Abstract
Background
Activating KRAS mutations are reported in up to 90% of pancreatic cancers. Refametinib potently inhibits MEK1/2, part of the MAPK signaling pathway. This phase I/II study evaluated the safety and efficacy of refametinib plus gemcitabine in patients with advanced pancreatic cancer.
Methods
Phase I comprised dose escalation, followed by phase II expansion. Refametinib and gemcitabine plasma levels were analyzed for pharmacokinetics. KRAS mutational status was determined from circulating tumor DNA.
Results
Ninety patients overall received treatment. The maximum tolerated dose was refametinib 50 mg twice daily plus standard gemcitabine (1000 mg/m2 weekly). The combination was well tolerated, with no pharmacokinetic interaction. Treatment-emergent toxicities included thrombocytopenia, fatigue, anemia, and edema. The objective response rate was 23% and the disease control rate was 73%. Overall response rate, disease control rate, progression-free survival, and overall survival were higher in patients without detectable KRAS mutations (48% vs. 28%, 81% vs. 69%, 8.8 vs. 5.3 months, and 18.2 vs. 6.6 months, respectively).
Conclusion
Refametinib plus gemcitabine was well tolerated, with a promising objective response rate, and had an acceptable safety profile and no pharmacokinetic interaction. There was a trend towards improved outcomes in patients without detectable KRAS mutations that deserves future investigation.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Pancreatic cancer is among the leading causes of cancer-related mortality worldwide [1], and activating KRAS mutations are reported in up to 90% of pancreatic cancers [2, 3]. These mutations cause constitutive activation of the mitogen-activated protein kinase signaling pathway (RAS-RAF-MEK-ERK) which, in turn, persistently activates downstream effectors leading to dysregulated cellular proliferation, survival, and metastasis [4–6]. Therefore, mitogen-activated protein kinase signaling is an important target for therapeutic inhibition [7]. However, only a subset of patients may benefit from single-agent treatment approaches as multiple pathways are commonly dysregulated.
Gemcitabine monotherapy has long been the standard of care for advanced pancreatic cancer and still represents an option (along with the oxaliplatin, irinotecan, fluorouracil, and leucovorin regimen, and gemcitabine plus albumin-bound paclitaxel) for first-line therapy in metastatic or locally advanced, unresectable disease [8–10]. However, the survival improvement with gemcitabine monotherapy is modest [11]. Previous phase II and III trials of gemcitabine combined with other cytotoxic agents have shown acceptable safety but inconsistent survival improvement versus monotherapy [10, 12–16]. The promising activity of cytotoxic combinations has also been associated with high toxicity [9, 10].
Refametinib (BAY 86-9766; Bayer Pharma AG, Berlin, Germany) is an orally available, potent, selective, allosteric (non-adenosine triphosphate competitive) inhibitor of MEK1/2 [17]. Refametinib has demonstrated both single-agent activity [18] and synergistic activity in combination with gemcitabine [19] in preclinical models of pancreatic cancer.
A single-arm, open-label, phase I/II study (NCT01251640) evaluated the safety and efficacy of refametinib plus gemcitabine in patients with advanced pancreatic cancer eligible for first-line gemcitabine. Phase I investigated the safety, tolerability, and pharmacokinetics of the combination; phase II evaluated the efficacy, safety, and biomarker analysis of the recommended phase II dose.
2 Methods
2.1 Ethical Approval and Informed Consent
The study protocol and all protocol amendments were reviewed and approved by independent ethics committees and institutional review boards for each study site before the start of the study and before implementation of the amendments. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
2.2 Study Design
This open-label, non-randomized, multicenter study (ClinicalTrials.gov number NCT01251640 (http://clinicaltrials.gov/show/NCT01251640)) comprised two phases: phase I evaluated three dose levels to determine the maximum tolerated dose and recommended phase II dose of refametinib plus gemcitabine; phase II evaluated the efficacy, safety, and biomarker analysis of the recommended phase II dose. The primary outcome measure in phase II was objective response rate (confirmed complete response and confirmed partial response) per blinded independent radiological review. Secondary outcome measures included disease control rate (complete response, partial response, and stable disease), progression-free survival, overall survival, toxicity, and determination of KRAS mutational status (wild type or mutant). Additional secondary measures were pre-planned correlation of KRAS mutational status with response and survival, and analysis of biomarkers relevant to RAS pathway activation or to pathways known to influence activity of RAS-RAF-dependent signal transduction.
Patients received standard gemcitabine intravenously at 1000 mg/m2 weekly on day 1; continuous oral treatment with refametinib twice daily began on day 2. Patients received refametinib plus gemcitabine for 7 out of 8 weeks (cycle 1), then 3 out of 4 weeks in subsequent cycles. Enrollment of up to 18 patients in phase I was planned. Dose escalation followed a 3 + 3 design. If no dose-limiting toxicity was seen within the first three patients at the starting dose level (refametinib 30 mg twice daily plus standard gemcitabine) and within the first 4 weeks of treatment, the next highest dose level (refametinib 50 mg twice daily plus standard gemcitabine) was to be initiated immediately. All three patients enrolled at the starting dose level were to continue treatment until they had received a full 8 weeks of therapy. If a dose-limiting toxicity occurred within the first three patients at the starting dose level and after the first 4 weeks of treatment, further recruitment to the higher dose cohort was to be paused and three additional patients were to be enrolled to the starting dose cohort. If no dose-limiting toxicities were observed in these additional three patients within 4 weeks of treatment, then enrollment to the higher dose level continued. If two or more patients out of a maximum of six showed dose-limiting toxicities at the starting dose level within cycle 1, the next lowest dose level would be investigated. The maximum tolerated dose was the highest dose level at which no more than one patient out of six experienced a dose-limiting toxicity. Following identification of the maximum tolerated dose, the data monitoring committee was to be involved in the definition of the recommended phase II dose.
Protocol-defined dose-limiting toxicities, as defined by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0, included grade 4 anemia; grade 4 neutropenia lasting more than 10 days; grade 3 or 4 neutropenia with fever greater than 38 °C; thrombocytopenia or grade 3 or 4 thrombocytopenia associated with serious bleeding; signs of serious bleeding; grade 3 or higher non-hematological toxicity; grade 3 or higher diarrhea if refractory to maximal anti-diarrheal therapy; grade 3 skin toxicity for more than 2 weeks with maximum supportive treatment; grade 4 skin toxicity (with subsequent removal from the study); missing more than 14 days of consecutive treatment; and increase in aspartate aminotransferase or alanine aminotransferase from grade 1 to grades 2–4 or from grade 2 (in patients with liver metastases) to grade 3 or 4 in the case of a second occurrence after a first recovery to baseline level taking more than 14 days, or a third occurrence.
In phase II, treatment with refametinib at the recommended phase II dose, plus standard gemcitabine 1000 mg/m2 weekly, began on cycle 1, day 1. Treatment continued until progressive disease, unacceptable toxicity, or other discontinuation criteria were met, as follows: initiation of a new anticancer regimen; development of a second malignancy; deterioration of Eastern Cooperative Oncology Group performance status to 4 or more; increased aspartate aminotransferase or alanine transaminase from grade 1 to grades 2–4 or from grade 2 (in patients with liver metastases) to grade 3 or 4 in the case of a second occurrence after a first recovery to baseline level taking more than 14 days, or a third occurrence; at the patient’s request; if continuation would be harmful to the patient’s health (as determined by the investigator); substantial non-compliance with study requirements; development of any intercurrent illness that may affect clinical status assessment or study endpoints; positive serum pregnancy test; use of illicit drugs or other substances that may contribute to toxicity; interruption in study drug administration because of drug-related toxicities for more than 22 days and/or delay of more than 22 days for gemcitabine; or if more dose reductions were required than allowed according to protocol.
2.3 Eligibility
Patients were eligible if aged 18 years or older and with histologically or cytologically confirmed, locally advanced or metastatic pancreatic adenocarcinoma not amenable to curative surgery or radiotherapy. Other eligibility criteria included life expectancy of 12 weeks or more; at least one unidimensional lesion measurable by computed tomography or magnetic resonance imaging (Response Evaluation Criteria in Solid Tumors version 1.1); resolution of all acute toxic effects of any prior local treatment to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 grade 1 or 0; Eastern Cooperative Oncology Group performance status of 2 or under; adequate laboratory criteria (platelet count ≥100 × 109/L; hemoglobin ≥10 g/dL; absolute neutrophil count ≥1.5 × 109/L; total bilirubin ≤1.5 mg/dL; alanine aminotransferase and aspartate aminotransferase each ≤2.5 × upper limit of normal [ULN; or ≤5 × ULN in case of liver metastases]; amylase and lipase ≤1.5 × ULN; serum creatinine ≤1.5 × ULN; prothrombin time or international normalized ratio and partial thromboplastin time ≤1.5 × ULN); and normal cardiac function as estimated by echocardiography.
2.4 Assessments
Screening included demographics and baseline characteristics, echocardiography, ophthalmic examination, plasma and tumor biopsy for genotyping and biomarker analysis, and tumor evaluation (blinded) by computed tomography or magnetic resonance imaging (Response Evaluation Criteria in Solid Tumors version 1.1). In phase I, serial blood samples for pharmacokinetic analysis of gemcitabine and refametinib and the respective inactive metabolites difluorodeoxyuridine and M-17 were collected up to 24 h post-infusion at cycle 1, days 1 and 22, and up to 8 hours post-dose at cycle 1, days 21 and 22. Blinded tumor assessments were performed independently every 8 weeks during treatment until progressive disease or the end of treatment. Confirmatory scans were performed 4 or more weeks after an objective tumor response (complete response or partial response) was documented. Investigator assessment of tumor scans was performed for all patients who received treatment. Safety (changes in laboratory values, vital signs, electrocardiogram, and physical examination) was assessed at screening, at cycle 1, day 1, and weekly thereafter. Adverse events and concomitant medications were assessed continuously from screening onwards. Following the end of treatment, patients entered a 30-day safety follow-up including adverse-event documentation. Survival follow-up was performed monthly up to 8 months after the first treatment of the last patient.
2.5 Pharmacokinetic Assessments
Plasma concentrations of all analytes were measured using fully validated high-pressure liquid chromatography with mass spectrometric detection, and pharmacokinetic parameters were calculated by non-compartmental analysis using WinNonlin® (Version 4.1; Pharsight Corporation, Princeton, NJ, USA).
2.6 Biomarker Studies
Circulating tumor DNA in plasma was analyzed for KRAS mutational status by beads, emulsions, amplification, and magnetics (BEAMing) technology (Covance Inc., Princeton, NJ, USA), with an assay cutoff of 0.02% mutant allele for positivity. Circulating microRNA from plasma was analyzed by quantitative polymerase chain reaction using a human microRNA panel (Exiqon, Woburn, MA, USA). Tumor biopsies were collected where available, as freshly frozen or formalin-fixed and paraffin-embedded. Histological analysis comprised hematoxylin and eosin staining and Ki67 immunolabeling. Targeted archival tumor gene next-generation sequencing was performed using the FoundationOne® panel (Foundation Medicine, Cambridge, MA, USA). Gene expression was analyzed using RNA isolated from tumor biopsy samples using the Ovation® formalin-fixed and paraffin-embedded circulating DNA synthesis kit (NuGen, San Carlos, CA, USA), and RNA sequencing was performed using an Ion Proton™ system (Life Technologies, Grand Island, NY, USA). Reads were mapped to hg19 using TopHat2 [20] with Bowtie2 [21]. Gene-level read counts and reads per kilobase of transcript per million values were calculated with Expressionist® Refiner Genome (Genedata, Lexington, MA, USA).
2.7 Statistical Analysis
Phase I data were analyzed descriptively. The primary efficacy endpoint in phase II tested the null hypothesis that the overall response rate would be 7% or less on the α level of 12.5% using a one-sided binomial test; assuming a true overall response rate of 17% under study treatment, exactly 60 patients treated at the recommended phase II dose were required to be analyzed for efficacy for a power of 90% (primary analysis set). The null hypothesis was to be rejected if seven or more patients in the primary analysis set experienced confirmed complete response or confirmed partial response. Other secondary efficacy endpoints in phase II were analyzed descriptively; corresponding p values are not confirmatory. Descriptive statistics and frequency tables were used for safety analysis for all patients who received at least one dose of study treatment (safety analysis set).
3 Results
3.1 Baseline Demographics and Disease Characteristics
Of the 24 patients enrolled in phase I, 20 were treated and evaluable for safety assessment (Fig. 1a). Ten patients were assigned to dose level 1 (refametinib 30 mg twice daily) and 10 to dose level 2 (refametinib 50 mg twice daily). In phase II, 107 patients were enrolled and screened; 80 were treated and evaluable for safety assessment (Fig. 1b), of whom 10 were originally enrolled at dose level 2 in phase I and are therefore accounted for twice. Overall, 55.6% of patients were male and the median age was 63 years (Table 1). Most patients (85.6%) had metastatic disease.
Patient disposition and flow in phase I (a) and phase II (b). aFour patients were replaced because of non-dose-limiting toxicity events before the end of the first cycle. bIncludes 10 patients from the 50 mg/kg twice daily dose level in phase I. cProtocol-defined reasons refer to the statistical requirement of exactly 60 treated patients to be centrally evaluated for the primary efficacy endpoint; patients excluded from the primary efficacy analysis were evaluated for response by investigator assessment. AE adverse event, BID twice daily other n=2
In phase II, of the 60 patients centrally evaluated for response (primary analysis set; Fig. 1b), 39 (65%) had KRAS mutations, as determined from circulating tumor DNA. Frequently observed KRAS mutations included G12D, G12V, and G12R; mutations in codon 38 or 436 were not observed. Molecular tumor characterization was performed in 23 out of 30 archival samples (77%) with sufficient tumor content. Tumor exome sequencing revealed KRAS mutations (G12D, G12R, G12V, Q61H, Q61R, A59G) in 15 out of 16 patient samples containing sufficient DNA (Fig. 2). Frequent co-occurring somatic mutations or amplifications in patients with KRAS mutations included TP53 (14 out of 15 [93%]), CDKN2A (5 out of 15 [33%]), C-MYC (4 out of 15 [27%]), and KAT6A (2 out of 15 [13%]). One patient with stable disease and low Ki67 H-score had two co-existing KRAS mutations (A59G, Q61R). Discordance was observed in KRAS mutational status, as determined by BEAMing technology, in three samples.
Nineteen samples with sufficient tumor content had sufficient RNA for analysis of gene expression. Messenger RNA expression data for all genes and for genes with published KRAS pathway signatures [22] were tested for correlation with response to treatment; visual inspection of principal component analysis and hierarchical clustering results showed no obvious correlation (data not shown; no statistical analysis was performed because of the small sample number). Correlation between copy number alteration and messenger RNA expression level was investigated for genes with copy number alteration in more than one patient, and expression of C-MYC and KAT6A correlated with gene amplification (Online Resource 1).
MicroRNA expression data were generated from baseline plasma samples. No individual difference in microRNA was observed for KRAS mutational status, response to treatment, or treatment (data not shown). An association between expression level and KRAS mutational status was observed for miR-96-5p, miR-214-3p, and miR-877 (Online Resource 2). The false discovery rate for each analysis was 0.33.
3.2 Exposure and Safety
In phase I, the mean daily dose of refametinib was 56.8 mg in the 30 mg cohort (range 40.7–59.8) and 91 mg in the 50 mg cohort (range 66.8–100). Mean refametinib treatment duration was 23.5 weeks (range 4.6–54) in the 30-mg cohort and 11.3 weeks (range: 2–32.3) in the 50-mg cohort. During phase I, treatment was tolerated at dose level 2 (refametinib 50 mg twice daily plus standard gemcitabine), which was declared the maximum tolerated dose and recommended phase II dose. During phase I, one patient in the 30-mg cohort experienced grade 3 deterioration of general status (dose-limiting toxicity), which led to dose interruption, remained unresolved, and was considered unrelated to treatment. This patient subsequently experienced grade 5 steatohepatitis which was deemed treatment-related (gemcitabine); a relationship to refametinib could not be excluded. One patient in the 50-mg cohort experienced grade 3 pneumonitis (dose-limiting toxicity), considered treatment-related, leading to dose interruption and study withdrawal. In phase I, four patients from each dose level were not evaluable for dose-limiting toxicities because they had not reached the end of one cycle of treatment or had received too low a dose of treatment.
In phase II, the mean daily dose of refametinib was 88 mg overall (range 52.7–100; relative dose intensity 88%); 66% of patients (53 out of 80) received an average dose of 81–100 mg daily. Mean refametinib treatment duration, excluding interruptions, was 14.7 weeks (range 0.9–51.3). The mean weekly gemcitabine dose was 895.6 mg/m2 (range 500–1000; relative dose intensity 90%); 95% of patients (76 out of 80) received 751–1000 mg/m2 per week. The mean gemcitabine treatment duration, excluding interruptions, was 11.6 weeks (range 1–37).
The main reasons for study discontinuation in phase II were adverse events not associated with progressive disease (39%) or radiological progression (33%) (Online Resource 3). All patients experienced at least one treatment-emergent adverse event; most experienced at least one grade 3 (49%) or grade 4 (23%) treatment-emergent adverse event. The most common grade 3 or 4 treatment-emergent adverse event was neutropenia (39%; 14% grade 4). Overall, 66% of patients experienced at least one serious adverse event, considered refametinib-related in 24% of patients and gemcitabine-related in 26% of patients. No grade 5 adverse events were considered refametinib-related, although one patient (1.3%) had a grade 5 adverse event considered gemcitabine-related. Frequent treatment-emergent adverse events, occurring in 20% or more of patients, are shown in Table 2. In phase II, five patients had pneumonitis (two each at grades 2 and 3, respectively, and one at grade 4), in addition to two patients in phase I (one at grade 2 and one at grade 3 [dose-limiting toxicity]).
3.3 Pharmacokinetics
In phase I, following multiple-dose oral administration, refametinib was well absorbed at both dose levels (30 mg twice daily and 50 mg twice daily), with comparable exposure without (cycle 1, day 21) and with (cycle 1, day 22) gemcitabine (Online Resource 4A). Refametinib and metabolite M-17 pharmacokinetic parameters were generally comparable with historical data in patients with other cancer types [23] (Online Resource 5). Gemcitabine exposure was comparable when administered without (cycle 1, day 1) and with (cycle 1, day 22) refametinib (Online Resource 4B). The pharmacokinetic parameters of gemcitabine and metabolite difluorodeoxyuridine are shown in Online Resource 6.
3.4 Efficacy
Of the 60 patients evaluated for response by independent radiological review, none had confirmed complete responses and 14 (23%) had confirmed partial responses, giving an objective response rate of 23%; the disease control rate was 73% (Table 3). The null hypothesis of objective response rate of 7% or less could thus be rejected. Seven patients had unconfirmed partial responses (12%) and no patients had unconfirmed complete responses.
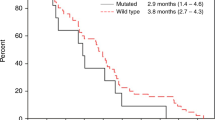
Median progression-free survival was 6.3 months and median overall survival was 8.9 months (Fig. 3).
There were 10 (13%) confirmed partial responses and no confirmed complete responses among all 80 investigator-assessed patients, giving an objective response rate of 13%. Another 10 patients (13%) had unconfirmed partial responses and 35 patients (44%) had stable disease; the disease control rate was 69%. No patients had an unconfirmed complete response. For the 80 investigator-assessed patients, median progression-free survival was 5.4 months (range 0–18.2; 95% confidence interval 4.0–7.1) and median overall survival was 8.4 months (range 0.5–18.2; 95% confidence interval 6.4–11.6).
3.5 Response by KRAS Mutational Status
Of the 60 patients evaluated for response by independent radiological review and for KRAS mutational status in circulating tumor DNA, KRAS mutations were detected in 39 patients (65%). Of these patients, 11 (28%) had partial responses (including unconfirmed partial responses) and 16 (41%) had stable disease; the disease control rate was 69% (27 out of 39). For patients without detectable KRAS mutations, 10 (48%) had partial responses (including unconfirmed partial responses) and seven (33%) had stable disease; the disease control rate was 81% (17 out of 21). KRAS wild-type allele frequency tended to correlate with better tumor response (Fig. 4).
A greater proportion of patients without detectable KRAS mutations (11 out of 20 [55%]) showed best change in target lesion size of 30% or more compared with patients with detectable KRAS mutations (13 out of 31 [43%]; blinded assessment) (Fig. 5a).
Change from baseline in target lesion size by KRAS mutational status (a), median PFS in KRAS subgroups (b), and median OS in KRAS subgroups (c) (primary analysis set). Nine out of the 60 patients in the primary analysis set were not evaluated for change in target lesion size, of whom two experienced protocol deviations. Six patients were not evaluable for change in carbohydrate antigen 19-9 from baseline. CI confidence interval, HR hazard ratio, NE not evaluable, OS overall survival, PFS progression-free survival
Median progression-free survival was 5.3 months and 8.8 months (Fig. 5b), and median overall survival was 6.6 months and 18.2 months (Fig. 5c), for patients with and without detectable KRAS mutations, respectively.
Of the 54 patients in the primary analysis set evaluable for change in serum level of carbohydrate antigen 19-9 from baseline, 29 showed a 50% or higher decrease (Online Resource 7), which did not appear to be associated with KRAS status. However, wild-type KRAS was associated with lower serum carbohydrate antigen 19-9 at baseline (p = 0.0236) and at cycle 1, day 29 (p = 0.0154) (Online Resource 8).
4 Discussion
This phase I/II study determined the maximum tolerated dose of refametinib plus gemcitabine and evaluated the efficacy and tolerability of the combination in patients with unresectable, locally advanced or metastatic pancreatic cancer, for whom gemcitabine-based therapy is indicated as first-line treatment.
The maximum tolerated dose in phase I was identified to be refametinib 50 mg twice daily plus standard gemcitabine, consistent with historical refametinib monotherapy data [23]. The combination appeared generally feasible, with the most frequent adverse events being grade 1 or 2. However, the incidence of grade 3 or 4 neutropenia in phase II (39%) was higher than in previous reports of gemcitabine in this patient population [9, 12, 14, 16]. In total, seven patients developed pneumonitis, a known toxicity of gemcitabine [24], although it remains possible that adding a MEK inhibitor may increase the incidence of pneumonitis, as seen in the phase II study of trametinib and gemcitabine (7 out of 80 cases vs. 2 out of 80 cases in the gemcitabine group) [25].
The primary efficacy endpoint in phase II was reached, with an objective response rate of 23% for the refametinib plus gemcitabine combination, which is more than twice as high as historical reports of gemcitabine monotherapy (range 5.4–10.5%) [11, 13, 26]. The overall disease control rate was consistent with historical reports of gemcitabine monotherapy (73% vs. 29.8–47.2%) [11, 13, 26]. Baseline demographics and disease characteristics were broadly similar to those seen in previous trials [13, 26]. The inclusion of patients with locally advanced, unresectable disease as well as those with metastatic disease remains representative of patients with advanced pancreatic cancer, and the evaluation of response rate in these patients was based on patients having at least one measurable lesion, consistent with recent trials [27–29].
Response, progression-free survival, and overall survival were similar to those reported for albumin-bound paclitaxel plus gemcitabine [10]. Partial response and overall survival were slightly lower than reported for the oxaliplatin, irinotecan, fluorouracil, and leucovorin regimen, although progression-free survival was similar [9]. Objective response rate and overall survival were also similar to those recently reported for the combination of trametinib and gemcitabine (objective response rate 22%; overall survival 8.4 months), along with the proportion of patients with detectable KRAS mutations (72%) [25]. In the trametinib and gemcitabine study, overall survival was greater with trametinib and gemcitabine than with gemcitabine and placebo in patients with mutant KRAS (n = 103; 8.3 vs. 6.7 months, respectively) and those with wild-type KRAS (n = 40; 8.6 vs. 5.9 months, respectively). In our study, median overall survival was also greater in patients without detectable KRAS mutations (18 vs. 6.6 months, respectively), as were median progression-free survival and objective response rate (8.8 vs. 5.3 months and 48% vs. 28%, respectively).
The proportion of patients with detectable KRAS mutations as determined from circulating tumor DNA was similar to that in a previous study in pancreatic cancer (62.6%) [30]. In the latter study, overall survival was greater in patients with wild-type versus mutant KRAS (413 vs. 276 days, respectively), suggesting a negative prognostic role for KRAS mutations detected in circulating tumor DNA.
Nevertheless, the predictive or prognostic role of KRAS following first-line gemcitabine-based therapy in pancreatic cancer remains unclear. Retrospective analysis of first-line gemcitabine-based therapy revealed a lower objective response rate in patients with mutant KRAS compared with wild-type KRAS (11% vs. 26%, respectively) [31]. Subgroup analysis revealed longer overall survival with gemcitabine and erlotinib in patients with wild-type KRAS (9.7 vs. 5.2 months), with no overall survival difference between KRAS mutational subgroups treated with other gemcitabine-based regimens (7.0 vs. 7.0 months) [31]. Conversely, subgroup analysis of a phase III study [14] reported similar overall survival in patients treated with gemcitabine and erlotinib irrespective of KRAS mutational status (6.1 vs. 6.0 months in wild type and mutant, respectively), while the mutant KRAS subgroup appeared to have greater benefit from gemcitabine monotherapy compared with the wild-type subgroup (7.4 vs. 4.5 months, respectively) [32].
Results from serum carbohydrate antigen 19-9 levels in both patient subsets were ambiguous and do not allow for firm conclusions. A negative impact of KRAS mutations and high serum carbohydrate antigen 19-9 levels on overall survival has been reported [33].
C-MYC amplification was prevalent in mutant KRAS tumors, consistent with previous observations, suggesting C-MYC pathway activation in these patients [34]. These data suggest that targeting C-MYC pathways may provide an alternative therapeutic strategy in the treatment of pancreatic adenocarcinoma [35].
KRAS mutational status also tended to correlate with miR-96-5 and miR-214-3 expression, roles for which have been described as a tumor suppressor in pancreatic adenocarcinoma [36] and in the regulation of growth and invasion of stem-like cells in a hepatocellular carcinoma model [37], respectively. However, the significance level must be interpreted with caution because of the sample size analyzed (800 microRNA species), the high false discovery rate, and the lack of corrections for multiple comparisons. Although preliminary, these data may support a role for circulating microRNAs as biomarkers of disease aggressiveness, warranting further investigation.
Although concordance between the mutational status in tumor specimens and circulating tumor DNA is generally very high, discordance between the mutational status in tumor and circulating tumor DNA from fresh plasma may occur and deserves further investigation [25, 38, 39]. Discordance was observed here in three samples between KRAS mutational status as determined by exome sequencing of tumor biopsies and BEAMing technology of fresh plasma. Although BEAMing technology is highly sensitive [40], sensitivity was not formally tested and false negatives could not be conclusively excluded in this small sample.
Refametinib is metabolized by liver enzymes CYP3A4 and CYP2C19, and is a substrate for glucuronidation by UGT2B7. Gemcitabine is metabolized by cytidine deaminase and is primarily eliminated in urine along with its metabolite difluorodeoxyuridine. As expected from these distinct metabolic and elimination pathways, no pharmacokinetic interactions were observed; refametinib and gemcitabine exposures were comparable when administered alone or in combination.
Overall, refametinib combined with gemcitabine is well tolerated in 8-weekly cycles up to the maximum tolerated dose, with no pharmacokinetic interaction. The primary endpoint of phase II was met: the combination showed a relatively high objective response rate in patients with advanced pancreatic cancer, with an acceptable safety profile. There was a trend towards improved survival in patients without detectable KRAS mutations compared with those with detectable KRAS mutations in circulating tumor DNA. This study also suggests that biomarker status in patients with KRAS mutations may provide predictive or prognostic information with regard to clinical benefit from refametinib plus gemcitabine.
References
Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 http://globocan.iarc.fr (2013). Accessed 5 Sept 2016.
Morris JP, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10:683–95.
Kanda M, Matthaei H, Wu J, et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730–3.
Eser S, Schnieke A, Schneider G, et al. Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer. 2014;111:817–22.
Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310.
Frémin C, Meloche S. From basic research to clinical development of MEK1/2 inhibitors for cancer therapy. J Hematol Oncol. 2010;3:8.
Friday BB, Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res. 2008;14:342–6.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma (Version 2.2014). http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (2014). Accessed 5 Sept 2016.
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25.
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703.
Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13.
Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513–8.
Nakai Y, Isayama H, Sasaki T, et al. A multicentre randomised phase II trial of gemcitabine alone vs gemcitabine and S-1 combination therapy in advanced pancreatic cancer: GEMSAP study. Br J Cancer. 2012;106:1934–9.
Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6.
Goncalves A, Gilabert M, François E, et al. BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol. 2012;23:2799–805.
Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212–7.
Iverson C, Larson G, Lai C, et al. RDEA119/BAY 869766: a potent, selective, allosteric inhibitor of MEK1/2 for the treatment of cancer. Cancer Res. 2009;69:6839–47.
Puehler F, Scholz A, Kissel M, et al. Allosteric MEK inhibitor BAY 86-9766 (RDEA119) shows anti-tumor efficacy in monotherapy and combination therapy in preclinical models of hepatocellular carcinoma and pancreatic cancer. Poster 151 presented at 22nd EORTC-NCI-AACR Symposium, Berlin, Germany, 16–19 November 2010.
Schmieder R, Puehler F, Neuhaus R, et al. The MEK inhibitor BAY 869766 inhibits tumor growth and metastatic spread, prolongs the survival and acts synergistically with standard of care drugs in models of hepatocellular carcinoma and pancreatic cancer. Mol Cancer Ther. 2011;10 (Suppl 1): (Abstract B247).
Kim D, Pertea G, Trapnell C, et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–9.
Loboda A, Nebozhyn M, Klinghoffer R, et al. A gene expression signature of RAS pathway dependence predicts response to PI3K and RAS pathway inhibitors and expands the population of RAS pathway activated tumors. BMC Med Genomics. 2010;3:26.
Weekes CD, Von Hoff DD, Adjei AA, et al. Multicenter phase I trial of the mitogen-activated protein kinase 1/2 inhibitor BAY 86-9766 in patients with advanced cancer. Clin Cancer Res. 2013;19:1232–43.
Barlési F, Villani P, Doddoli C, et al. Gemcitabine-induced severe pulmonary toxicity. Fundam Clin Pharmacol. 2004;18:85–91.
Infante JR, Somer BG, Park JO, et al. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer. 2014;50:2072–81.
Rothenberg ML, Moore MJ, Cripps MC, et al. A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer. Ann Oncol. 1996;7:347–53.
Borad MJ, Reddy SG, Bahary N, et al. Randomized phase II trial of gemcitabine plus TH-302 versus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2015;33:1475–81.
Stein SM, James ES, Deng Y, et al. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer. 2016;114:737–43.
van Zweeden AA, van der Vliet HJ, Wilmink JW, et al. Phase I clinical trial to determine the feasibility and maximum tolerated dose of panitumumab to standard gemcitabine-based chemoradiation in locally advanced pancreatic cancer. Clin Cancer Res. 2015;21:4569–75.
Kinugasa H, Nouso K, Miyahara K, et al. Detection of K-ras gene mutation by liquid biopsy in patients with pancreatic cancer. Cancer. 2015;121:2271–80.
Kim ST, Lim DH, Jang KT, et al. Impact of KRAS mutations on clinical outcomes in pancreatic cancer patients treated with first-line gemcitabine-based chemotherapy. Mol Cancer Ther. 2011;10:1993–9.
da Cunha Santos G, Dhani N, Tu D, et al. Molecular predictors of outcome in a phase 3 study of gemcitabine and erlotinib therapy in patients with advanced pancreatic cancer. National Cancer Institute of Canada Clinical Trials Group Study PA.3. Cancer. 2010;116:5599–607.
Ogura T, Yamao K, Hara K, et al. Prognostic value of K-ras mutation status and subtypes in endoscopic ultrasound-guided fine-needle aspiration specimens from patients with unresectable pancreatic cancer. J Gastroenterol. 2013;48:640–6.
Birnbaum DJ, Adélaïde J, Mamessier E, et al. Genome profiling of pancreatic adenocarcinoma. Genes Chromosomes Cancer. 2011;50:456–65.
Lin Q, Aihara A, Chung W, et al. LRH1 promotes pancreatic cancer metastasis. Cancer Lett. 2014;350:15–24.
Yu S, Lu Z, Liu C, et al. miRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Cancer Res. 2010;70:6015–25.
Xia H, Ooi LL, Hui KM. MiR-214 targets β-catenin pathway to suppress invasion, stem-like traits and recurrence of human hepatocellular carcinoma. PLoS One. 2012;7:e44206.
Higgins MJ, Jelovac D, Barnathan E, et al. Detection of tumor PIK3CA status in metastatic breast cancer using peripheral blood. Clin Cancer Res. 2012;18:3462–9.
Ignatiadis M, Lee M, Jeffrey SS. Circulating tumor cells and circulating tumor DNA: challenges and opportunities on the path to clinical utility. Clin Cancer Res. 2015;21:4786–800.
Li M, Diehl F, Dressman D, et al. BEAMing up for detection and quantification of rare sequence variants. Nat Methods. 2006;3:95–7.
Acknowledgments
The authors would like to thank Tanja Torbica, PhD, at Complete HealthVizion for assistance in the preparation and revision of the draft manuscript, based on detailed discussion and feedback from all the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by Bayer HealthCare Pharmaceuticals, Inc. John Bridgewater is partly supported by the UCLH/UCL Biomedical Research Centre.
Conflict of Interest
HR has performed a consulting or advisory role for Boehringer Ingelheim, Bayer, GlaxoSmithKline, Bristol-Myers Squibb, Roche, and Sanofi-Aventis. JJ has performed a consulting or advisory role for Eli Lilly, Pfizer, AstraZeneca, and Celgene; participated in a speaker bureau for Roche; received research funding from GlaxoSmithKline, Roche, and Novartis; and received travel, accommodation, and other expenses from Boehringer Ingelheim. MH has received honoraria from Celgene, research funding from Roche and Boehringer Ingelheim, and travel, accommodation, and other expenses from Celgene and Boehringer Ingelheim. MP has received honoraria and research funding from Bayer. PR has received honoraria from Roche, Sirtex, Bayer, and Celgene; performed a consulting or advisory role for Roche, Sirtex, Sanofi-Aventis, Celgene, Baxter, Merck Serono, and Daiichi Sankyo; received research funding from Sanofi-Aventis, Bayer, Roche, and Merrimack; and received travel, accommodation, and other expenses from Roche, Merck Serono, Celgene, and Sanofi-Aventis. JB is partly supported by the UCLH/UCL Biomedical Research Centre. He has performed a consulting or advisory role for Roche, Merck Serono, and AstraZeneca, and has received travel, accommodation, and other expenses from Merck Serono and the European Society for Medical Oncology. BM has received honoraria from, and has performed a consulting or advisory role for, Bayer. SC has performed a consulting or advisory role for Roche, Sanofi-Aventis, and Celgene. PM has received honoraria from Ipsen, Bayer, and Celgene, and has received research funding from Ipsen. DVB has received travel, accommodation, and other expenses from Amgen. MG, KR, AS, and HS are employees of Bayer Pharma AG. VLG is an employee of Bayer S.p.A. PRa is an employee of Bayer HealthCare Pharmaceuticals, Inc. and has stock or other ownership interests in Bayer HealthCare Pharmaceuticals, Inc. MT and BHC are employees of Bayer HealthCare Pharmaceuticals, Inc. J-LVL, UMM, CW, PS, AZ, WS, and SD have no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 553 kb)
Rights and permissions
About this article
Cite this article
Van Laethem, JL., Riess, H., Jassem, J. et al. Phase I/II Study of Refametinib (BAY 86-9766) in Combination with Gemcitabine in Advanced Pancreatic cancer. Targ Oncol 12, 97–109 (2017). https://doi.org/10.1007/s11523-016-0469-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-016-0469-y