Abstract
Common scab of potato caused by pathogenic Streptomyces species poses an economic burden to commercial potato cultivation. Despite decades of research, no molecular markers linked to this trait are currently available, making the development of scab resistant potato varieties inefficient. We evaluated common scab lesion type and tuber coverage in 198 individuals segregating for common scab resistance in replicated field trials with high natural disease pressure over two growing seasons. To identify genetic features associated with resistance, we genotyped the tetraploid (4x) population and parental lines (Kalkaska and Tundra) using the Illumina Infinium 8303 Potato Array and conducted genome-wide association analysis. Here, we report two significant single nucleotide polymorphism (SNP) markers on chromosome 1 associated with both scab traits in both years as well as a SNP marker on chromosome 2 associated with scab tuber coverage. Gene expression profiling of the parents of this population in response to Thaxtomin A treatment revealed differential expression of genes in tubers putatively involved in cell wall biosynthesis and hormone signaling.
Resumen
La roña común de la papa, causada por especies patogénicas de Streptomyces, representa una carga económica al cultivo comercial de papa. A pesar de décadas de investigación, actualmente no se encuentran disponibles marcadores moleculares ligados a este carácter, haciendo ineficiente el desarrollo de variedades de papa resistentes a la roña. Evaluamos el tipo de lesión de la roña común y cubrimiento de tubérculo en 198 individuos segregantes para resistencia a la roña común en ensayos de campo repetidos con alta presión natural de la enfermedad en dos ciclos de cultivo. Para identificar características genéticas asociadas con la resistencia, genotipamos a la población tetraploide (4x) y a las líneas parentales (Kalkaska y Tundra) usando la matriz de papa Illumina infinitum 8303 y se condujo el análisis de la asociación amplia del genoma. Aquí reportamos dos marcadores significativos de polimorfismo de nucleótido individual (SNP) en el cromosoma 1 asociados tanto a caracteres de la roña en ambos años como al marcador SNP en el cromosoma 2 asociado con el cubrimiento de la roña en el tubérculo. El perfil de la expresión génica de los progenitores de esta población en respuesta a tratamiento con Thaxtomin A reveló expresión diferencial de genes en tubérculos putativamente involucrados en la biosíntesis de pared celular y en señalamiento hormonal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cultivated potato, Solanum tuberosum L. Group Tuberosum (2n = 4x = 48), is the fourth most important food crop worldwide, with production following rice, wheat, and maize (FAOSTAT 2017). Potato production is hampered by many diseases including those caused by oomycetes, fungi, bacteria, and viruses. Decades of research have led to the discovery and mapping of numerous resistance (R) genes for diseases such as late blight, potato virus Y, and Verticillium (Gebhardt and Valkonen 2001; Holterman et al. 2017; Solomon-Blackburn and Barker 2001; Tai et al. 2018). However, one disease that continues to be problematic is potato common scab caused by saprophytic, filamentous, soil-borne bacteria in the genus Streptomyces.
Common scab of potato occurs throughout the potato growing regions of the world and is characterized by superficial raised or pitted lesions that result in reduced marketability of tubers. The type and tuber coverage of scab lesions caused by pathogenic Streptomyces species is determined by a complex of factors including environmental conditions, pathogen species, and time of infection (Braun et al. 2017a). Potato tubers are considered damaged by the United States Department of Agriculture when the aggregate surface area of surface scab is greater than 25% or when removal of pitted scab lesions causes a loss of more than 5% of the total tuber weight. Although separate data regarding economic losses due to common scab alone in the United States is not recorded, the presence of scab lesions on fresh-market or processing potatoes can cause them to be rejected by processors and packaging companies. Management practices to control common scab, including adjustment of soil pH, maintaining high soil moisture and soil fumigation, are not consistently effective (reviewed in Dees and Wanner 2012). Common scab resistance of potato is a very important trait for potato varieties and an emphasis of most potato breeding programs in the United States and throughout the world. Although several scab resistant/tolerant potato varieties are available, there is little information regarding their mechanism/s of resistance despite over 50 years of research and a wealth of knowledge regarding the genetics of resistance to many other potato diseases.
The genetic basis of common scab resistance has been difficult to unravel due to several factors. Common scab resistance is influenced to a large extent by environmental conditions (Bradshaw et al. 2008; Jansky et al. 2019; Wiersema 1974) and there is high variability of pathogen distribution and soil environment both within and between field trials. Common scab resistance in tetraploid potato is hypothesized to be quantitative in nature (Dees et al. 2016; Driscoll et al. 2009) but heritability studies in diploid potato species suggest relatively few genes controlling this trait (Alam 1972; Cipar and Lawrence 1972; Murphy et al. 1995). Heritability values reported in tetraploid studies vary widely (Bradshaw et al. 2008; Haynes et al. 2009; Navarro et al. 2015; Zorrilla et al. 2014), potentially due in part to differences in genetic backgrounds, various Streptomyces strains, and screening methods. To date, few quantitative trait loci (QTL) associated with common scab resistance have been identified. Using a linkage mapping approach, two QTLs on chromosomes 2 and 6 accounting for 15% of the variability in scab resistance in a tetraploid S. tuberosum population were detected (Bradshaw et al. 2008), and a single QTL on chromosome 11 was associated with scab resistance derived from the diploid species S. chacoense (Braun et al. 2017b). Recent genetic studies aimed to combat the limitations of biparental linkage mapping have only incrementally improved our understanding of genes underlying scab resistance. In a genome wide association study (GWAS) of 143 tetraploid clones, significant single nucleotide polymorphisms (SNP) associated with scab resistances were identified on chromosomes 2, 4, and 12 (Yuan et al. 2019). A genomic selection study utilizing 370 tetraploid individuals and nine years of field scab phenotyping data suggests an additive resistance model with a major effect SNP on chromosome 9 (Enciso-Rodriguez et al. 2018). However, no molecular markers linked to this trait are currently available, making the development of scab resistant varieties inefficient.
A primary pathogenicity determinant in common scab-causing Streptomyces genotypes is the production of the phytotoxin Thaxtomin A (Loria et al. 2008). Thaxtomin A inhibits cellulose biosynthesis (King et al. 2001; Scheible et al. 2003), which may serve to compromise potato tuber cell walls and facilitate rapid cell penetration. Alternatively, it is possible that Thaxtomin A targets auxin signaling by disrupting auxin efflux polar transport (Tegg et al. 2013) or interacting directly at auxin receptors in the host plant (Navarro et al. 2006; Tegg et al. 2005). Certain plant pathogenic species of Streptomyces produce other toxins, such as coronafacic acid derivatives and a necrosis inducing peptide (Nec1), as well as the enzyme α-tomatinase that detoxifies the phytoanticipin α-tomatine (Bignell et al. 2013; Bukhalid et al. 1998; Seipke and Loria 2008). Thus, various pathogen-produced factors may also play roles in virulence and disease development.
Physical, chemical and transcriptional response mechanisms of resistance to common scab have been proposed. Increased tuber phellem cell layers, phellem suberization (Thangavel et al. 2016) and smaller lenticel size have been reported in resistant clones (Atiq et al. 2013). Conversion of Thaxtomin A to a less toxic form by glycosylation-mediated detoxification is another potential mechanism of resistance (Acuna et al. 2001). Recently, appropriate timing of a sustained transcriptional defense response has been reported as a hallmark of scab resistance (Dees et al. 2016).
We report here on the identification of significant single nucleotide polymorphism (SNP) markers associated with common scab resistance in Michigan in two years by genome wide association analysis in a tetraploid mapping population and gene expression profiling of the parents of this population in response to Thaxtomin A treatment.
Materials and Methods
Plant Material
A tetraploid Solanum tuberosum population (MSV507, n = 198) derived from a cross between the chip-processing varieties Tundra x Kalkaska was used for GWAS analysis. Tundra is susceptible to common scab while Kalkaska is resistant to pathogenic Streptomyces sp. present in Michigan soils. Replicated field trials (4 replicates) of the parental lines and MSV507 mapping population progeny were grown at the MSU Montcalm Research Center Common Scab Nursery (MRCSN) in both 2011 and 2012 under irrigation in a replicated block design, using the best management practices. The MRCSN soil is a Montcalm sandy loam with heavy pathogen pressure, which results in consistent infection across replications. Each replication consisted of a 10-hill plot planted at 23 cm (9 in.) in-row spacing. Tubers were harvested into bags and washed in the laboratory prior to scab evaluation. Scab lesion type and tuber surface coverage assessments were taken for up to 20 randomly selected tubers of each plot (N = 16,000). To describe the presence of surface and pitted scab, each plot was given a scab lesion type rating ranging from 0 to 6 (0 = No scab lesions; 1 = Superficial lesions, discrete; 2 = Superficial lesions, coalescing; 3 = Raised lesions, discrete; 4 = Raised lesions, coalescing; 5 = Pitted lesions, discrete; 6 = Pitted lesions, coalescing) adapted from (Wanner 2006). To identify any discrete genetic factors that may be controlling tuber lesion-covered surface, a component of tuber marketability, each plot was also given a tuber scab surface coverage score ranging from 0 to 6 (0 = no visible disease on tuber surface, 0.5 = < 1%; 1 = ≥ 1–2%; 2 = ≥ 2.1–5%; 3 = ≥ 5.1–10.%; 4 = ≥ 10.1–25%; 5 = ≥ 25.1–50%; 6 = ≥ 50%) (http://www.spongospora.ethz.ch/LaFretaz/scoringtable.htm). Data were analyzed in JMP® (Version Pro 13. SAS Institute Inc., Cary, NC). Means were calculated from the individual 20 tuber ratings for scab lesion type and scab tuber coverage for each replicate. The overall means of the four replicates for scab lesion type rating and scab tuber coverage score for each individual were computed and used for GWAS analysis.
SNP Genotyping
Genomic DNA was extracted from fresh leaf tissue of the two parental lines and the 198 F1 individuals following the Qiagen DNeasy Kit instructions. Both parents and all 198 progeny were SNP genotyped using the Infinium 8303 Potato Array (Felcher et al. 2012). The sample genotypes were called using a custom Perl script for five cluster calling developed for tetraploids based upon theta value thresholds (Hirsch et al. 2013). The genotype data were filtered to remove non-informative SNPs with the following characteristics: no clustering observed, >10% clones with no genotype call, >2 hits on the potato doubled monoploid Version 4.03 pseudomolecules (PM), 2 hits that were on different PM, no genotype call for one or both parents, or unexpected segregation. After filtering, 3075 informative markers were used for association analysis (Table S1).
GWAS Analysis
Association analysis was performed for scab tuber coverage and scab lesion type in both years with 3075 SNPs using the GWASpoly package in R version 3.1.1 (Rosyara et al. 2016). Four genetic models were tested for each dataset including tetraploid general, tetraploid additive, simplex dominant (all three heterozygotes are equivalent to one of the homozygotes) and duplex dominant (the duplex genotype has a common effect with the simplex and nulliplex genotypes or the triplex and quadriplex genotypes). A 1000x permutation test was run for each trait and year to establish a LOD threshold corresponding to a genome wide false positive rate of 5%. Manhattan plots were produced using GWASpoly. The proportion of phenotypic variance explained by significant SNPs was estimated by the coefficient of determination (R2). Genotypes of significant SNPs were coded in accordance with the gene action (i.e. simplex dominant or duplex dominant) and the R2 for each trait was calculated using the linear regression model in R. Contextual sequences of candidate SNPs were used in a BLAST search of DM1–3 pseudomolecules (Version 4.03) in the SpudDB database (Hirsch et al. 2014; http://solanaceae.plantbiology.msu.edu/) to identify putative candidate genes.
Expression Profiling
Tissue culture plantlets of Kalkaska (R) and Tundra (S) were grown in 25 × 150 mm culture tubes on modified MS media at 23–27 °C under fluorescent lights (30 μmol·m−2·s−1) with a 16 h photoperiod. When roots were well established, plants were transferred to a soil-less, hydroponic nutrient film (NFT) system (Khatri et al. 2010). During the first three weeks of growth in the NFT system, plants were fed with a 50% nutrient solution, pH 5.7–6.0 (IonicGrow, Hydrodynamics International, Lansing, MI) and maintained at a 16 h photoperiod under high-pressure sodium lights. For the remainder of the experiment, the photoperiod was reduced to 10 h to induce tuberization. Two days prior to treatment, tubers (3 to 5 mm in diameter) were lifted from the surface of the NFT system and placed in Whatman™ Grade 1 filter-paper lined 100 mm Petri dishes to prevent contamination of the system. Tubers used for the control treatment and tubers for Thaxtomin A treatment were never from the same plant and plants were discarded after the tubers were harvested to prevent further use.
Thaxtomin A was prepared by growing Streptomyces scabies in oatmeal broth (Loria et al. 2001). The cultures were allowed to grow for one week with shaking. The broth was filtered through three layers of cheesecloth followed by vacuum filtration through Whatman #1 filter paper. The filtrates were extracted 3X v/v with ethyl acetate. The ethyl acetate extracts were combined, dried with anhydrous sodium sulfate prior to removal of the solvent by rotary evaporation. Thaxtomin A was isolated from the extract by preparative TLC (King et al. 1989). A treatment solution of 50 μM Thaxtomin A in 2% ethanol was prepared. Sterilized Whatman™ Grade 1 filter paper discs (7 mm) were created using a sterile hole punch and soaked in either the control solution (2% ethanol) or 50 μM Thaxtomin A (in 2% ethanol) and air-dried. These treated discs were then placed onto the selected tubers with sterile forceps. Tuber slices 2-3 mm thick were taken from tissue directly beneath the treatment disc at 2, 4, 6, 8, 18, 20 and 24 h after treatment, immediately frozen in liquid nitrogen and stored at −80 °C. As this was the first examination of gene expression profiles in response to Thaxtomin A treatment, we selected these time points to gain insight into early tuber responses. Due to the small size of the tubers, 3 tubers were used for each treatment x time-point to ensure enough tissue for RNA extraction. RNA was extracted using a modified hot phenol protocol (Davidson et al. 2011).
The Ambion Turbo DNA-free system (Thermo Fischer Scientific, Waltham, MA) was used to remove genomic DNA. RNA quality was analyzed using an Agilent bioanalyzer (Agilent Technologies, Santa Clara, CA). Illumina cDNA libraries were prepared using the TruSeq DNA sample prep kit and sequenced in single-end mode (50 nt) on the Illumina Hiseq 2000 platform (Illumina, San Diego, CA). Raw reads were quality checked using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) and processed with Trimmomatic (v0.35) (MINLEN = 36, LEADING = 3, TRAILING = 3, SLIDINGWINDOW = 4,20) to remove adapters, primers and low-quality bases (Bolger et al. 2014). Filtered reads were mapped to the doubled monoploid S. tuberosum group Phureja clone DM1–3 (DM) pseudomolecules (Version 4.03; http://solanaceae.plantbiology.msu.edu/) using HISAT2 (Kim et al. 2019). Uniquely mapping reads overlapping the DM 4.03 gene models (http://solanaceae.plantbiology.msu.edu/) were counted with HTSeq v0.11.2 (Anders et al. 2015) in unstranded mode. Read counts were then used to conduct differential expression analysis with the R package edgeR v3.24.3 (Robinson et al. 2010). Differential expression between control and thaxtomin treatments was accomplished by combining the experimental factors genotype (Tundra or Kalkaska) and condition (treatment or control) into a single factor. As there was no replication of each condition in the individual genotypes at each timepoint, time was not included as a factor in the model. A multidimensional scaling plot was generated to compare samples using the plotMDS function in edgeR. The contrast function in edgeR was used to compare Kalkaska treatment samples compared to Kalkaska control samples and Tundra treatment samples to Tundra control samples. The quasi-likelihood F-test method was used to identify differentially expressed transcripts with a p value cut-off of 0.01. Custom Venn diagrams of differentially expressed transcripts were created using the VIB Bioinformatics and Evolutionary Genomics online tool (http://bioinformatics.psb.ugent.be/webtools/Venn/). Heatmaps of log2 fold change values of differentially expressed genes were created using the pheatmap package v1.0.12 in R.
Data Availability
Raw sequence reads are available in the NCBI Sequence Read Archive under BioProject ID PRJNA637104.
Results
Scab Phenotyping
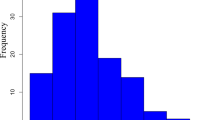
We observed a continuous distribution with positive skewedness for scab lesion type and tuber coverage in the 198 progeny assessed in 2011 and 2012 (Fig. 1, Table S1). Individuals with lower scores for both traits than resistant parent Kalkaska were observed in both years, indicative of genetic contributions toward resistance from both parental lines. The scab tuber coverage was more variable in 2012 (0.80–5.12) than in 2011 (1.09–4.94) (Fig. 1). Both mean scab tuber coverage and lesion type based on the mean of 20 tubers subsampled from each plot were significantly higher in 2011 (2.66 and 2.69, respectively) than in 2012 (2.15 and 2.32, respectively; P < 0.0001 and P < 0.0016, respectively). However, there was a significant positive correlation between traits both within and across years (Table 1). The highest correlations were between scab lesion type and tuber coverage within the 2012 growing season (0.80) and between scab lesion type in 2011 and 2012 growing seasons (0.77) (Table 1).
Distribution of scab tuber surface coverage scores (a) and scab lesion type scores (b) in 198 Kalkaska x Tundra tetraploid progeny assessed in replicated field trials in two growing seasons. Scab lesion type ratings ranged from 0 to 6 (0 = No scab lesions; 1 = Superficial lesions, discrete; 2 = Superficial lesions, coalescing; 3 = Raised lesions, discrete; 4 = Raised lesions, coalescing; 5 = Pitted lesions, discrete; 6 = Pitted lesions, coalescing) adapted from (Wanner 2006). Tuber scab coverage scores ranged from 0 to 6 (0 = no visible disease on tuber surface, 0.5 = < 1%; 1 = ≥ 1–2%; 2 = ≥ 2.1–5%; 3 = ≥ 5.1–10.%; 4 = ≥ 10.1–25%; 5 = ≥ 25.1–50%; 6 = ≥ 50%) (http://www.spongospora.ethz.ch/LaFretaz/scoringtable.htm). Data represent the mean of four plots of 20 sub-sampled tubers. Parental values for Kalkaska (K) and Tundra (T) are indicated. Outlier box plots for each distribution are shown and include the median sample value (line within the box plot) and a confidence diamond containing the mean and upper and lower 95% of the mean. Charts created in JMP® (Version Pro 13. SAS Institute Inc., Cary, NC)
GWAS Analysis
A single QTL defined by two significant SNPs (solcap_snp_c1_5267 and solcap_snp_c1_14089) on chromosome 1 between nucleotide positions 73,706,321-75,333,653 bp was identified for common scab lesion type in the simplex dominant (1-dom-ref) model in both years (Table 2, Fig. 2). In the 1-dom-ref model, the reference allele (A) is dominant and all three heterozygotes (AAAB, AABB, and ABBB) have equivalent phenotypic effect as the homozygous genotype of the reference allele (AAAA). A distinct SNP (solcap_snp_c1_12345) on chromosome 2 was found to be associated with common scab tuber coverage in 2011 in the duplex dominant (2-dom-alt) model, but not in 2012 (Table 2). Under the 2-dom-alt model, the alternate allele is dominant and the duplex alternate allele genotype (AABB) has the same effect as the triplex and quadriplex genotypes (BBBB and ABBB). The significant SNP (solcap_snp_c1_12345) identified on chromosome 2 lies within gene PGSC0003DMG400010445 (27,408,577-27,417,302 bp) annotated as a serine/threonine protein kinase.
Manhattan plots of significant SNPs along the 12 chromosomes and chromosome 00 for scab lesion type in 2011 (a) and 2012 (b) and scab tuber coverage in 2011 (c) detected using the simplex dominant (1-dom-ref) and duplex dominant (2-dom-alt) models. In the 1-dom-ref model, it is assumed that the reference allele (A) is dominant and that all three heterozygous genotypes (ABBB, AABB, and AAAB) have the same effect as the homozygous reference allele genotype (AAAA). Under the 2-dom-alt model, the alternate allele (B) is dominant and the duplex alternate allele genotype (AABB) has the same effect as the triplex and quadriplex genotypes (BBBB and ABBB). Plots were generated using the GWASpoly R package
Differential Expression Analysis
A total of 345 genes were differentially expressed between Kalkaska treatment and control samples while 306 genes were differentially expressed between Tundra treatment and control samples (α = 0.01) (Fig. 3a). Differentially expressed genes were distributed across all 12 chromosomes in both genotypes (Fig. 3b). To investigate if the regions mapped to common scab resistance in this study were involved in a Thaxtomin A response, we examined the expression of genes in close proximity to our significant SNPs on chromosome 1 and 2. Genes containing the three significant SNPs identified by GWAS in this study were not differentially expressed between the two genotypes at these times. However, there are several genes annotated as extensin proteins (PGSC0003DMG400019439, PGSC0003DMG400022518, PGSC0003DMG400022517, PGSC0003DMG400022515, PGSC0003DMG400022516, and PGSC0003DMG400022514) within the QTL region on chromosome 1. We observed significant down-regulation of one of the extensins in this region (PGSC0003DMG400022514) in susceptible Tundra tubers treated with Thaxtomin A (Table S2). Two genes in close proximity to the protein kinase containing the significant SNP associated with scab tuber coverage on chromosome 2 were found to be differentially expressed in tubers treated with Thaxtomin A: an ankyrin repeat containing protein (PGSC0003DMG400010444; 27,480,863- 27,482,073 bp) down-regulated in treated Kalkaska tubers and a CHP-rich zinc finger protein (PGSC0003DMG400010439; 27,540,399- 27,542,051 bp) up-regulated in treated Tundra tubers (Table S2).
Differential expression observed in common scab resistant variety Kalkaska and susceptible variety Tundra in response to Thaxtomin A treatment. a Venn diagram showing the overlap of differentially expressed genes among the two varieties across the full time course. b The log2 counts per million (CPM) of the 599 differentially expressed genes in Thaxtomin A treatment (T) and control (C) tuber samples of each variety (Kalkaska; K, Tundra; T) at sampled timepoints 2, 4, 6, 10, 18, 20 and 24 h post treatment are shown in the heatmap. Transcripts are ordered by position on the twelve chromosomes of the DM v4.03 assembly. The relative position of significant SNPs identified by GWAS analysis in this study are shown with arrows on the left of the heatmap. The SNP prefix ‘solcap_snp_’ has been omitted for each of the three SNPs to conserve space. The color key to the right of the heatmap indicates log2 CPM values
Multi-dimensional scaling plot analysis did not reveal a distinct pattern of expression that separated the treatment samples from control samples at each timepoint for the two varieties examined (Fig. S1). In the resistant variety Kalkaska, we observed differential expression of genes involved in cell wall biosynthesis and modification, including two pectate lyases (PGSC0003DMG402000859; PGSC0003DMG400015815) and cellulose synthase (PGSC0003DMG400013398), as well as genes associated with hormone signaling (Table S2). Several genes of interest were upregulated in treatment samples of both varieties were an ERF1 protein (PGSC0003DMG400026049), a trypsin proteinase inhibitor (PGSC0003DMG400024067), and a WRKY transcription factor (PGSC0003DMG400023196) (Table S2).
Discussion
Scab resistance in Kalkaska is derived from Lemhi Russet in the maternal lineage (Douches et al. 2009; Pavek et al. 1981). Lemhi Russet scab resistance is donated by grandparent USDA X96–56, originating from a cross between Earlaine and a Solanum demissium x S. tuberosum hybrid (Love 1999; Stevenson et al. 1955). The continuous distribution of scab lesion type and tuber coverage in the population used in this study and the small effect of the two genomic regions associated with scab that we identified (Table 2) are indicative of quantitative inheritance in this S. tuberosum germplasm. We report a novel QTL on chromosome 1 associated with scab lesion type in two field seasons with simplex dominance effect and a significant marker for scab tuber coverage on chromosome 2 with duplex dominance. As this SNP is 9.1 Mb upstream of the SNP (solcap_snp_c2_17864) associated with common scab resistance on chromosome 2 identified by Yuan et al. (2019), it is possible it is associated with a distinct genetic factor. The SNP identified in this study on chromosome 2 is within a serine/threonine protein kinase, proteins that are involved in late blight defense response in potato (Fantino et al. 2017; Yang et al. 2018), but was only detected in one field season. Of the few published studies mapping genetic features underlying scab resistance in potato, chromosome 2 is the only region identified repeatedly (Bradshaw et al. 2008; Yang et al. 2018). Coupled together with previous reports of QTL on chromosomes 2,4,6,9,11 & 12, each contributing minor effect to scab resistance (Bradshaw et al. 2008; Braun et al. 2017b; Enciso-Rodriguez et al. 2018; Yang et al. 2018), our findings underscore the difficulty of developing molecular markers linked to scab resistance and recommend the trait as a candidate for genomic selection.
There are numerous different approaches for measuring the extent and severity of scab infection to distinguish between resistant and susceptible genotypes. Unlike other potato diseases such as blights, soft rots and rusts, phenotyping scab is complicated by a wide variability of infection in tubers from a single plant. The methodology in this study used the mean of 20 individual tubers in a plot to assess lesion type and tuber lesion-covered surface area. This approach allows discrimination between the two responses, which although correlated, may be governed by separate interactions between the host plant and the pathogen (Goth et al. 1993; Kinkel et al. 1998; Wilson 2001). That we identified unique genomic regions associated with scab tuber coverage and scab lesion type supports this theory.
Assessing scab infection of tubers grown in fields naturally infested with Streptomyces may suffer from erratic distribution of the pathogen, the presence of multiple isolates that vary in virulence, and varying effects of environmental factors. Because Thaxtomin A has been strongly associated with virulence, it is possible that this toxin may be useful as a screen for resistance without the confounding factors associated with field screening in infested fields. Propagation of potato seedlings in vitro on media containing Thaxtomin A demonstrated a relationship between toxin inhibition of growth and scab resistance (Hiltunen et al. 2011; Hiltunen et al. 2006; Lawrence et al. 1990). However, in other research, no correlation between common scab resistance and thaxtomin sensitivity was found (Tegg and Wilson 2010). Somatic potato cells that survived treatment with Thaxtomin A were used to generate plants with the goal of developing new resistant lines. While this approach did result in lines resistant to the disease, not all of the disease resistant lines showed tolerance to treatment with the toxin. However, there was an association of thaxtomin tolerance with those lines expressing the greatest disease resistance. This suggests that while tolerance to Thaxtomin is important in resistance, other host factors may be involved (Wilson et al. 2009, 2010).
Results from this study indicate differences in the transcriptional response between resistant cultivar Kalkaska and susceptible cultivar Tundra activated by Thaxtomin A exposure in the early and later stages of a 24-h period. To our knowledge, this was the first examination of expression response to Thaxtomin A in potato tubers. Previous expression profiling of tubers inoculated with Streptomyces at different tuber developmental stages demonstrated the importance of launching an early and sustained defense response in scab resistant variety ‘Beate’ (Dees et al. 2016). Interestingly all of the 52 genes differentially expressed in both Kalkaska and Tundra tubers treated with Thaxtomin A in this study were upregulated in both varieties (Fig. 3a, Table S2). Without replication at each of timepoints assessed in this study it is unclear whether increased expression of these genes occurs simultaneously in both genotypes.
We observed differentially expressed genes involved in cell wall biosynthesis and hormone signaling. Of particular interest was a gene encoding an extensin protein located within the QTL region associated with scab lesion type on chromosome 1 and down-regulated in the tubers of susceptible cultivar Tundra. Extensins are a superfamily of cell wall glycoproteins involved in a variety of functions, including plant defense (Castilleux et al. 2018; Rashid 2016). Deposition and cross-linking of extensin proteins strengthen the cell wall and may restrict pathogen growth (Lamport et al. 2011; Rashid 2016). In potato, a strong up-regulation of extensin transcripts has been reported in tubers 1–5 days after wounding (Neubauer et al. 2012). Reduced extensin expression in Tundra may contribute to compromised cell wall integrity.
The phytohormones ethylene and jasmonic acid are associated with the regulation of biotic stress response and may act synergistically (Bari and Jones 2009; Derksen et al. 2013). Jasmonic acid signaling in particular has been correlated with resistance to necrotrophic pathogens (Antico et al. 2012; Glazebrook 2005) and requires conjugation to isoleucine in order to serve as a signal molecule (Katsir et al. 2008; Thines et al. 2007). The increased expression of a gene encoding a jasmonic acid-amino acid-conjugating enzyme (PGSC0003DMG401000095) on chromosome 1 near the QTL region in treated Kalkaska samples (Table S2) could represent induced jasmonic acid signaling in this variety. Moreover, we observed unique expression profiles of ethylene responsive factor (ERF) proteins and the early auxin response SMALL AUXIN UP RNA (SAUR) family proteins between varieties Kalkaska and Tundra.
Whether the differentially expressed genes identified by exogenous application of thaxtomin to tubers grown in a controlled environment are representative of a resistance response in the field requires further study. Clarke et al. (2019) suggest that Streptomyces species-dependent resistance in potato cultivars may be linked to the quantity of thaxtomin production. It would be informative to determine if there is a thaxtomin dose-dependent transcriptional response in resistant cultivar Kalkaska that differs from Tundra. In addition, it is important to note that pathogenic species of Streptomyces are known to produce other toxins and virulence factors (e.g., coronafacic acid derivatives and the necrosis inducing peptide Nec1 (Bignell et al. 2013; Bukhalid et al. 1998)). Thus, it is possible that in vitro screening may need to include these factors alone or in combination with Thaxtomin A for accurate in vitro screening of common scab resistance and susceptibility.
Conclusions
Here, we provide further evidence for quantitative inheritance of common scab resistance in tetraploid potato and illustrate the difficulty of developing molecular markers to select for this trait. The expression profiles of tubers we generated from a scab resistant and scab susceptible cultivar in response to Thaxtomin A treatment allowed identification of several differentially expressed genes located within our QTL on chromosome 1 and near our candidate SNP on chromosome 2 and demonstrate the possibility of using a pathogenicity determinant to investigate genes involved in the scab resistance response.
References
Acuna, I.A., G.A. Strobel, B.J. Jacobsen, and D.L. Corsini. 2001. Glucosylation as a mechanism of resistance to thaxtomin A in potatoes. Plant Science 161: 77–88.
Alam, Z. 1972. Inheritance of scab resistance in 24-chromosome potatoes. 6764–6764.
Anders, S., P.T. Pyl, and W. Huber. 2015. HTSeq—a python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169. https://doi.org/10.1093/bioinformatics/btu638.
Antico, C.J., C. Colon, T. Banks, and K.M. Ramonell. 2012. Insights into the role of jasmonic acid-mediated defenses against necrotrophic and biotrophic fungal pathogens. Frontiers in Biology 7: 48–56.
Atiq, M., A. Khalid, W. Hussain, A. Nawaz, S. Asad, and T. Ahmad. 2013. Genetic potential of potato germplasm against common scab disease caused by streptomyces scabies. Pakistan Journal of Phytopathology 25: 27–30.
Bari, R., and J.D. Jones. 2009. Role of plant hormones in plant defence responses. Plant Molecular Biology 69: 473–488.
Bignell, D.R., J.K. Fyans, and Z. Cheng. 2013. Phytotoxins produced by plant pathogenic streptomyces species. Journal of Applied Microbiology 116: 223–235.
Bolger, A.M., M. Lohse, and B. Usadel. 2014. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120. https://doi.org/10.1093/bioinformatics/btu170.
Bradshaw, J.E., C.A. Hackett, B. Pande, R. Waugh, and G.J. Bryan. 2008. QTL mapping of yield, agronomic and quality traits in tetraploid potato (Solanum tuberosum subsp. Tuberosum). Theoretical and Applied Genetics 116: 193–211. https://doi.org/10.1007/s00122-007-0659-1.
Braun, S., A. Gevens, A. Charkowski, C. Allen, and S. Jansky. 2017a. Potato common scab: A review of the causal pathogens, management practices, varietal resistance screening methods, and host resistance. American Journal of Potato Research 94: 283–296.
Braun, S.R., J.B. Endelman, K.G. Haynes, and S.H. Jansky. 2017b. Quantitative trait loci for resistance to common scab and cold-induced sweetening in diploid potato. The Plant Genome: 10.
Bukhalid, R.A., S.Y. Chung, and R. Loria. 1998. Nec1, a gene conferring a necrogenic phenotype, is conserved in plant-pathogenic Streptomyces spp. and linked to a transposase pseudogene. Molecular Plant Microbe Interactions 11: 960–967.
Castilleux, R., B. Plancot, M. Ropitaux, A. Carreras, J. Leprince, I. Boulogne, M.-L. Follet-Gueye, Z.A. Popper, A. Driouich, and M. Vicré. 2018. Cell wall extensins in root–microbe interactions and root secretions. Journal of Experimental Botany 69: 4235–4247.
Cipar, M., and C. Lawrence. 1972. Scab resistance of haploids from two Solanum tuberosum cultivars. American Potato Journal 49: 117–119.
Clarke, C.R., C.G. Kramer, R.R. Kotha, L.A. Wanner, D.L. Luthria, and M. Kramer. 2019. Cultivar resistance to common scab disease of potato is dependent on the pathogen species. Phytopathology 109: 1544–1554.
Davidson, R.M., C.N. Hansey, M. Gowda, K.L. Childs, H. Lin, B. Vaillancourt, R.S. Sekhon, N. de Leon, S.M. Kaeppler, and N. Jiang. 2011. Utility of RNA sequencing for analysis of maize reproductive transcriptomes. The Plant Genome 4: 191–203.
Dees, M.W., and L.A. Wanner. 2012. In search of better management of potato common scab. Potato Research 55: 249–268.
Dees, M., E. Lysøe, M. Alsheikh, J. Davik and M. Brurberg. 2016. Resistance to Streptomyces turgidiscabies in potato involves an early and sustained transcriptional reprogramming at initial stages of tuber formation. Molecular Plant Pathology 703–713.
Derksen, H., C. Rampitsch, and F. Daayf. 2013. Signaling cross-talk in plant disease resistance. Plant Science 207: 79–87.
Douches, D.S., J. Coombs, R. Hammerschmidt, W. Kirk, and C. Long. 2009. Kalkaska: A round white chip-processing potato variety with common scab resistance. American Journal of Potato Research 86: 347–355. https://doi.org/10.1007/s12230-009-9089-8.
Driscoll, J., J. Coombs, R. Hammerschmidt, W. Kirk, L. Wanner, and D. Douches. 2009. Greenhouse and field nursery evaluation for potato common scab tolerance in a tetraploid population. American Journal of Potato Research 86: 96–101.
Enciso-Rodriguez, F., D. Douches, M. Lopez-Cruz, J. Coombs and G. De Los Campos. 2018. Genomic selection for late blight and common scab resistance in tetraploid potato (Solanum tuberosum). G3: Genes|Genomes|Genetics 200273.
Fantino, E., M. Segretin, F. Santin, F. Mirkin, and R. Ulloa. 2017. Analysis of the potato calcium-dependent protein kinase family and characterization of StCDPK7, a member induced upon infection with Phytophthora infestans. Plant Cell Reports 36: 1137–1157.
FAOSTAT. 2017. Food and agriculture organization of the united nations. www.faostat.fao.org.
Felcher, K.J., J.J. Coombs, A.N. Massa, C.N. Hansey, J.P. Hamilton, R.E. Veilleux, C.R. Buell, and D.S. Douches. 2012. Integration of two diploid potato linkage maps with the potato genome sequence. PLoS One 7: e36347. https://doi.org/10.1371/journal.pone.0036347.
Gebhardt, C., and J.P. Valkonen. 2001. Organization of genes controlling disease resistance in the potato genome. Annual Review of Phytopathology 39: 79–102.
Glazebrook, J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Review of Phytopathology 43: 205–227.
Goth, R., K. Haynes and D. Wilson. 1993. Evaluation and characterization of advanced potato breeding clones for resistance to scab by cluster analysis. Plant Disease 77 (9): 911.
Haynes, K., B. Christ, C. Burkhart, and B. Vinyard. 2009. Heritability of resistance to common scab in diploid potatoes. American Journal of Potato Research 81: 165–170.
Hiltunen, L.H., I. Laakso, V. Chobot, K.S. Hakala, A. Weckman, and J.P. Valkonen. 2006. Influence of thaxtomins in different combinations and concentrations on growth of micropropagated potato shoot cultures. Journal of Agricultural and Food Chemistry 54: 3372–3379.
Hiltunen, L.H., M. Alanen, I. Laakso, A. Kangas, E. Virtanen, and J.P.T. Valkonen. 2011. Elimination of common scab sensitive progeny from a potato breeding population using thaxtomin a as a selective agent. Plant Pathology 60: 426–435.
Hirsch, C.N., C.D. Hirsch, K. Felcher, J. Coombs, D. Zarka, A. Van Deynze, W. De Jong, R.E. Veilleux, S. Jansky, P. Bethke, D.S. Douches, and C.R. Buell. 2013. Retrospective View of North American Potato ( L.) Breeding in the 20 and 21 Centuries.Genes|Genomes|Genetics 3 (6):1003–1013.
Hirsch, C.D., J.P. Hamilton, K.L. Childs, J. Cepela, E. Crisovan, B. Vaillancourt, C.N. Hirsch, M. Habermann, B. Neal and C.R. Buell. 2014. Spud DB: A resource for mining sequences, genotypes, and phenotypes to accelerate potato breeding. The Plant Genome 7: 1–12. https://doi.org/10.3835/plantgenome2013.12.0042.
Holterman, M., E. Bakker, S. van den Elsen, T. Borm, H. Pomp, M. Sterken, J. Helder and A. Goverse. 2017. The development of a high-throughput marker platform for genome-wide mapping of NB-LRR resistance genes in potato. In 5th Plant Genomics & Gene Editing Congress: Europe.
Jansky, S., K. Haynes, and D. Douches. 2019. Comparison of two strategies to introgress genes for resistance to common scab from diploid Solanum chacoense into tetraploid cultivated potato. American Journal of Potato Research 96: 255–261.
Katsir, L., A.L. Schilmiller, P.E. Staswick, S.Y. He, and G.A. Howe. 2008. Coi1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proceedings of the National Academy of Sciences 105: 7100–7105.
Khatri, B.B., R.S. Tegg, P.H. Brown, and C.R. Wilson. 2010. Infection of potato tubers with the common scab pathogen Streptomyces scabiei in a soil-less system. Journal of Phytopathology 158: 453–455.
Kim, D., J.M. Paggi, and S.L. Salzberg. 2019. Graph-based genome alignment and genotyping with hisat2 and hisat-genotype. Nature Biotechnology 37: 907–915.
King, R.R., C.H. Lawrence, M.C. Clark, and L.A. Calhoun. 1989. Isolation and characterization of phytotoxins associated with Streptomyces scabies. Journal of the Chemical Society, Chemical Communications 13: 849–850.
King, R.R., C.H. Lawrence, and J.A. Gray. 2001. Herbicidal properties of the thaxtomin group of phytotoxins. Journal of Agricultural and Food Chemistry 49: 2298–2301.
Kinkel, L.L., J.H. Bowers, K. Shimizu, E.C. Neeno-Eckwall, and J.L. Schottel. 1998. Quantitative relationships among thaxtomin a production, potato scab severity, and fatty acid composition in streptomyces. Canadian Journal of Microbiology 44: 768–776.
Lamport, D., M. Kieliszewski, Y. Chen, and M. Cannon. 2011. Update on the extensin super- family. Role of the extensin superfamily in primary cell wall architecture. Plant Physiology 156: 9–11.
Lawrence, C., M. Clark, and R. King. 1990. Induction of common scab symptoms in aseptically cultured potato tubers by the vivotoxin, thaxtomin. Phytopathology 80: 606–608.
Loria, R., Clark, C. A., Bukhalid, R. A., & Fry, B. A. (2001). Gram-positive bacteria: 504 streptomyces. In N. W. Schaad, J. B. Jones & W. Chun (Eds.), Laboratory guide for 505 identification of plant pathogenic bacteria (pp. 236–249.). St. Paul/Minnesota: APS Press.
Loria, R., D.R. Bignell, S. Moll, J.C. Huguet-Tapia, M.V. Joshi, E.G. Johnson, R.F. Seipke, and D.M. Gibson. 2008. Thaxtomin biosynthesis: The path to plant pathogenicity in the genus streptomyces. Antonie Van Leeuwenhoek 94: 3–10.
Love, S.L. 1999. Founding clones, major contributing ancestors, and exotic progenitors of prominent north American potato cultivars. American Journal of Potato Research 76: 263–272.
Murphy, A.M., H. De Jong, and G.C. Tai. 1995. Transmission of resistance to common scab from the diploid to the tetraploid level via 4x-2x crosses in potatoes. Euphytica 82: 227–233.
Navarro, L., P. Dunoyer, F. Jay, B. Arnold, N. Dharmasiri, M. Estelle, O. Voinnet, and J.D. Jones. 2006. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312: 436–439.
Navarro, F., K. Rak, E. Banks, B. Bowen, C. Higgins, and J. Palta. 2015. Strategies for selecting stable common scab resistant clones in a potato breeding program. American Journal of Potato Research 92: 326–338.
Neubauer, J.D., E.C. Lulai, A.L. Thompson, J.C. Suttle, and M.D. Bolton. 2012. Wounding coordinately induces cell wall protein, cell cycle and pectin methyl esterase genes involved in tuber closing layer and wound periderm development. Journal of Plant Physiology 169: 586–595.
Pavek, J., D. Corsini, J. Garner, S. Michener, W. Sparks, G. Carnahan, C. Stanger, A. Mosley, M. Johnson, and G. Carter. 1981. Lemhi russet: A new high yielding potato variety with wide adaptation, attractive tubers, and high internal quality. American Potato Journal 58: 619–625.
Rashid, A. 2016. Defense responses of plant cell wall non-catalytic proteins against pathogens. Physiological and Molecular Plant Pathology 94: 38–46.
Robinson, M., D. McCarthy, and G. Smyth. 2010. EdgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 26: 139–140.
Rosyara, U., W. De Jong, D. Douches, and J. Endelman. 2016. Software for genome-wide association studies in autopolyploids and its application to potato. Plant Genome 9: 1–10.
Scheible, W.-R., B. Fry, A. Kochevenko, D. Schindelasch, L. Zimmerli, S. Somerville, R. Loria, and C.R. Somerville. 2003. An Arabidopsis mutant resistant to thaxtomin a, a cellulose synthesis inhibitor from Streptomyces species. The Plant Cell 15: 1781–1794.
Seipke, R.F., and R. Loria. 2008. Streptomyces scabies 87-22 possesses a functional tomatinase. Journal of Bacteriology 190: 7684–7692.
Solomon-Blackburn, R.M., and H. Barker. 2001. A review of host major-gene resistance to potato viruses X, Y, a and V in potato: Genes, genetics and mapped locations. Heredity 86: 8–16.
Stevenson, F., J.G. McLean, W. Hoyman, and R. Akeley. 1955. Early gem: A new early, russet-skin, scab-resistant variety of potato adapted to the early potato-producing sections of Idaho and to certain sections of North Dakota. American Potato Journal 32: 79–85.
Tai, H.H., D. De Koeyer, M. Sønderkær, S. Hedegaard, M. Lagüe, C. Goyer, L. Nolan, C. Davidson, K. Gardner, and J. Neilson. 2018. Verticillium dahliae disease resistance and the regulatory pathway for maturity and tuberization in potato. The Plant Genome 11: 1–15.
Tegg, R., L. Melian, C. Wilson, and S. Shabala. 2005. Plant cell growth and ion flux responses to the streptomycete phytotoxin thaxtomin A: Calcium and hydrogen flux patterns revealed by the non-invasive mife technique. Plant Cell Physiology 46: 638–648.
Tegg, R.S., S.N. Shabala, T.A. Cuin, N.W. Davies, and C.R. Wilson. 2013. Enhanced resistance to the cellulose biosynthetic inhibitors, thaxtomin A and isoxaben in arabidopsis thaliana mutants, also provides specific co-resistance to the auxin transport inhibitor, 1-npa. BMC Plant Biology 13: 76.
Thangavel, T., R. Tegg, and C. Wilson. 2016. Toughing it out – Disease resistant potato mutants have enhanced tuber skin defenses. Phytopathology 106: 474–483.
Thines, B., L. Katsir, M. Melotto, Y. Niu, A. Mandaokar, G. Liu, K. Nomura, S. He, G.A. Howe, and J. Browse. 2007. Jaz repressor proteins are targets of the scf coi1 complex during jasmonate signalling. Nature 448: 661–665.
Tegg, R.S., and C.R. Wilson. 2010. Relationship of resistance to common scab disease and tolerance to thaxtomin A toxicity within potato cultivars. European Journal of Plant Pathology 128 (2):143–148.
Wanner, L. 2006. A survey of genetic variation in streptomyces isolates causing potato common scab in the United States. Bacteriology 96: 1363–1371. https://doi.org/10.1094/PHYTO-96-1363.
Wiersema, H. 1974. Testing for resistance to common scab. Potato Research 17: 356–357.
Wilson, C. 2001. Variability within clones of potato cv. Russet burbank to infection and severity of common scab disease of potato. Journal of Phytopathology 149: 625–628.
Wilson, C., G. Luckman, R. Tegg, Z. Yuan, A. Wilson, A. Eyles, and A. Conner. 2009. Enhanced resistance to common scab of potato through somatic cell selection in cv. Iwa with the phytotoxin thaxtomin a. Plant Pathology 58: 137–144.
Wilson, C.R., R.S. Tegg, A.J. Wilson, G.A. Luckman, A. Eyles, Z.Q. Yuan, L.H. Hingston, and A.J. Conner. 2010. Stable and extreme resistance to common scab of potato obtained through somatic cell selection. Phytopathology 100: 460–467.
Yang, X., X. Guo. Y. Yang, P. Ye, X. Xiong, J. Liu, D. Dong, and G. Li. 2018. Gene profiling in late blight resistance in potato genotype sd20. International Journal of Molecular Sciences 19.
Yuan, J., B. Benoît, D.K. David, R. Umesh, W. Zixiang and L.E. Martin. 2019. Genome-wide association study of resistance to potato common scab. Potato Research. https://doi.org/10.1007/s11540-019-09437-w.
Zorrilla, C., F. Navarro, S. Vega, K. Bamberg, and J. Palta. 2014. Identification and selection for tuber calcium, internal quality and pitted scab in segregating ‘Atlantic’x ‘Superior’reciprocal tetraploid populations. American Journal of Potato Research 91: 673–687.
Funding
This work was supported by funds from the U.S. Department of Agriculture, National Institute of Food and Agriculture, Agriculture and Food Research Initiative Plant Breeding, Genetics, and Genome grant 2009-85606-05673 to David Douches. This project was also supported by Project GREEEN (Generating Research and Extension to meet Economic and Environmental Needs) funding awarded to Project Director David Douches and funds from the Solanaceae Coordinated Agriculture Project (SOLCAP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaiser, N., Coombs, J., Felcher, K. et al. Genome-Wide Association Analysis of Common Scab Resistance and Expression Profiling of Tubers in Response to Thaxtomin A Treatment Underscore the Complexity of Common Scab Resistance in Tetraploid Potato. Am. J. Potato Res. 97, 513–522 (2020). https://doi.org/10.1007/s12230-020-09800-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-020-09800-5