, Yu J, Arnett D, Ordovas J, Buckler E (2010) Mixed linear model approach adapted for genome-wide association studies. Nat Genet 42:355–360
Zhao W, Liu D, Yu X (2008) First report of potato scab caused by Streptomyces turgidiscabies in China. Plant Dis 92:1587
Acknowledgements
The authors would like to thank the potato breeding technical staff at the Fredericton Research and Development Centre of AAFC as well as staff at the Benton Ridge Breeding Substation for their assistance in field plot establishment and data collection. The contribution of long-time breeding team members, A. Murphy and D. Wilson (both retired), is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yuan, J., Bizimungu, B., De Koeyer, D. et al. Genome-Wide Association Study of Resistance to Potato Common Scab. Potato Res. 63, 253–266 (2020). https://doi.org/10.1007/s11540-019-09437-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11540-019-09437-w