Abstract
Commercial potato minituber production systems aim at high tuber numbers per plant. This study investigated by which mechanisms planting density (25.0, 62.5 and 145.8 plants/m2) of in vitro derived plantlets affected minituber yield and minituber number per plantlet. Lowering planting density resulted in a slower increase in soil cover by the leaves and reduced the accumulated intercepted radiation (AIR). It initially also reduced light use efficiency (LUE) and harvest index, and thus tuber weights per m2. At the commercial harvest 10 weeks after planting (WAP), LUE tended to be higher at lower densities. This compensated for the lower AIR and led to only slightly lower tuber yields. Lowering planting density increased tuber numbers per (planted) plantlet in all grades. It improved plantlet survival and increased stem numbers per plant. However, fewer stolons were produced per stem, whereas stolon numbers per plant were not affected. At lower densities, more tubers were initiated per stolon and the balance between initiation and later resorption of tubers was more favourable. Early interplant competition was thought to reduce the number of tubers initiated at higher densities, whereas later-occurring interplant competition resulted in a large fraction of the initiated tubers being resorbed at intermediate planting densities. At low planting densities, the high number of tubers initiated was also retained. Shortening of the production period could be considered at higher planting densities, because tuber number in the commercial grade > 9 mm did not increase any more after 6 WAP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In potato (Solanum tuberosum L.) seed production systems, in vitro derived plantlets are commonly used for production of clean seed stock (Jones 1988; Struik and Lommen 1990; Van der Zaag 1990; Pruski 2007). The plantlets can be planted directly into the field (Thornton and Knutson 1986; Tadesse et al. 2001; Tadesse 2007) or first used for production of small minitubers in greenhouses (Sipos et al. 1988; Lommen and Struik 1992c; Seabrook et al. 1995; Struik 2007). Large-scale, commercial production systems for minitubers typically grow in vitro derived plantlets in organic soil or artificial substrates in containers at densities of around 100–200 plants per m2 during a growing period of 10–18 weeks (Grigoriadou and Leventakis 1999; Pruski et al. 2003). They rarely yield more than two minitubers per plant in commercial grades (Grigoriadou and Leventakis 1999; T. Stolte, personal communication). Since in vitro plants are expensive, this low number per plant increases the cost price per minituber considerably.
At high densities, in vitro derived plants initiate only a few tubers, most of which grow to harvestable sizes (Lommen and Struik 1992b). Higher tuber numbers per plant can be achieved by introducing one or more repetitive harvests during the growing period (Lommen and Struik 1992c) either in soil or in hydroponics. The tuber removal stimulates the initiation of new tubers (Lommen and Struik 1992a) or the growth of otherwise resorbed, very small tubers to harvestable sizes (Lommen and Struik 1992b). The size distribution of minitubers produced, however, is a function of the number of tubers produced and the tuber yield. Repetitive harvesting consequently leads to relatively small tubers, and in addition has a high labour demand. It therefore may be less attractive for commercial production. Since smaller minitubers show larger losses during storage than larger minitubers (Lommen 1993), and a poorer performance and yield after field planting (Lommen and Struik 1994, 1995; Karafyllidis et al. 1997), the production of larger minitubers is preferred. An increase in tuber number per plant should thus be accompanied by a simultaneous increase in tuber yield per plant.
Decreasing planting density may be a prospectful way of increasing the number of minitubers per plant without reducing their size. Higher planting densities are well known to increase the numbers of minitubers per m2, but—comparable to seed tuber crops—to reduce the minituber number per plant and their size (Lommen and Struik 1992c; Roy et al. 1995; Abdulnour et al. 2003). Less is known about the mechanisms through which minituber numbers and their sizes are affected by planting density.
The aims of the present research were (1) to investigate the possibilities of increasing the minituber production per plant in a large-scale, commercial production system by lowering the planting density and (2) to study through which mechanisms planting density affects minituber production, concentrating on (a) processes determining tuber yield, (b) crop components determining tuber number and (c) the retention of tubers during aging of the crop. This will contribute to optimizing production conditions and help to determine the most economical plant density for reducing the cost price of the minitubers produced.
Material and Methods
In Vitro Production of Plantlets
Experiments were carried out with plantlets of cvs. Junior (maturity score 9, ‘very early’), Bintje (maturity score 6.5, ‘mid-early to mid-late’) and Aziza (maturity score 5, ‘late’) as part of a commercial production system. In vitro plants were multiplied routinely at NAK-AGRO (Emmeloord, The Netherlands) by subculturing single node cuttings every 4 weeks as described by Lommen and Struik (1992a). The temperature in the growth room was 21−23 °C and light was supplied by fluorescent tubes (Philips TL-54) at a photosynthetic photon flux of 11−10 μmol s-1 m-2 (400−700 nm) for 16 h per day. Plantlets were multiplied on medium containing 4.4 g l-1 M&S basal salts and vitamins (Murashige and Skoog 1962), 8.0 g l-1 agar, 25 g l-1 sucrose and 0.01 g l-1 Alar 64% (daminozide). Culture tubes contained one plantlet per tube on 10 ml medium. After the last in vitro multiplication, 25 single node cuttings were grown in a plastic jar (10-cm diameter, 5-cm height) containing 75 ml of the same medium for 14 days and were then planted.
Planting
The in vitro plantlets were planted at 25.0, 62.5 and 145.8 plants per m2 in 60 cm × 40 cm × 15 cm (length × width × height) trays with permeable sides and bottom. The trays contained 10 cm of potting compost consisting of a peat/clay mixture (1:1). Thirty-five planting holes (five rows × seven holes) per tray were mechanically pressed in the potting compost in a rectangular spacing. Each planting hole was 5 cm deep and 2 cm in diameter. Planting was done by hand. The plant density of 145.8 plants per m2 was obtained by planting an in vitro plantlet in every hole of a tray (five rows × seven holes). The plant density of 62.5 plants per m2 was obtained by planting 15 in vitro plantlets per tray, in which the row spacing remained the same, but only three positions per row were planted (five rows × three holes). The plant density of 25.0 plants per m2 was obtained by planting six plants per tray, in which the number of rows planted per tray was further decreased to two (two rows × three holes). Holes that remained unplanted were filled with potting compost from the same trays. The trays were watered by hand immediately after planting and moved to a glasshouse for further growth. Day temperatures varied between 18 and 25 °C and night temperatures were kept above 10 °C. The relative humidity was between 80 and 90%. Day length changed during the experiment from 15 h through 16.5 h to 16 h. Plants were watered with a rain system in the first 2 weeks after planting (WAP) at least twice a day depending on the daily temperature. Thereafter, water was supplied twice a day with an ebb–flood system. Watering was stopped 1 week before the final harvest. Plants were fertilized with calcium ammonium nitrate at 11 g N m-2. Planting was done on 6 May 2000, and destructive harvests were done at 6 and 10 WAP. No additional lighting was used.
Experimental Design
The experimental unit was one tray. Within each cultivar, the experiment was set up as a split-plot design with five blocks, in which the planting density treatment was applied to the main plots and the harvest time to the subplots. Every tray was surrounded by a tray with the same planting density to eliminate border effects. After the destructive harvest at 6 WAP empty spots were filled with adjacent trays.
Observations and Calculations
The percentage of green ground cover was estimated weekly by means of a grid of 40 cm × 60 cm divided into 99 squares (11 × 9), viewed directly from above (Haverkort et al. 1991). Daily ground cover was calculated by linear interpolation between weekly values. Photosynthetically active radiation (PAR) outside the glasshouse was calculated as 50% of total incident global solar radiation. The glasshouse structure also prevented approximately 25% of the incoming PAR from reaching the crop, so a conversion factor of 0.5 × 0.75 = 0.375 was used to calculate the PAR that reached the crop from the total incident global solar radiation measured outside the glasshouse. Accumulated intercepted radiation (AIR) was calculated over the whole season by multiplying the daily ground cover by the daily PAR and summing up the daily values. Light use efficiency (LUE) was calculated as the dry weight production (excluding roots) divided by the AIR. At the destructive harvest 6 WAP the number of surviving plants, the number of below-ground originating stems and the number of main stolons per tray were counted. One tray contained 35, 15 and six plants for the respective planting densities. Dry weights of the total of stems, leaves and stolons were determined. Tuber numbers in three grades were determined: mesh grade ≤ 9 mm, 9 mm < mesh grade ≤ 17 mm and mesh grade > 17 mm. Fresh and dry weights of all tubers were determined. Total dry weight was calculated as the sum of the dry weight of the stems, leaves, stolons and tubers (excluding roots). The harvest index (HI) was the fraction of tuber dry weight to the total dry weight. The dry matter concentration of the tubers was the fraction of the tuber dry weight to the tuber fresh weight. Note the difference between the denominations used: ‘planted plant’, which is the same as the planting density, and ‘plant’, which refers to plants that were actually present at the moment of harvesting.
Statistical Analysis
Data from destructive harvests were subjected to analysis of variance using Genstat release 8.0. Analyses were carried out for each harvest date separately, except when data were compared between harvests. Within a harvest date, the experiment was analysed as randomized complete block experiment combined over cultivars; across harvest dates, the experiment was analysed as split-plot experiment combined over cultivars. Significances of cultivar effects were tested in the cultivar–block stratum; significances of effects of planting density and planting density × cultivar interactions were tested in the cultivar–block–planting density stratum. When data were analysed over both harvest dates, significances of harvest date and harvest date × cultivar, harvest date × planting density and harvest date × cultivar × planting density interactions were tested in the cultivar–block–planting density–harvest date stratum. Relevant least significant differences (P < 0.05) were calculated when the main effects of planting density, planting density × cultivar and/or planting density × cultivar × harvest date interactions were significant (P < 0.05) or indicated a tendency (0.05 ≤ P < 0.10).
Results
Yield Formation
At lower planting densities, ground cover increased more slowly and 100% ground cover was reached later than at higher densities for all cultivars (Fig. 1). The percentage of ground cover started to decrease between 8 and 9 WAP for cvs. Bintje and Junior and between 9 and 10 WAP for cv. Aziza. This decrease was faster for cv. Junior than for cvs. Bintje and Aziza. The decrease in ground cover was the same at all planting densities.
At 6 WAP, AIR, LUE, total dry weight, HI, tuber dry weight, dry matter concentration of the tubers and tuber fresh weight were all lower at lower planting densities (Table 1). At 10 WAP, AIR was still lower at lower plant densities. However, the very early cv. Junior now showed a tendency towards a higher LUE at lower planting densities, whereas the differences in LUE between planting densities for the mid-early cv. Bintje and late cv. Aziza could not be established as significant (Table 2). Total dry weight was not different between planting densities for cvs. Junior and Bintje but showed a tendency to decrease with decreasing planting density in cv. Aziza (Table 2). Planting density had no significant effect on HI at 10 WAP and the effects on tuber dry weight were comparable to those on total dry weight. At lower planting densities, dry matter concentration of the tubers was higher than at higher planting densities for cv. Junior, was not significantly different from that at higher densities for cv. Bintje and was lower than at higher planting densities for cv. Aziza (Table 2). Tuber fresh weight showed a tendency to decrease with decreasing planting density (Table 2).
Yield Components
The fraction of plants surviving transplanting was higher at lower planting density but the actual plant number per m2 still remained lower (Table 3). Also the number of stems per plant was higher at lower planting density, whereas the number of stems per m2 was lower (Table 3). Decreasing planting density, however, significantly decreased the number of stolons per stem (Table 3) but had no significant effect on the number of stolons per plant (Table 4). The number of tubers initiated at 6 WAP per stolon was highest at the lower and intermediate planting densities, but on a per m2 basis tuber numbers were lower at lower planting densities (Table 3). The retention of the initiated tubers—assessed as the fraction of tubers initiated at 6 WAP that were retained between 6 and 10 WAP—was best at the lowest planting density (Table 3). The intermediate planting density—at which high numbers of tubers were initiated—showed the poorest retention of tubers. Plants at the highest plant density—at which much lower tuber numbers were initiated—retained an intermediate fraction of tubers, not significantly different from the value at the highest and intermediate planting densities (Table 3). The number of tubers per m2 at 10 WAP was lower at lower planting densities, but the fresh weight per tuber was higher (Table 3). Tuber fresh weight production per m2 at 10 WAP still showed a tendency to be lower at lower planting densities (Table 3). Decreasing the planting density significantly increased the numbers of tubers > 0, > 9 and > 17 mm per stolon, per stem and per plant (Table 4) for all three grades at 10 WAP.
Changes in Tuber Number and Size Between 6 and 10 WAP
At 6 WAP, the overall trend was that the number of tubers > 0, > 9 and > 17 mm per planted plant decreased with increasing planting density, but especially in the later cultivars the differences between the lowest and intermediate planting densities were small and in none of the cultivars significant. In the late cv. Aziza the number of tubers > 9 and > 17 mm did not even differ significantly between all planting densities.
The changes in number of tubers > 0 mm per planted plant between 6 and 10 WAP reflected the changes in the number of tubers initiated and retained per stolon treated as described above. For tubers > 0 mm, the relatively high number initiated at 6 WAP at 25.0 plants per m2 did not change significantly between 6 and 10 WAP, whereas the relatively high number initiated at 62.5 plants per m2 decreased between 6 and 10 WAP. The lower number of tubers initiated per planted plant at 6 WAP at 145.8 plants per m2 did not change significantly between 6 and 10 WAP.
The relatively high number of tubers > 9 mm per planted plant present at a planting density of 25.0 plants per m2 at 6 WAP increased significantly between 6 and 10 WAP. At 62.5 plants per m2, the relatively high number present at 6 WAP did not increase further: it even decreased in the early cv. Junior and did not change in the other cultivars. Relatively lower numbers of tubers > 9 mm were present per planted plant at 6 WAP at 145.8 plants per m2 and no significant changes occurred between 6 and 10 WAP (Fig. 2).
The cumulative number of tubers in three grades per planted plant at 6 and 10 weeks after planting at three planting densities for in vitro derived plantlets from three cultivars. Cumulative number of tubers > 0, > 9 and > 17 mm with different letters within the same cultivar are significantly different according to the least significant difference at the 5% level. WAP weeks after planting, PD planting density
The number of tubers > 17 mm per planted plant increased significantly from 6 to 10 WAP in all three cultivars at 25.0 planted plants per m2. At 62.5 plants per m2, this number only increased significantly for cvs. Bintje and Aziza and did not increase any more for the early cv. Junior at 62.5 plants per m2, and no significant changes were found at 145.8 plants per m2 (Fig. 2).
The general trend after 10 WAP was that the numbers of tubers > 0, > 9 and > 17 mm per planted plant, i.e. the multiplication factors for tubers in different grades, were significantly higher at lower planting density in all cultivars, although not all differences within individual cultivars indeed could be assessed as significant (Fig. 2).
Discussion
Crop Yield Parameters
Six WAP, tuber dry weight per m2 was considerably lower at lower planting densities (Table 1). This was not just because of a reduced AIR at lower planting densities, but also because of a lower LUE and a lower HI (Table 1). The reduced AIR was caused directly by the slower ground cover by green foliage at lower planting densities (Fig. 1), but the lower LUE and HI were unexpected. The lower HI indicates that plants at low planting density were allocating relatively fewer assimilates to the tubers. This suggests that the plants at lower densities were sink-limited, meaning that they did not have enough tubers to allocate assimilates to or that the capacity of the existing tubers to absorb assimilates was limited. It is less likely that tuber initiation was delayed, because plants at the lower density had more tubers initiated per plant than those at higher densities: Tuber numbers initiated per stolon were higher (Table 3) and the total number of stolons per plant did not differ significantly (Table 4). Sink-limitation can also explain why LUE was lower at low plant density: The photosynthetic capacity of potato increases after formation of tubers (Lorenzen and Ewing 1990). In addition, carbohydrate-rich tubers are produced more efficiently from assimilates than protein-rich leaves. Differences between planting densities in tuber fresh weight per m2 were smaller than those in tuber dry weight per m2 because the tuber dry matter concentration was lower at lower planting densities (Table 1).
Ten WAP, at the moment of commercial harvest, the differences in AIR as observed 6 WAP (Table 1) had persisted (Table 2) because soil cover in all cultivars and at all planting densities was already 100% 6 WAP and differences between planting densities in the decline during senescence were negligible (Fig. 1). For the earlier cultivars, Junior and Bintje, however, the lower AIR at lower planting densities did not result in lower tuber dry weights per m2 (Table 2), because LUE over the total period of 10 weeks tended to be higher for plants growing at the lower densities, especially in cv. Junior, whereas no significant differences in HI occurred between planting densities (Table 2). The higher LUE thus counteracted the negative effect of the lower AIR on total and tuber dry yield in earlier cultivars (Table 2). A reason for the higher LUE at lower densities could be higher respiration rates at high planting densities because of excessive leaf production, and consequently a reduction in net assimilation (cf. Khurana and McLaren 1982). The later cultivar, Aziza, did not show a higher LUE at the lowest planting density, likely because tuber sink limitation reduced LUE more at lower planting density in later than in earlier cultivars. In this cultivar, total and tuber dry weight production per m2 at 10 WAP followed the trend in AIR, i.e. a lower production at lower densities (Table 2). Effects of planting density on tuber dry matter concentration varied over cultivars, with fresh tuber yield as a result showing a trend of being slightly lower at lower planting densities. In comparable systems, this small decrease in tuber yield per m2 with decreasing planting densities below approximately 100 plants per m2 was also reported by Wiersema (1986) and Roy et al. (1995).
Yield Components
Lowering planting density significantly increased the number of tubers per plant in all grades (Table 4) in concordance with effects commonly found in in vitro derived plantlets (Lommen and Struik 1992c; Roy et al. 1995; Abdulnour et al. 2003). In crops from seed tubers, the number of tubers produced per plant is determined by the number of tubers per stolon, the number of stolons per stem and the number of stems per plant (Haverkort et al. 1990). In in vitro derived plants, the higher number of tubers per plant at lower planting density was a direct effect of the increased number of tubers per stolon (Table 4) and the increased number of stems per plant (Table 3). The number of stolons per stem even decreased at a lower planting density (Table 3), thus resulting in a negative contribution to the increase of the number of tubers per plant. This seems to be in contrast with what was reported by Haverkort et al. (1990), who found that plants from seed tubers with more stems per plant also tend to produce more stolons per plant. In in vitro derived plants at the low planting density, some of the earlier-produced stolons likely turned upwards to become stems, thus forming extra stems and reducing the number of stolons. Lowering planting density had no significant effect on the number of stolons per plant (Table 4), which is in concordance with what was reported by Fonseka et al. (1996). The number of tubers per stem increased with decreasing planting density (Table 4); thus, the decrease of the number of stolons per stem did not totally counteract the increase of the number of tubers per stolon at lower planting density.
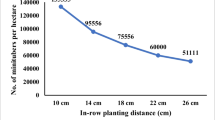
As expected, lowering the planting density led to a lower number of tubers per m2 (Roy et al. 1995; Abdulnour et al. 2003), but because a higher fresh weight per tuber partly counteracted this effect, there was only weak decrease in the final tuber fresh yield per m2 with decreasing planting density (Table 3). The lower number of tubers per m2 at lower planting density resulted from the much lower number of plants planted per m2. The higher plant survival (Table 3) and more tubers per plant at lower planting density did not compensate for the lower number of plants. The better plant survival was likely because of a lower competition for light, water and nutrition (cf. Rex et al. 1987; Roy et al. 1995).
Tuber Initiation and Retention with Time
Plant density greatly affected the change in tuber number between 6 and 10 WAP. The number of tubers > 0 mm per planted plant or stolon did not decrease as much with time at the lowest planting density as at the higher densities, especially 62.5 planted plants per m2 (Fig. 2, Table 3). Plants at 62.5 planted plants per m2 apparently were unable to produce enough assimilates in a later phase of growth to maintain all tubers initiated at 6 WAP, thus making these plants source-limited. As discussed before, the lower LUE at lower planting densities at 6 WAP (Table 1) likely resulted from sink-limitation. That may explain why no decrease in the number of tubers > 0 mm per planted plant was found at the lowest planting density: all tubers initiated were maintained and even grew to a larger size (Fig. 2).
The fraction of tubers retained during aging (Table 3) and the decrease in the number of tubers (Fig. 2) indicated larger changes at the intermediate density than at the highest density. This is likely because plants at the highest planting density had initiated a lower number of tubers per stolon in an early stage and thus also had to support a low number of tubers later, while the plants at the intermediate planting density initiated an almost comparable number of tubers per stolon as at the lowest planting density (Table 3). At the highest density early competition may have reduced light interception per plant and consequently the number of tubers initiated (cf. O’Brien et al. 1998). Plants at the intermediate density will have experienced the increasing interplant competition later than those at the highest density, and were unable to maintain all the initiated tubers, leading to a higher resorption.
Decreasing the Length of the Production Period
In all cultivars, the individual tuber weights within the commercial size classes increased between 6 and 10 WAP (not shown). Larger minitubers have better growth and produce more and larger tubers when planted in the field (Lommen and Struik 1994, 1995; Karafyllidis et al. 1997). Glasshouse-grown minitubers, however, are usually sold by number in certain size classes, and not by weight within those classes. Tubers > 9 mm are considered large enough to sell, but tubers > 17 mm are preferable because they perform better.
When a larger size does not result in higher prices, an approach to reduce costs for a production company might be to decrease the length of the production period for minitubers produced at the higher planting densities. At the low planting density the number of tubers > 9 and > 17 mm increased significantly between 6 and 10 WAP in all cultivars. At 62.5 plants per m2, however, the number of tubers > 9 mm did not increase any more between 6 and 10 WAP (Fig. 2) and for cv. Junior even decreased. Also at 142.8 plants per m2, the number of tubers > 9 mm did not change. Thus, decreasing the production period from 10 to 6 weeks would not lead to a significantly lower number of tubers > 9 mm per planted plant at 62.5 and 145.8 plants per m2. For tubers > 17 mm, the number increased at 62.5 plants per m2 between 6 and 10 WAP for the two latest cultivars, whereas there was no change for cv. Junior. At 145.8 plants per m2, there were no changes in any of the cultivars. Thus, reducing the production period from 10 to 6 weeks would not lead to a significantly lower number of tubers > 17 mm at 145.8 and at 62.5 plants per m2 for the earliest cultivar, but will result in fewer tubers > 17 mm in the later cultivars. This is in line with a later and/or longer tuber filling period in later cultivars.
Practical Implications
Lowering planting density was a good way to both increase the number of tubers per planted plant in commercial grades (> 9 mm) and simultaneously increase the fresh weight per tuber. Decreasing planting density from 145.8 to 25.0 plants per m2 led to twice as many tubers of harvestable size per plantlet, which in turn decreases the labour and equipment demand for producing the in vitro plantlets by half.
However, decreasing planting density from 145.8 to 25.0 plants per m2 on average reduced the number of tubers per m2 to one third. Thus, to be able to produce the same number of minitubers with a planting density of 25.0 plants per m2, the area used for production has to be 3 times larger. This will increase the costs per minituber again.
Whether decreasing planting density is profitable will be a trade-off between the benefits of a lower labour demand for production of in vitro plantlets, and thus the price of an in vitro derived plant, the increased costs that accompany an increased area needed for production of the same number of minitubers, and the probably higher prices owing to the better quality of minitubers produced.
Abbreviations
- AIR:
-
Accumulated intercepted radiation
- CV:
-
Cultivar
- DMC:
-
Dry matter concentration
- DW:
-
Dry weight
- FW:
-
Fresh weight
- HI:
-
Harvest index
- LUE:
-
Light use efficiency
- PAR:
-
Photosynthetically active radiation
- PD:
-
Planting density
- WAP:
-
Weeks after planting
References
Abdulnour J, Roy G, Desjardins Y (2003) Effect of supplemental lighting, substrate (potting mix) volume and plant densities on potato minituber production during winter greenhouse culture in Quebec. Acta Hortic 619:53–58
Fonseka HD, Asanuma K, Kusutani A, Ghosh AK, Ueda K (1996) Growth and yield of potato cultivars in spring cropping I. Plant morphology, growth, assimilate partitioning and yield under two planting densities. Jpn J Crop Sci 65:269–276
Grigoriadou K, Leventakis N (1999) Large scale commercial production of potato minitubers, using in vitro techniques. Potato Res 42:607–610 doi:10.1007/BF02358178
Haverkort AJ, van de Waart M, Bodlaender KBA (1990) Interrelationships of the number of initial sprouts, stems, stolons and tubers per potato plant. Potato Res 33:269–274 doi:10.1007/BF02358456
Haverkort AJ, Uenk D, Veroude H, van de Maart M (1991) Relationships between ground cover, intercepted solar radiation, leaf area index and infrared reflectance of potato crops. Potato Res 34(1):113–121 doi:10.1007/BF02358105
Jones ED (1988) A current assessment of in vitro culture and other rapid multiplication methods in North America and Europe. Am Potato J 65:209–220 doi:10.1007/BF02854453
Karafyllidis DI, Georgakis DN, Stavropoulos NI, Vezyroglou IA, Nianiou EX (1997) Effect of planting density and size of potato minitubers on their yielding capacity. Acta Hortic 462:943–949
Khurana SC, McLaren JS (1982) The influence of leaf area, light interception and season on potato growth and yield. Potato Res 25:329–342 doi:10.1007/BF02357290
Lommen WJM (1993) Post-harvest characteristics of potato minitubers with different fresh weights and from different harvests. II. Losses during storage. Potato Res 36:273–282 doi:10.1007/BF02361793
Lommen WJM, Struik PC (1992a) Influence of a single non-destructive harvest on potato plantlets grown for minituber production. Neth J Agric Sci 40:21–41
Lommen WJM, Struik PC (1992b) Production of potato minitubers by repeated harvesting: Plant productivity and initiation, growth and resorption of tubers. Neth J Agric Sci 40:342–358
Lommen WJM, Struik PC (1992c) Production of potato minitubers by repeated harvesting: Effects of crop husbandry on yield parameters. Potato Res 35:419–432 doi:10.1007/BF02357598
Lommen WJM, Struik PC (1994) Field performance of potato minitubers with different fresh weights and conventional seed tubers: crop establishment and yield formation. Potato Res 37:301–313 doi:10.1007/BF02360523
Lommen WJM, Struik PC (1995) Field performance of potato minitubers with different fresh weights and conventional seed tubers: Multiplication factors and progeny yield variation. Potato Res 38:159–169 doi:10.1007/BF02357929
Lorenzen JH, Ewing EE (1990) Changes in tuberization and assimilate partitioning in potato (Solanum tuberosum L.) during the first 18 days of photoperiod treatment. Ann Bot (Lond) 66:457–464
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497 doi:10.1111/j.1399-3054.1962.tb08052.x
O’Brien PJ, Firman DM, Allen EJ (1998) Effects of shading and seed tuber spacing on initiation and number of tubers in potato crops (Solanum tuberosum). J Agric Sci 130:431–449 doi:10.1017/S0021859698005541
Pruski K (2007) The canon of potato science: 22. In vitro multiplication through nodal cuttings. Potato Res 50:293–296 doi:10.1007/s11540-008-9050-0
Pruski K, Astatkie T, Duplessis P, Lewis T, Nowak J, Struik PC (2003) Use of jasmonate for conditioning of potato plantlets and microtubers in greenhouse production of minitubers. Am J Potato Res 80:183–193
Rex BL, Russel WA, Wolfe HR (1987) The effect of spacing of seed pieces on yield, quality and economic value for processing of Shepody potato in Manitaba. Am Potato J 64:177–189 doi:10.1007/BF02853461
Roy RD, Machado VS, Alam SMM, Ali A (1995) Greenhouse production of potato (Solanum tuberosum L. cv. Désirée) seed tubers using in vitro plantlets and rooted cuttings in large propagation beds. Potato Res 38:61–68 doi:10.1007/BF02358070
Seabrook JEA, Percy JE, Douglass LK, Tai GCC (1995) Photoperiod in vitro affects subsequent yield of greenhouse-grown potato tubers. Am Potato J 72:365–373 doi:10.1007/BF02849333
Sipos J, Nowak J, Hicks G (1988) Effect of daminozide on survival, growth and yield of micropropagated potatoes. Am Potato J 65:353–364 doi:10.1007/BF02853531
Struik PC (2007) The canon of potato science: 25. Minitubers. Potato Res 50:305–308 doi:10.1007/s11540-008-9051-z
Struik PC, Lommen WJM (1990) Production, storage and use of micro- and minitubers. In: Proceedings 11th Triennial Conference of the European Association for Potato Research, Edinburgh, pp 122−133
Tadesse Mehari (2007) The canon of potato science: 23. Transplants. Potato Res 50:297–299 doi:10.1007/s11540-008-9078-1
Tadesse Mehari, Lommen WJM, Struik PC (2001) Effects of nitrogen pre-treatment of transplants from in vitro produced potato plantlets on transplant growth and yield in the field. Neth J Agric Sci 49:67–79
Thornton MK, Knutson WK (1986) Effect of transplant container volume and growing season length on field performance of micropropagated potatoes. Am Potato J 63:399–410 doi:10.1007/BF02854103
Van der Zaag DE (1990) The implications of micropropagation for the future of seed production systems in Europe. In: Proceedings 11th Triennial Conference of the European Association for Potato Research, Edinburgh, pp 28−45
Wiersema SG (1986) A method of producing seed tubers from true potato seed. Potato Res 29:225–237 doi:10.1007/BF02357653
Acknowledgements
We would like to thank NAK-AGRO Emmeloord for the possibility to carry out this research under commercial conditions and their permission to publish the results. Special thanks go to T. Stolte and T. Meulendijks for their cooperation and ideas.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van der Veeken, A.J.H., Lommen, W.J.M. How Planting Density Affects Number and Yield of Potato Minitubers in a Commercial Glasshouse Production System. Potato Res. 52, 105–119 (2009). https://doi.org/10.1007/s11540-008-9124-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11540-008-9124-z