Abstract
The evaluation of response to treatment is a critical step for determining the effectiveness of oncology drugs. Targeted therapies such as tyrosine kinase inhibitors and mammalian target of rapamycin inhibitors are active drugs in patients with metastatic renal cell carcinoma (mRCC). However, treatment with this type of drugs may not result in significant reductions in tumor size, so standard evaluation criteria based on tumor size, such as Response Evaluation Criteria in Solid Tumors (RECIST), may be inappropriate for evaluating response to treatment in patients with mRCC. In fact, targeted therapies apparently yield low response rates that do not reflect increased disease control they may cause and, consequently, the benefit in terms of time to progression. To improve the clinical and radiological evaluation of response to treatment in patients with mRCC treated with targeted drugs, a group of 32 experts in this field have reviewed different aspects related to this issue and have put together a series of recommendations with the intention of providing guidance to clinicians on this matter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over 64,000 new renal cell carcinomas (RCCs) are annually detected in the USA, and 13,000 people will die from the disease. Most RCCs are discovered incidentally on medical imaging, and a great percentage of them may be treated by surgery, but one third of patients will present either with locally advanced tumor or with metastases [1]. In addition, another third of patients may develop metastatic disease after initial treatment.
Traditionally, RCC have been remarkably resistant to chemotherapy and radiotherapy. Over the last years, there have been important advances about pathophysiological processes in RCC: von Hippel–Lindau (VHL) gene mutations or methylations, angiogenesis alterations, evasion of apoptosis, or sustained angiogenesis. These advances have enabled the emergence of new drugs designed to target and interfere with specific aberrant biological pathways.
Four main histological subtypes of RCC have been described: clear cell (75 %), papillary (15 %), chromophobe (5 %), and oncocytoma (5 %). These histological subtypes also have implications for prognosis and treatment response. Clear cell RCC shows a worse prognosis than papillary or chromophobe tumors and responds very well to antiangiogenic agents, while response of papillary RCC to these agents is limited. Antivascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) agents are effective mainly in clear cell RCC because VEGF is elevated in the majority of clear cell tumors as a result of inactivation of the VHL gene leading to overexpression of the hypoxia-inducible factor (HIF), which induces the expression of a number of angiogenesis-related factors.
The protein transcript of the VHL gene (pVHL) plays a central role in the pathogenesis of clear cell RCC. In a normoxic state, pVHL allows degradation of HIF. HIF-α is responsible for inducing expression of genes, which produce various ligands of membrane receptors associated with angiogenesis and proliferation, such as VEGF, the platelet-derived growth factor (PDGF), and the tumor growth factor (TGF-α). While HIF is mostly active in hypoxic conditions, VHL-defective RCC shows constitutive activation of HIF even in oxygenated environments. pVHL complex inactivation by mutation or loss of expression of the VHL gene causes intracellular accumulation of HIF-α that stimulates the transcription of genes regulating VEGF, PDGF, and TGF-α [2]. However, within the three variants of HIF, HIF-2α is a key feature in the pathogenesis of RCC, with HIF1α and HIF3α playing only minor roles [3].
Besides, a major stimulus of cancer angiogenesis is tissue hypoxia via HIF. The mammalian target of rapamycin (mTOR) pathway enhances the translation of HIF1α messenger ribonucleic acid, thereby increasing the overall angiogenic and vasculogenic effect although, as mentioned previously, HIF1α playing only a secondary role in the majority of RCC.
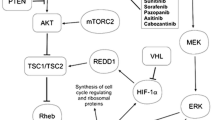
Consequently, receptors for VEGF, PDGF, and mTOR pathway are rational targets in the treatment of RCC [4–8]. The administration of targeted therapies, such as bevacizumab, tyrosine kinase inhibitors (TKIs), and mTOR inhibitors, is associated with significant clinical benefits in patients with metastatic RCC (mRCC) (Fig. 1).
Relationship between transduction pathways, tumor hallmarks, and specific biologic pathways targeted by therapies in renal cancer. Akt is a serine-threonine protein kinase, cKIT is a proto-oncogene. EGFR epidermal growth factor receptor, HER2 human epidermal growth factor receptor 2, HIF hypoxia-inducible factor, mTOR mammalian target of rapamycin, PDGF platelet-derived growth factor, PI3K phosphatidylinositol 3-kinase, TKIs tyrosine kinase inhibitors, VEGF vascular endothelial growth factor, VEGF/PDGF receptors vascular endothelial growth factor and platelet-derived growth factor receptors
Antiangiogenic-targeted therapies are significantly active in patients with mRCC. Overproduction of VEGF is a key feature in this type of tumors, which results in the activation of angiogenesis that explains the hypervascular nature of RCC. Since 2006, new options for the treatment of mRCC have included agents targeting tumor angiogenesis or pathways mediating growth and proliferation. Among these agents are TKIs, such as sunitinib, pazopanib, sorafenib, axitinib, or tivozanib. They have a broad range of targets, including VEGF and PDGF receptors, with some differences in affinity and bioavailability between them. Although TKIs can lead to shrinkage of the tumor, in most patients, a stabilization of the disease is only obtained, according to the Response Evaluation Criteria in Solid Tumors (RECIST). However, all these agents have showed some clinical benefit in phase III clinical trials, mainly in progression free survival (PFS) [9]. Different drugs specifically target VEGF and VEGFR. Bevacizumab is an antibody that exclusively targets VEGF, inhibiting the interactions of this ligand with all of the receptors to which it binds. Several phase III trials have demonstrated significant clinical benefit with the combination of bevacizumab plus interferon (IFN) with respect to IFN as monotherapy in terms of overall response rate and PFS but not in overall survival (OS), probably due to cross-over or to the addition of other targeted therapies [10, 11]. In addition, ramucirumab is an antiangiogenic agent that inhibits VEGFR2, an important receptor involved in tumor angiogenesis [12].
Regarding to mTOR inhibitors, two drugs are used nowadays in the management of mRCC: temsirolimus and everolimus. Temsirolimus is an intravenous mTOR inhibitor that has showed a benefit in OS in first-line mRCC, in patients in the poor prognosis group according to Motzer criteria [13]. Everolimus is another mTOR inhibitor that has demonstrated superiority over placebo in mRCC patients progressing after at least one previous TKI [14]. In spite of treatment efficacy in patients treated with targeted therapies in most of cases, there may not be significant reductions in tumor size. For this reason, standard evaluation criteria based on tumor size, such as RECIST, may not be the best for evaluating the effectiveness of targeted drugs in patients with mRCC because they suggest apparently low response rates that do not reflect increased disease control they may cause and, consequently, the benefit in terms of time to progression (TTP) [15].
In general, RECIST 1.1 provides a standardized and practical method to assess response to treatment and to define disease progression in patients with solid tumors. However, other criteria have been published with the aim to improve response assessment in patients with renal cancer (Table 1). In this regard, there is a change from a pure anatomical evaluation of tumor burden, i.e., assessments based on changes in the size of the tumor, to a qualitative evaluation based on contrast enhancement of target lesions [16]. Antiangiogenic agents often cause decreased tumor vascularity and necrosis, leading to a reduction in the attenuation and enhancement of the lesions, which should be determined by the implementation of these novel criteria. Additionally, functional imaging techniques seem to be sensitive methods for detecting clinically relevant antiangiogenic drug-induced modifications in mRCC. Among them, dynamic contrast enhanced (DCE) imaging techniques, such as perfusion computed tomography (CT), DCE-magnetic resonance imaging (DCE-MRI), and DCE-ultrasound (DCE-US) have been demonstrated to more accurately predict response to TKIs in patients with mRCC [17–22] (Table 2). Lastly, clinicians must bear in mind that patients with mRCC treated with targeted therapies may achieve clinical benefits regardless the achievement of response according to radiological criteria, i.e., RECIST. All these features emphasize the need of additional approaches for the evaluation of response to treatment in patients with mRCC undergoing treatment with targeted therapies.
The EVALUATION project was initiated by two main coordinators (one medical oncologist and one radiologist) who suggested to a group of six national experts in the field to issue a series of recommendations for optimizing the clinical and radiological evaluation of response in patients with mRCC treated with targeted drugs. These experts were involved in the field of RCC for more than 5 years and treated at least 15 new patients per year. A questionnaire consisting of nine questions, divided into four sections, was prepared in a national face-to-face working meeting that took place in May 2012. Subsequently, in four regional face-to-face working meetings that took place in September 2012, the questionnaire was evaluated by a group of eight different experts and answered by consensus. Each regional meeting was coordinated by 2 national experts or coordinators, and involved 6 additional regional experts, making a total of 32 specialists who participated. The contribution by radiologists and medical oncologists was equal at every step of the project. Moreover, radiologists and medical oncologists were generally matched in pairs as they were working at the same hospital and were in fact the responsible physicians for diagnosing and treating patients with mRCC at the same hospital. Once the answers from each regional meeting were collected by national experts, they met again in October 2012 along with project coordinators to construct the final consensus for each of the original nine questions and to provide the most relevant bibliography that supported their agreed statements.
This article is divided into four different sections, and its purpose is to provide the best recommendations from currently available scientific evidence on the nine questions initially formulated.
Evaluation criteria for treatment response in mRCC
Should the response to treatment of targeted drugs be assessed in a way that is different from classical cytotoxic agents?
In 1981, on the initiative of the World Health Organization (WHO), Miller et al. [23] proposed uniform criteria for reporting response, recurrence, and disease-free interval, as well as the grading of acute and subacute toxicity, in the treatment of cancer. The WHO criteria were widely used until 2000, when RECIST were first published and adopted for the assessment of treatment response in patients with cancer. In 2009, a new version of RECIST, namely, RECIST 1.1, was published with the aim of resolving certain questions and issues raised following the use of the first version [24].
Nowadays, radiologists and oncologists agree that RECIST 1.1 are the standard criteria for the radiological evaluation of response in patients treated with classical chemotherapy agents. This has been supported by both clinical practice and clinical research. As a general rule, tumor response to treatment according to RECIST usually translates as better patient outcome in terms of TTP and/or OS in patients with cancer. However, this correlation is not always observed. This has been supported by results of a meta-analysis carried out by Buyse et al. [25] on individual data from 3791 patients with metastatic colorectal cancer treated with fluoropyrimidines. In addition, response rates are not invariably true surrogates for clinical benefit in patients with cancer [26]. A true surrogate should reflect the full effect of treatment benefits on patients and there is no evidence that response rate does this. Thus, it is important to highlight that some patients with apparently nonresponding tumors may nonetheless experience benefit due to oncology therapy.
At present, two of the treatments most frequently administered to patients with mRCC are TKIs and mTOR inhibitors. As it has been mentioned above, the effect of these two therapies is mainly based on targeting proangiogenic factors, such as PDGFR, VEGFR, and HIF. TKIs and mTOR inhibitors induce early and extensive necrosis yet without a significant decrease in tumor size [22, 27–29]. These considerations suggest that RECIST 1.1 is inadequate for the assessment of efficacy in patients treated with targeted drugs.
Based on these findings, this panel of experts recommends that response evaluation in patients with mRCC treated with these drugs should have a different approach from that taken with classical chemotherapy agents. In the same way, the evaluation of response to treatment with targeted drugs in mRCC should not be only based on the changes in the size of the lesion.
Which criteria should be used to evaluate the response to treatment in patients with mRCC?
Although RECIST 1.1 is a basic tool in the evaluation of response to treatment in oncology, there is a need for tumor response criteria better adapted to molecular targeted therapy-related changes (Table 1).
The Choi criteria were first developed for the evaluation of response in patients with gastrointestinal stromal tumors treated with imatinib because RECIST tended to underestimate imatinib-induced tumor response in this setting. The Choi criteria take into account changes in tumor attenuation, which can be detected through contrast-enhanced computed tomography (CECT) scans and reflect tumor density. With regard to patients with mRCC, van der Veldt et al. [30] carried out a comparison between the Choi criteria and RECIST in 55 patients treated with sunitinib. In the first evaluation of response to treatment carried out in this trial, partial response (PR) according to the Choi criteria defined a significantly larger population of patients with greater treatment benefit in terms of PFS and OS than RECIST (p< 0.001 for both). However, when only patients with PR and stable disease (SD) ≥12 weeks were considered, the predictive value of RECIST was substantially increased. The reliability of the Choi criteria is particularly limited in the assessment of patients presenting lesions <15 mm or with heterogeneous or poor-enhanced lesions at baseline CT. Hence, the Choi criteria would be useful in the assessment of patients with mRCC undergoing treatment with sunitinib. However, the use of these criteria does not change the management of this population compared to RECIST evaluation (Fig. 2).
Evaluation of tumoral response with RECIST 1.1 and MASS criteria in metastatic renal cancer with antiangiogenic therapy. Serial computed tomography (CT) scanning corresponding to a metastatic deposit: basal CT image (a) and CT images 1 month (b) and 2 months (c) following the administration of sunitinib. Comparison based on the longest diameter and density values in Hounsfield units (HU). Based on RECIST, CT exam performed 1 month following therapy correlated to progressive disease. However, in this case, increased diameter was secondary to extensive hemorrhagic necrosis due to antiangiogenic treatment. Although this was a nonenhancing lesion (when comparing enhanced to unenhanced scans—not showed), little decrease in median tumor density (from 61 to 49 HU) was due to the presence of hemorrhagic changes. These findings would be considered a favorable response according to MASS criteria. Two months posttherapy, partial response may be considered with a decreasing size. Besides, there was a decreasing median tumor density (from 49 to 34 HU). However, new solid enhancing areas (arrowheads) in a previous homogeneously nonenhancing mass represent unfavorable response with SACT or MASS criteria
A retrospective study carried out by Nathan et al. [15] compared the response to treatment according to RECIST, Choi, and modified Choi criteria (also named Nathan criteria) and correlated with TTP in 20 patients with mRCC treated with either sunitinib or cediranib. Images obtained through CECT scans were analyzed. Results revealed that neither response defined by RECIST nor response defined by conventional Choi criteria correlated with TTP in these patients. However, the detection of changes in both size and enhancement of arterial perfusion in patients with mRCC treated with a TKI better predicted the outcome of these patients than standard RECIST or Choi criteria.
In another retrospective study carried out by Smith et al. [31], 53 patients with mRCC were treated with the antiangiogenic agents sorafenib or sunitinib. An evaluation of changes in both tumor size and attenuation, as well as the detection of unique patterns of contrast enhancement through CECT scan, were the objectives of this study. Data obtained from this study were used to develop the Size and Attenuation Computed Tomography (SACT) criteria. The evaluation of response to therapy was then compared using three imaging criteria, namely, RECIST, modified Choi/Nathan, and SACT criteria. Response according to the assessment of imaging techniques was correlated with TTP and disease-specific survival. According to SACT criteria, a favorable response with a sensitivity of 75 % and a specificity of 100 % was found in patients with PFS >250 days, in comparison with a sensitivity and a specificity of 16 and 100 %, respectively, for RECIST, and of 93 and 44 %, respectively, for modified Choi criteria. Objectively measuring changes in both tumor size and attenuation of the lesions shown on the first CECT scan after treatment initiation with an antiangiogenic targeted agent considerably improved assessment of response in patients with mRCC. However, due to the small size of the study population analyzed, further research was warranted.
Smith et al. [32] attempted to correct some of the deficiencies observed in SACT criteria and to simplify them. These improvements led to the development of Mass, Attenuation, Size and Structure (MASS) criteria, which take into account changes in lesion size, attenuation, and internal structure. CT exams were evaluated on routine portal venous phase. Indeed, the importance of marked decreased attenuation (a decreased in attenuation of 40 HU or greater in one or more than one predominantly solid enhancing lesions), marked central necrosis (>50 %), and size changes in early tumor response assessment was confirmed. These results, together with other findings associated with tumor progression such as the appearance of new metastases and a marked central filling (new enhancement in a previously nonenhancing mass with homogeneous low attenuation), were included into the MASS criteria. According to the MASS criteria, response to treatment is divided into three categories, namely, favorable, indeterminate, and unfavorable response. These three categories differ significantly from one another with respect to TTP (p< 0.0001, log-rank test) and disease-specific survival (p< 0.0001, log-rank test). In addition, standard CECT examinations of 84 patients with mRCC treated with a TKI were retrospectively evaluated using MASS, RECIST, SACT, and modified Choi criteria. According to the MASS criteria, a favorable response with a sensitivity of 86 % and a specificity of 100 % in identifying patients with a good clinical outcome was found, i.e., patients with PFS >250 days, in comparison with 17 % (p< 0.03) sensitivity and 100 % specificity for a PR according to RECIST. Hence, the MASS criteria seem to more accurately predict response than RECIST, SACT, and modified Choi criteria in metastatic target lesions and might better predict disease outcome in these patients.
In some of the previously described studies, a key aspect in the evaluation of response to treatment with antiangiogenic therapies in mRCC is the early determination of whether these agents are effective in patients or not. A retrospective study carried out in 70 patients with mRCC treated with an antiangiogenic agent showed that, according to the Early Posttherapy Imaging Changes (EPTIC) criteria [33], a reduction in the sum of the longest unidimensional diameter of the tumor of 10 % obtained in the first CT scan after treatment initiation, performed approximately 1–2 months after therapy initiation, could be defined as an early PR, which could identify patients who would subsequently achieve a longer treatment benefit.
Typically, most abdominal imaging is performed during the portal venous phase. However, multiphasic CT scans are sometimes performed on other types of high-vascular tumors to improve radiologists' ability to consistently and reproducibly measure these lesions. An evaluation of the arterial phase is required to allow radiologists, not only to display all the lesions present but also to detect modifications in tumor vascularity. Patients with mRCC develop hypervascular metastases, some of which can only be identified through a CT scan carried out over the arterial phase of enhancement. Therefore, an implementation of a biphasic CT technique, comprising the arterial phase in thorax and superior abdomen, as well as the portal phase of enhancement in abdomen–pelvis is advisable in patients with mRCC [34–36]. This proposal is also supported by modified Choi criteria [15].
In summary, over the last decades, the assessment of response to treatment in patients with mRCC has been carried out using RECIST as the primary criteria. Available data supported the implementation of RECIST 1.1 not only in clinical research but also in clinical practice. However, both sets of RECIST criteria have some limitations in the evaluation of patients treated with molecular targeted therapies. These therapies apparently lead to disease stabilization rather than to substantial tumor regression. Such limitations in RECIST criteria have encouraged that, in addition to RECIST, complementary data based on imaging findings should be considered. In this sense, patients with more highly vascular renal tumors have beneficial outcome when treated with VEGF pathway inhibitors. Pretreatment evaluation of tumor enhancement or parameters obtained using functional imaging techniques (such as K trans or blood flow/volume) show promising as predictive and/or prognostic indicators [37]. Beside this, different studies have shown strong statistical relationships between early changes (4–16 weeks after the initiation of the therapy) in imaging-based data (size, density in Hounsfield units, or functional parameters) and clinical outcome in mRCCs treated with antiangiogenic therapy [30, 31, 33, 37]. Based on these data, this panel of experts recommends that response assessment in patients with mRCC treated with targeted therapies should include an early vascular response evaluation and that changes in size, density, or functional imaging-based parameters for target lesions should be reported.
Response to treatment, clinical benefit, and survival in mRCC
Do patients with mRCC who achieve disease stabilization benefit from treatment?
Antiangiogenic agents rarely achieve a reduction of 30 % in the sum of the longest diameters (SLD) of target lesions required for an objective response according to RECIST. Nonetheless, these drugs have demonstrated improvements in terms of PFS. In a study carried out in 334 patients with mRCC treated with sunitinib [38]. Thiam et al. determined a threshold of treatment evaluation that best reflected the outcome of these patients. Thresholds from −45 % to +10 % in tumor size modification were tested and correlated with treatment efficacy in terms of PFS. In this study, a variation of −10 % in the SLD identified patients with mRCC accurately and rapidly benefiting from their treatment with sunitinib. This evidence is also supported by the retrospective analysis performed on data from the RECORD-1 trial, a phase III trial comparing response to treatment in 416 patients with mRCC treated with everolimus or placebo, who had previously progressed to an antiangiogenic therapy with sunitinib or sorafenib. A series of tumor response thresholds [39], determined by the highest reduction in the SLD of target lesions, was correlated with significant improvements in terms of PFS. This analysis demonstrated that a reduction ≥5 % in SLD is a better predictor of benefit in terms of PFS than objective response according to RECIST.
In summary, disease stabilization is frequently observed in patients with mRCC treated with an antiangiogenic agent. Moreover, this outcome often leads to an extension in terms of TTP and PFS. Based on this, this panel of experts considers that disease stabilization should be regarded as a treatment benefit. In line with this, patients with mRCC presenting disease symptoms may demonstrate clinical improvement after antiangiogenic therapy initiation, even if no response according to RECIST has been demonstrated. In these patients, clinical improvement should be regarded as a clinical response to treatment.
Should the achievement of a response be the main objective of the treatment given to patients with mRCC?
In spite of the fact that patients may achieve benefits regardless of the response achieved according to radiological criteria, there is some evidence that correlates radiological response with benefits in clinical outcomes such as PFS. This evidence was supported, as previously mentioned, by Thiam et al. [38], who showed that small reductions in tumor size also translate into benefits in terms of PFS. Tumor vascularity is also a potential predictor of treatment outcome in patients with mRCC treated with antiangiogenic agents such as sunitinib or sorafenib. In addition, contrast enhancement of tumors determined by CT scans is also significantly correlated with microvascular density and treatment outcomes as Han et al. [21] demonstrated in a study of 46 patients with mRCC receiving antiangiogenic treatment. In this retrospective analysis, tumor enhancement was significantly associated with benefits in terms of time to size reduction (p= 0.03) and PFS (p= 0.028).
The definition of response according to Choi criteria takes into account not only a reduction in tumor size but also a decrease in tumor attenuation. Van der Veldt et al. [30] compared RECIST and Choi criteria and demonstrated that both were helpful for predicting treatment outcomes in patients with mRCC receiving sunitinib. However, it is important to note that response to treatment according to imaging criteria also means a reduction in tumor size that enables patients to undergo resection. Another important aspect is the assumption that patients presenting early disease progression must not have obtained treatment benefit, so that clinicians are able to carry out an early treatment modification. Lastly, there is a general agreement about the fact that the main objective in the evaluation of the response for an individual oncology drug is to bring forward the benefit in terms of PFS, which should be also reflected in terms of OS. However, for patients with mRCC receiving successive treatment lines, the translation of benefits in terms of PFS into benefits in terms of OS is not always achieved.
Based on all these considerations, this panel of experts agrees that improvements in quality of life, and secondary in PFS and OS, are the main objectives of treatment administration in patients with mRCC.
Are there any laboratory parameters or clinical features that may anticipate disease progression in a patient who has achieved SD?
Over the last years, one of the main goals of research in oncology has been to identify clinical features and laboratory parameters with prognostic value. In fact, some of them provide classifications that may enable clinicians to choose the optimal treatment for every patient. Prognostic classifications have been developed in several clinical trials with patients with mRCC. In one of these trials, a retrospective study analyzing data of 670 patients with mRCC, five prognostic factors were identified and a model of prognostic stratification was then developed [40]. Thus, prior to treatment, some features were recognized to be associated with a shorter survival, namely, low Karnofsky performance status, low hemoglobin levels, high serum calcium, time from diagnosis to treatment initiation, and an absence of prior nephrectomy. Based on these features, three risk groups were established, which were separated by steps of 6 months or more in terms of OS (Motzer criteria). Heng and colleagues [41] subsequently proposed a new prognostic classification, analyzing only data from patients treated with new drugs. These risk categories are currently being used in the context of clinical trials, and also in clinical practice.
In a retrospective analysis of an international database with 3,748 patients with mRCC, the International Kidney Cancer Working Group identified nine prognostic factors and developed a validated model for survival based on them [42]. Factors contributing to the prognostic index were treatment, performance status, number of metastatic sites, time from diagnosis to treatment initiation, pretreatment hemoglobin levels, white blood count, as well as lactate dehydrogenase (LDH), alkaline phosphatase and serum calcium levels. As a result, a model was devised that divided patients among three risk groups that were later validated using an independent data set of 645 patients treated with a TKI [42].
These and other prognostic factors may indicate disease progression, but there is general agreement that no validated risk factor can reliably determine disease progression. These biomarkers do not have enough supporting evidence to enable clinicians to carry out a treatment modification or a treatment withdrawal in an individual patient. As an example, serum levels of LDH are a biomarker for guiding clinicians in terms of treatment choice. Serum LDH is an enzyme involved in the mammalian target of rapamycin complex 1 (mTORC1) and in the process of tumor hypoxia and necrosis. High serum levels of LDH are related to poor prognosis in patients with cancer. With the aim of testing this biomarker as a prognostic and a predictive factor, Armstrong et al. [43] retrospectively evaluated pretreatment and posttreatment serum LDH in 404 poor-risk patients with mRCC treated with temsirolimus. Serum levels of LDH were demonstrated to have a prognostic and predictive value as a biomarker of benefits in terms of OS in this population treated with an mTORC1 inhibitor. Nonetheless, further research is warranted to confirm these results. However, it is now generally recognized that no treatment modification should be decided based only on abnormal laboratory values, with the exception of those attributable to toxicity associated with the oncology treatment. On the whole, when radiological and clinical data are considered in an individual patient, it is usually accepted that clinical data are more relevant than radiological data in the decision to withdraw or maintain an oncology treatment.
Based on these data, this panel of experts agrees that there is no laboratory parameter or clinical feature according to which disease progression may be predicted in patients with mRCC. However, some alterations in the clinical symptoms of the patient may indicate disease progression. Nonetheless, clinical symptoms should be correlated with radiological data before taking any decision on treatment for each individual patient.
Baseline evaluation and follow-up strategies in patients with mRCC
Is the radiological evaluation of response to treatment standardized by consistent response criteria throughout patient follow-up?
Tumor response evaluation is based on changes in the number and size of primary or secondary target lesions [44]. The reliability of these evaluations depends on the quality of the comparative radiological measurements of the target lesions. Thus, potential bias may arise due to the absence of standardization in the imaging technique used during assessment. The absence of standardization can involve aspects such as the phase of enhancement (arterial or portal) evaluated using the CT scan, volume and flow of the contrast injected to the patient for the correct imaging evaluation, poor selection of target lesions during the baseline evaluation, and inaccurate measurements of these lesions. Therefore, a thorough approach is essential in each tumor response evaluation in order to avoid bias and obtain accurate results. On the other hand, some essential patient information is necessary for radiologists to obtain an optimal response evaluation in patients with mRCC. This information should include the name or type of drug administered to the patient, treatment initiation date, data regarding the best response achieved with the current therapy, as well as the date of treatment modification, if applicable.
One of the key requirements in the evaluation of response to treatment using RECIST 1.1 is the selection of target lesions [24]. According to these criteria, target lesions should be decided during the baseline evaluation of the patient and only these target lesions should be subsequently followed up. One potential limitation in the implementation of RECIST 1.1 is to include the primary tumor as one of the target lesions, considering that there is a disproportionate effect of large renal tumors relative to their metastases in the results of the evaluation of response to treatment by RECIST 1.1 [45]. Additionally, it is important to highlight that, to date, no definite frequency and duration of the whole evaluation process have been established for patients with mRCC [35].
Imaging follow-up protocols should take into account several clinical scenarios. As a general rule, an imaging evaluation should be done prior to treatment initiation. Subsequent evaluations, every 3 months, should be performed in the thorax, abdomen, and pelvic regions, as well as additional assessments in brain, skeleton, etc. as required, depending on the symptoms of each individual patient. Nonetheless, in the era of molecular targeted therapy, newer cancer- and therapy-specific criteria will play an important role in the personalization of cancer care [46]. This task should be carried out by multidisciplinary teams, in which radiologists and oncologists play a major role. Although there are few publications on how these specialists should apply medical practice in this setting [47, 48], it is generally recognized that multidisciplinary teams represent an improvement in terms of communication, coordination, and decision making among specialists, which enables clinicians to accomplish personalized treatment plans according to tumor type, biomarkers, and other patient features.
Radiologists should complete their evaluation according to RECIST 1.1 with the addition of other useful data in their report, such as modifications in the radiological density of the lesions, necrosis >50 %, or the presence of new enhancement in a previously homogeneously hypoattenuating nonenhancing mass.
In this regard, this panel of experts supports the need to develop a basic template for reporting results of the evaluation of response to treatment using CT scan in patients with mRCC. Selection criteria for both, target lesions and target lymph nodes, will be consistent in each baseline evaluation. Therefore, the template should include the main characteristics of the lesions and lymph nodes, as well as delimitations of size, diameter length, and number of targeted lesions. Additionally, the template needs to include the sum of diameters of all target lesions. With regard to the follow-up evaluations, this form has to specify the need of using RECIST 1.1 [24], although this panel of experts highlights the necessity of performing determinations of changes in the enhancement of target lesions also, according to MASS or Nathan criteria [15, 32].
Finally, functional and molecular imaging techniques may be useful tools for the assessment of response to targeted therapies. However, their implementation may lead not only to more accuracy but also to an increased complexity in this process [49]. Based on these, this panel of experts agrees that radiologists and oncologists involved in the diagnosis and treatment of patients with mRCC should have a wide knowledge of the specific characteristics of mRCC, as well as on the individual effects that each targeted therapy has on these patients, because these drugs are modifying the paradigm of the evaluation of tumor response in this setting. Specialists should also be updated in terms of new treatment evaluation criteria and their implementation in the evaluation of response to treatment in patients with mRCC. In addition, the standardization of a common language to be used among specialists, especially with the purpose of comparing the results of multicenter clinical trials, is a pending issue that should be addressed soon [50, 51].
How often should patients with mRCC be evaluated for tumor response by imaging techniques? Are there any patients requiring more frequent evaluations?
Progress in genetics and oncology has expanded the array of therapies for patients with mRCC, significantly improving their outcomes. Imaging techniques play a primary role in the evaluation of response to treatment in this setting [52]. According to RECIST 1.1, frequency of tumor reevaluation should be protocol specific and adapted to the type and schedule of treatment. As a general rule, an evaluation of response to treatment should be performed every three cycles of treatment. However, for those patients treated with sunitinib, assessments every two cycles are more appropriate. On the other hand, the effect of radiation on patients during the implementation of imaging techniques is a relevant issue. Therefore, optimizing the use of radiation is an aspect to be considered when determining the requirement of an additional evaluation for an individual patient. There is a general rule on this matter for keeping radiation as low as reasonably achievable (ALARA criterion) for each evaluation of treatment response [53].
Another relevant aspect is that a first follow-up scan performed approximately 1 month after the initiation of therapy enables clinicians to distinguish between patients who are refractory to a particular therapy and those who achieve a good response and prognosis according to EPTIC criteria [33]. Patients with mRCC who develop toxicity from their first treatment administration can also benefit from an early first evaluation aimed at determining whether a treatment modification is convenient for them. Patients require an additional evaluation of response to treatment when disease progression is suspected, based on modifications of some clinical features. In contrast, a longer period between evaluations of response to treatment should be considered in patients considered long-term survivors or those who have not shown modifications in their clinical features or who have presented SD in several subsequent evaluations.
Taking into account these considerations, this panel of experts concludes that there is a general consensus on the convenience of scheduling treatment response evaluations every 3 months in patients with mRCC. However, this general rule should be tailored to the individual clinical features of each patient, such as clinical deterioration and toxicity among others.
During the evaluation of treatment response, should images from the last evaluation be compared with the immediately previous one or with those from all previous evaluations, including the baseline evaluation?
To assess objective response to treatment or disease progression, it is necessary to estimate the overall tumor burden at baseline and compare it with subsequent measurements. There is a general agreement among clinicians about the necessity of implementing RECIST 1.1 accurately regarding this issue [24]. In addition, it is recommended that, during the baseline imaging evaluation of the patient, target lesions should be clearly defined and identified. In subsequent treatment response evaluations, this panel of experts agrees that it should be enough to compare the latest tumor images with those obtained in the immediately previous assessment when the result of the evaluation is clear enough. In case of doubt, comparisons should be established between images of as many evaluations as required with the purpose of detecting slight changes in the size of the target lesions. Detection of slight changes could enable clinicians to establish a disease progression or a response to treatment in a patient with mRCC. In addition, RECIST 1.1 requires the comparison of tumor images from the latest evaluation with images of one or more previous evaluations of response to treatment, depending on the features of each individual patient.
Novel imaging techniques to evaluate the response to treatment in mRCC
Do novel imaging techniques have a real value in the evaluation of response to treatment in patients with mRCC?
Novel functional and molecular imaging techniques may be useful tools in the evaluation of patients with cancer (Table 2). Several trials have assessed the role of these techniques in the evaluation of treatment response in patients with mRCC treated with antiangiogenic agents, as well as their ability to predict patients' outcomes [20–22, 43, 54–58]. One of the main negative aspects associated with functional and molecular imaging techniques is the pending issue of their standardization in terms of use and acquisition for the clinical practice [49]. Research has been carried out with this purpose and also with the aim of optimizing the use of functional and molecular techniques in the context of clinical trials and daily clinical practice [49, 59–62], and CT perfusion certainly fulfills the criteria necessary for being considered a robust response biomarker [62, 63]. Unfortunately, there is not sufficient standardization or scientific evidence to abandon anatomical assessment of tumor burden. However, specific vascular parameters such as flow velocity, relative vascular volume, and relative blood flow rate can be quantified by DCE-US [61]. In addition, DCE-CT parameters may be considered as surrogates for physiological and molecular processes underlying tumor angiogenesis [62, 64].
Moreover, consensus guidelines have been developed, which aim to promote a broader application of DCE-CT in the evaluation of tumor vascularity. According to these guidelines, tumor angiogenesis can be robustly assessed by DCE-CT (Fig. 3). Perfusion CT is one of the most useful functional techniques in renal cancer. Perfusion CT is a feasible technique to assess tissue perfusion parameters in patients with mRCC, which correlate positively with microvascular density and may reflect angiogenesis of renal cancer [65]. Perfusion CT also correlates to tumor aggressiveness. Perfusion fraction and blood volume values are significantly higher in high-grade mRCC than in low-grade mRCC [66]. Besides, perfusion CT may have a prognostic value in mRCC. In this setting, patients with tumors with high blood flow at baseline appear to have a shorter PFS (or worse prognosis). Finally, perfusion CT may be a tool for predicting response to antiangiogenic therapy. Fournier et al. [20] reported that baseline perfusion CT parameters were higher in responder patients to antiangiogenic therapy.
Patient with mRCC treated with antiangiogenic therapy. a A 48-year-old man with a clear cell renal cancer (arrowheads) and a metastatic deposit in the left adrenal gland (arrow) in the pretreatment period. Conventional computed tomography (CT) image and perfusion CT parametric maps corresponding to blood flow (BF), blood volume (BV), and permeability-surface (PS) area product. Perfusion CT allows for a quantitative evaluation of tumor angiogenesis. Values of functional parameters in the adrenal metastasis were BF= 182 ml/min/100 g, BV= 21 mil/100 g, and PS= 27 ml/min/100 g. b Perfusion CT evaluation 13 days posttherapy with sunitinib in the same patient. Conventional CT image and perfusion CT parametric maps corresponding to BF, BV, and PS area product show early marked decrease in the values of functional parameters of the metastatic deposit (BF= 76 ml/min/100 g, BV= 7.1 ml/100 g, and PS= 18 ml/min/100 g)
DCE-MRI has become an important means for the analysis of how new therapies affect tumor vasculature, either as a direct target or indirectly [60]. Hence, DCE-MRI together with pharmacokinetic models is an appropriate approach for the assessment of response to novel treatments in this setting.
Besides, imaging techniques such as diffusion weighted-MRI (DW-MRI) allow clinicians to assess the thermally driven motion of water in tissues [67]. There are several microscopic organizational features that affect tissue water diffusivity, including cell density, extracellular space tortuosity, integrity of cellular membranes, tissue organization (e.g., glandular formation), and tissue perfusion. However, the basic biological premise of DW-MRI technique is that malignant tissues present, in general, high cellular density with more cellular membranes per volume unit, so that water diffusion is more impeded in tumors than in benign/normal tissues. Using DW-MRI, radiologists may obtain quantitative images of the diffusion, the apparent diffusion coefficient (ADC). In this setting, DW-MRI may show ADC changes, which may predict response to treatment [68]. Discrete reductions of ADC values have been described with antivascular therapies, but increased ADC is observed if there is significant tumor necrosis caused by the antivascular treatment. However, there is still a scarce experience with the use of DW-MRI in the evaluation of renal cancer response to therapy [69–71]. Finally, recent technologic advances have enabled the development of whole-body DW imaging. The clinical utility of whole-body MRI in patients with RCC is limited. The main advantage of whole-body MRI is its high diagnostic accuracy for musculoskeletal metastases [72].
2-[18F]fluoro-2-deoxy-d-glucose ([18F]FDG) PET has a limited role in the evaluation of RCC because this tumor usually shows a low glucose metabolism activity. However, when RCC is [18F]FDG avid, PET may have a prognostic value and can be applied to monitor response evaluation of targeted therapies [56, 73, 74]. In these patients, the maximum standardized uptake value (SUVmax) correlated with decreased survival [56]. Besides, there was a decrease in the maximum standardized uptake value (SUVmax) after the first treatment cycle [74]. However, data seem to be contradictory, and Kayani et al. [56] reported that metabolic response at 4 weeks was not predictive of patient's outcome, but metabolic progression (i.e., increase in SUVmax) at week 16 did correlate with inferior outcomes.
Additional PET radiotracers should continue to be evaluated to obtain the best biological readout. In this setting, Liu et al. have evaluated early changes in proliferation after sunitinib treatment in mRCC using a thymidine analogue. These authors evidenced that a change in SUV total at week 4 appeared to be associated with the degree of tumor response seen on the first disease assessment scan. Beside this, a proliferative increase in the target lesions during therapy withdrawal was seen in all patients [75]. Integrins play an important role in cell–cell and cell–matrix interactions. Between them, integrin αvβ3 presents a key role in tumor angiogenesis by facilitating endothelial cell migration. The pentapeptide cyclo (–Arg–Gly–Asp–DPhe–Val–) (RGD) has been developed for the evaluation of αvβ3 expression based on its high affinity and selectivity for these integrins [76]. This radiotracer seems to be promising in the evaluation of angiogenesis [77], but its use in RCC has been limited [78].
Finally, several targeted drugs have been radiolabeled as PET tracers, including [89Zr]bevacizumab and [18F] sunitinib [79, 80]. These specific PET tracers may provide a unique opportunity for personalized treatment planning.
Based on these data, this panel of experts agrees that there are several promising novel techniques for the assessment of response to treatment in patients with mRCC treated with targeted therapies; however, DCE imaging techniques and PET seem to be the most promising technique in mRCC. Functional and molecular imaging techniques provide continuous quantitative evaluation parameters to overcome the inherent limitations of the rigid categories of RECIST 1.1. Nonetheless, at present, the real value of these techniques is still to be defined. Their obvious complexity, which is mainly due to their limited implementation in the evaluation of response to treatment in this setting, as well as the pending matter of their standardization are the main reasons why, to date, these novel techniques have not achieved relevance in the clinical practice.
Future challenges
In this paper, a series of recommendations on certain issues has been proposed in the light of the currently available scientific evidence and the expertise of the panel, with the intention of providing guidance to clinicians to improve the clinical and radiological evaluation of response to treatment in patients with mRCC treated with targeted drugs. However, there are several other issues that have not been addressed, but these will be further clarified in the near future, and are mentioned below.
One of the most important issues from a clinical point of view is the need for a consensus to define a patient with mRCC as a “slow progressor.” Although it is generally recognized that patients known as “slow progressors” are usually obtaining a clinical benefit from their current treatment, there is also a growing need among oncologists to establish a boundary in terms of tumor growth speed in order to carry out a treatment change. This limit in terms of tumor growth should differentiate between those patients who obtain a clinical benefit from their antiangiogenic treatment, in spite of the fact that an increase in tumor size is observed, and those who do not. Therefore, if slow disease progression is observed in a patient with mRCC while maintaining a substantial clinical benefit derived from treatment, it does not necessarily imply a treatment modification, but it requires an individualized evaluation of the patient.
Another pending issue is an analysis of the effectiveness of resection of the primary tumor in patients with mRCC. Despite the fact that tumor resection improves the outcome of patients treated with antiangiogenic agents, treatment efficacy has been only demonstrated in those resected patients who have also been treated with cytokines, i.e., interleukin-2 or interferon. With the purpose of demonstrating the efficacy of antiangiogenic agents in resected patients, two outstanding ongoing clinical trials are evaluating the role of nephrectomy, following the development of these novel agents (CARMENA-ClinicalTrials.gov Identifier: NCT00930033; EORTC SURTIME-ClinicalTrials.gov Identifier: NCT01099423).
The effectiveness of an antiangiogenic treatment in patients with mRCC presenting either an unresectable primary tumor or unresectable large metastases is another pending issue. The value of neadjuvant therapy in locally advanced or metastatic disease needs to be established. On the other hand, clinicians must be aware of the imaging modifications associated with the administration of each antiangiogenic agent, with the purpose of optimizing the evaluation of response to treatment in this setting. Lastly, paradoxical response, which is very frequent in patients with RCC, is a subject still under discussion. Paradoxical response does not represent a unique type of response, but several types, and its management should be approached on an individual basis.
References
Cohen HT, McGovern FJ (2005) Renal-cell carcinoma. N Engl J Med 353:2477–2490. doi:10.1056/NEJMra043172
Brugarolas J (2007) Renal-cell carcinoma—molecular pathways and therapies. N Engl J Med 356:185–187. doi:10.1056/NEJMe068263
Li L, Kaelin WG Jr (2011) New insights into the biology of renal cell carcinoma. Hematol Oncol Clin North Am 25:667–686. doi:10.1016/j.hoc.2011.04.004
Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, Latif F, Liu S, Chen F, Duh FM et al (1994) Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet 7:85–90. doi:10.1038/ng0594-85
Iliopoulos O, Levy AP, Jiang C, Kaelin WG Jr, Goldberg MA (1996) Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci U S A 93:10595–10599
Koul H, Huh JS, Rove KO, Crompton L, Koul S, Meacham RB, Kim FJ (2011) Molecular aspects of renal cell carcinoma: a review. Am J Cancer Res 1:240–254
Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271–275. doi:10.1038/20459
Na X, Wu G, Ryan CK, Schoen SR, di'Santagnese PA, Messing EM (2003) Overproduction of vascular endothelial growth factor related to von Hippel–Lindau tumor suppressor gene mutations and hypoxia-inducible factor-1 alpha expression in renal cell carcinomas. J Urol 170:588–592. doi:10.1097/01.ju.0000074870.54671.98
Bedke J, Gouttefangeas C, Singh-Jasuja H, Stevanovic S, Behnes CL, Stenzl A (2013) Targeted therapy in renal cell carcinoma: moving from molecular agents to specific immunotherapy. World J Urol. doi:10.1007/s00345-013-1033-3
Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar B, Bajetta E, Gorbunova V, Bay JO, Bodrogi I, Jagiello-Gruszfeld A, Moore N (2007) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370:2103–2111. doi:10.1016/S0140-6736(07)61904-7
Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J, Small EJ (2008) Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 26:5422–5428. doi:10.1200/JCO.2008.16.9847
Albiges L, Salem M, Rini B, Escudier B (2011) Vascular endothelial growth factor-targeted therapies in advanced renal cell carcinoma. Hematol Oncol Clin North Am 25:813–833. doi:10.1016/j.hoc.2011.04.006
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O'Toole T, Lustgarten S, Moore L, Motzer RJ (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356:2271–2281. doi:10.1056/NEJMoa066838
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grunwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372:449–456. doi:10.1016/S0140-6736(08)61039-9
Nathan PD, Vinayan A, Stott D, Juttla J, Goh V (2010) CT response assessment combining reduction in both size and arterial phase density correlates with time to progression in metastatic renal cancer patients treated with targeted therapies. Cancer Biol Ther 9:15–19
Shinagare AB, Krajewski KM, Jagannathan JP, Ramaiya NH (2012) Genitourinary imaging: part 2, role of imaging in medical management of advanced renal cell carcinoma. AJR Am J Roentgenol 199:W554–W564. doi:10.2214/AJR.12.9233
Flaherty KT, Rosen MA, Heitjan DF, Gallagher ML, Schwartz B, Schnall MD, O'Dwyer PJ (2008) Pilot study of DCE-MRI to predict progression-free survival with sorafenib therapy in renal cell carcinoma. Cancer Biol Ther 7:496–501
Hahn OM, Yang C, Medved M, Karczmar G, Kistner E, Karrison T, Manchen E, Mitchell M, Ratain MJ, Stadler WM (2008) Dynamic contrast-enhanced magnetic resonance imaging pharmacodynamic biomarker study of sorafenib in metastatic renal carcinoma. J Clin Oncol 26:4572–4578. doi:10.1200/JCO.2007.15.5655
Lamuraglia M, Escudier B, Chami L, Schwartz B, Leclere J, Roche A, Lassau N (2006) To predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib: pilot study using dynamic contrast-enhanced Doppler ultrasound. Eur J Cancer 42:2472–2479. doi:10.1016/j.ejca.2006.04.023
Fournier LS, Oudard S, Thiam R, Trinquart L, Banu E, Medioni J, Balvay D, Chatellier G, Frija G, Cuenod CA (2010) Metastatic renal carcinoma: evaluation of antiangiogenic therapy with dynamic contrast-enhanced CT. Radiology 256:511–518. doi:10.1148/radiol.10091362
Han KS, Jung DC, Choi HJ, Jeong MS, Cho KS, Joung JY, Seo HK, Lee KH, Chung J (2010) Pretreatment assessment of tumor enhancement on contrast-enhanced computed tomography as a potential predictor of treatment outcome in metastatic renal cell carcinoma patients receiving antiangiogenic therapy. Cancer 116:2332–2342. doi:10.1002/cncr.25019
van der Veldt AA, Meijerink MR, van den Eertwegh AJ, Boven E (2010) Targeted therapies in renal cell cancer: recent developments in imaging. Target Oncol 5:95–112. doi:10.1007/s11523-010-0146-5
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47:207–214
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. doi:10.1016/j.ejca.2008.10.026
Buyse M, Thirion P, Carlson RW, Burzykowski T, Molenberghs G, Piedbois P (2000) Relation between tumour response to first-line chemotherapy and survival in advanced colorectal cancer: a meta-analysis. Meta-Analysis Group in Cancer. Lancet 356:373–378
Fleming TR, DeMets DL (1996) Surrogate end points in clinical trials: are we being misled? Ann Intern Med 125:605–613
Flaherty KT (2007) Sorafenib in renal cell carcinoma. Clin Cancer Res 13:747s–752s. doi:10.1158/1078-0432.CCR-06-2063
Rixe O, Bukowski RM, Michaelson MD, Wilding G, Hudes GR, Bolte O, Motzer RJ, Bycott P, Liau KF, Freddo J, Trask PC, Kim S, Rini BI (2007) Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol 8:975–984. doi:10.1016/S1470-2045(07)70285-1
Rodriguez Faba O, Breda A, Rosales A, Palou J, Algaba F, Maroto Rey P, Villavicencio H (2010) Neoadjuvant temsirolimus effectiveness in downstaging advanced non-clear cell renal cell carcinoma. Eur Urol 58:307–310. doi:10.1016/j.eururo.2010.03.005
van der Veldt AA, Meijerink MR, van den Eertwegh AJ, Haanen JB, Boven E (2010) Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Br J Cancer 102:803–809. doi:10.1038/sj.bjc.6605567
Smith AD, Lieber ML, Shah SN (2010) Assessing tumor response and detecting recurrence in metastatic renal cell carcinoma on targeted therapy: importance of size and attenuation on contrast-enhanced CT. AJR Am J Roentgenol 194:157–165. doi:10.2214/AJR.09.2941
Smith AD, Shah SN, Rini BI, Lieber ML, Remer EM (2010) Morphology, Attenuation, Size, and Structure (MASS) criteria: assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. AJR Am J Roentgenol 194:1470–1478. doi:10.2214/AJR.09.3456
Krajewski KM, Guo M, Van den Abbeele AD, Yap J, Ramaiya N, Jagannathan J, Heng DY, Atkins MB, McDermott DF, Schutz FA, Pedrosa I, Choueiri TK (2011) Comparison of four early posttherapy imaging changes (EPTIC; RECIST 1.0, tumor shrinkage, computed tomography tumor density, Choi criteria) in assessing outcome to vascular endothelial growth factor-targeted therapy in patients with advanced renal cell carcinoma. Eur Urol 59:856–862. doi:10.1016/j.eururo.2011.01.038
Jain Y, Liew S, Taylor MB, Bonington SC (2011) Is dual-phase abdominal CT necessary for the optimal detection of metastases from renal cell carcinoma? Clin Radiol 66:1055–1059. doi:10.1016/j.crad.2011.06.002
Patel U, Sokhi H (2012) Imaging in the follow-up of renal cell carcinoma. AJR Am J Roentgenol 198:1266–1276. doi:10.2214/AJR.11.8381
Raptopoulos VD, Blake SP, Weisinger K, Atkins MB, Keogan MT, Kruskal JB (2001) Multiphase contrast-enhanced helical CT of liver metastases from renal cell carcinoma. Eur Radiol 11:2504–2509. doi:10.1007/s003300100853
O'Connor JP, Jayson GC (2012) Do imaging biomarkers relate to outcome in patients treated with VEGF inhibitors? Clin Cancer Res 18:6588–6598. doi:10.1158/1078-0432.CCR-12-1501
Thiam R, Fournier LS, Trinquart L, Medioni J, Chatellier G, Balvay D, Escudier B, Dromain C, Cuenod CA, Oudard S (2010) Optimizing the size variation threshold for the CT evaluation of response in metastatic renal cell carcinoma treated with sunitinib. Ann Oncol 21:936–941. doi:10.1093/annonc/mdp466
Oudard S, Thiam R, Fournier LS, Medioni J, Lamuraglia M, Scotte F, Fabre E, Kim D, Kpamegan E, Panneerselvam A, Cuenod CA (2012) Optimisation of the tumour response threshold in patients treated with everolimus for metastatic renal cell carcinoma: analysis of response and progression-free survival in the RECORD-1 study. Eur J Cancer 48:1512–1518. doi:10.1016/j.ejca.2012.01.027
Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J (1999) Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 17:2530–2540
Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, Venner P, Knox JJ, Chi KN, Kollmannsberger C, McDermott DF, Oh WK, Atkins MB, Bukowski RM, Rini BI, Choueiri TK (2009) Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 27:5794–5799. doi:10.1200/JCO.2008.21.4809
Manola J, Royston P, Elson P, McCormack JB, Mazumdar M, Negrier S, Escudier B, Eisen T, Dutcher J, Atkins M, Heng DY, Choueiri TK, Motzer R, Bukowski R (2011) Prognostic model for survival in patients with metastatic renal cell carcinoma: results from the international kidney cancer working group. Clin Cancer Res 17:5443–5450. doi:10.1158/1078-0432.CCR-11-0553
Armstrong AJ, George DJ, Halabi S (2012) Serum lactate dehydrogenase predicts for overall survival benefit in patients with metastatic renal cell carcinoma treated with inhibition of mammalian target of rapamycin. J Clin Oncol 30:3402–3407. doi:10.1200/JCO.2011.40.9631
Ollivier L, Leclère J, Thiesse P, Di Stefano D, Vincent C (2007) Évaluation de la réponse thérapeutique en cancérologie: le rôle de l'imagerie morphologique. Bull Cancer 94:171–177
Schwartz LH, Mazumdar M, Wang L, Smith A, Marion S, Panicek DM, Motzer RJ (2003) Response assessment classification in patients with advanced renal cell carcinoma treated on clinical trials. Cancer 98:1611–1619. doi:10.1002/cncr.11712
Nishino M, Jagannathan JP, Krajewski KM, O'Regan K, Hatabu H, Shapiro G, Ramaiya NH (2012) Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol 198:737–745. doi:10.2214/AJR.11.7483
Castel P, Tassy L, Lurkin A, Blay JY, Meeus P, Mignotte H, Faure C, Ranchere-Vince D, Bachelot T, Guastalla JP, Sunyach MP, Guerin N, Treilleux I, Marec-Berard P, Thiesse P, Ray-Coquard I (2012) Multidisciplinarity and medical decision, impact for patients with cancer: sociological assessment of two tumour committees' organization. Bull Cancer 99:E34–E42. doi:10.1684/bdc.2012.1559
Lamb BW, Sevdalis N, Taylor C, Vincent C, Green JS (2012) Multidisciplinary team working across different tumour types: analysis of a national survey. Ann Oncol 23:1293–1300. doi:10.1093/annonc/mdr453
Figueiras RG, Padhani AR, Goh VJ, Vilanova JC, Gonzalez SB, Martin CV, Caamano AG, Naveira AB, Choyke PL (2011) Novel oncologic drugs: what they do and how they affect images. Radiographics 31:2059–2091. doi:10.1148/rg.317115108
Negrier S, Ollivier L, Di Stefano-Louineau D, Escudier B, Savary J, Lasset C, Thiesse P (2000) Reliability of the response rate in oncology: analysis of the causes for variation. Bull Cancer 87:927–934
Suzuki C, Jacobsson H, Hatschek T, Torkzad MR, Boden K, Eriksson-Alm Y, Berg E, Fujii H, Kubo A, Blomqvist L (2008) Radiologic measurements of tumor response to treatment: practical approaches and limitations. Radiographics 28:329–344. doi:10.1148/rg.282075068
Katabathina VS, Lassau N, Pedrosa I, Ng CS, Prasad SR (2012) Evaluation of treatment response in patients with metastatic renal cell carcinoma: role of state-of-the-art cross-sectional imaging. Curr Urol Rep 13:70–81. doi:10.1007/s11934-011-0233-x
European ALARA Network. http://www.eu-alara.net/. Accessed 10 Oct 2013
Faria SC, Ng CS, Hess KR, Phongkitkarun S, Szejnfeld J, Daliani D, Charnsangavej C (2007) CT quantification of effects of thalidomide in patients with metastatic renal cell carcinoma. AJR Am J Roentgenol 189:378–385. doi:10.2214/AJR.07.2164
Hugonnet F, Fournier L, Medioni J, Smadja C, Hindie E, Huchet V, Itti E, Cuenod CA, Chatellier G, Oudard S, Faraggi M (2011) Metastatic renal cell carcinoma: relationship between initial metastasis hypoxia, change after 1 month's sunitinib, and therapeutic response: an 18 F-fluoromisonidazole PET/CT study. J Nucl Med 52:1048–1055. doi:10.2967/jnumed.110.084517
Kayani I, Avril N, Bomanji J, Chowdhury S, Rockall A, Sahdev A, Nathan P, Wilson P, Shamash J, Sharpe K, Lim L, Dickson J, Ell P, Reynolds A, Powles T (2011) Sequential FDG-PET/CT as a biomarker of response to Sunitinib in metastatic clear cell renal cancer. Clin Cancer Res 17:6021–6028. doi:10.1158/1078-0432.CCR-10-3309
Maksimovic O, Schraml C, Hartmann JT, Bitzer M, Claussen CD, Pintoffl J, Horger M (2010) Evaluation of response in malignant tumors treated with the multitargeted tyrosine kinase inhibitor sorafenib: a multitechnique imaging assessment. AJR Am J Roentgenol 194:5–14. doi:10.2214/AJR.09.2744
Williams R, Hudson JM, Lloyd BA, Sureshkumar AR, Lueck G, Milot L, Atri M, Bjarnason GA, Burns PN (2011) Dynamic microbubble contrast-enhanced US to measure tumor response to targeted therapy: a proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapy. Radiology 260:581–590. doi:10.1148/radiol.11101893
Aboagye EO, Gilbert FJ, Fleming IN, Beer AJ, Cunningham VJ, Marsden PK, Visvikis D, Gee AD, Groves AM, Kenny LM, Cook GJ, Kinahan PE, Myers M, Clarke L (2012) Recommendations for measurement of tumour vascularity with positron emission tomography in early phase clinical trials. Eur Radiol 22:1465–1478. doi:10.1007/s00330-011-2311-3
Leach MO, Morgan B, Tofts PS, Buckley DL, Huang W, Horsfield MA, Chenevert TL, Collins DJ, Jackson A, Lomas D, Whitcher B, Clarke L, Plummer R, Judson I, Jones R, Alonzi R, Brunner T, Koh DM, Murphy P, Waterton JC, Parker G, Graves MJ, Scheenen TW, Redpath TW, Orton M, Karczmar G, Huisman H, Barentsz J, Padhani A (2012) Imaging vascular function for early stage clinical trials using dynamic contrast-enhanced magnetic resonance imaging. Eur Radiol 22:1451–1464. doi:10.1007/s00330-012-2446-x
Leen E, Averkiou M, Arditi M, Burns P, Bokor D, Gauthier T, Kono Y, Lucidarme O (2012) Dynamic contrast enhanced ultrasound assessment of the vascular effects of novel therapeutics in early stage trials. Eur Radiol 22:1442–1450. doi:10.1007/s00330-011-2373-2
Miles KA, Lee TY, Goh V, Klotz E, Cuenod C, Bisdas S, Groves AM, Hayball MP, Alonzi R, Brunner T (2012) Current status and guidelines for the assessment of tumour vascular support with dynamic contrast-enhanced computed tomography. Eur Radiol 22:1430–1441. doi:10.1007/s00330-012-2379-4
Goh V, Ng QS, Miles K (2012) Computed tomography perfusion imaging for therapeutic assessment: has it come of age as a biomarker in oncology? Invest Radiol 47:2–4. doi:10.1097/RLI.0b013e318229ff3e
Garcia-Figueiras R, Goh VJ, Padhani AR, Baleato-Gonzalez S, Garrido M, Leon L, Gomez-Caamano A (2013) CT perfusion in oncologic imaging: a useful tool? AJR Am J Roentgenol 200:8–19. doi:10.2214/AJR.11.8476
Chen Y, Zhang J, Dai J, Feng X, Lu H, Zhou C (2010) Angiogenesis of renal cell carcinoma: perfusion CT findings. Abdom Imaging 35:622–628. doi:10.1007/s00261-009-9565-0
Zhang YL, Ren J, Yu BL, Qu K, Wang K, Qiang YQ, Li CX, Sun XW, Li Z (2012) Utility of CT perfusion imaging for grading of clear cell renal cell carcinoma. Hereditary Genet 1:1–5. doi:10.4172/2161-1041.1000105
Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, Dzik-Jurasz A, Ross BD, Van Cauteren M, Collins D, Hammoud DA, Rustin GJ, Taouli B, Choyke PL (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11:102–125
Padhani AR, Koh DM (2011) Diffusion MR imaging for monitoring of treatment response. Magn Reson Imaging Clin N Am 19:181–209. doi:10.1016/j.mric.2010.10.004
Desar IM, ter Voert EG, Hambrock T, van Asten JJ, van Spronsen DJ, Mulders PF, Heerschap A, van der Graaf WT, van Laarhoven HW, van Herpen CM (2011) Functional MRI techniques demonstrate early vascular changes in renal cell cancer patients treated with sunitinib: a pilot study. Cancer Imaging 11:259–265. doi:10.1102/1470-7330.2011.0032
Gary R, Mac Vicar, Frank Miller, Rahul Rustogi, Zong-Ming Chen, Brenda K. Martone, Kuzel. T (2013) Correlation of pathologic findings after brief neoadjuvant sorafenib with results of dynamic-contrast enhanced (DCE) and diffusion-weighted magnetic resonance imaging (DW-MRI) in patients with locally advanced or metastatic clear cell renal cell carcinoma (RCC). J Clin Oncol 31 (Suppl 6): abstract 466
Leary A, Pickering LM, Larkin JMG, Leach MO, Gore ME, Sohaib A, Collins DJ, Koh D (2011) Quantitative diffusion-weighted (DW) MR imaging of microcapillary perfusion and tissue diffusivity as biomarkers of response of renal cell carcinoma (RCC) to treatment with sunitinib. ASCO Meeting Abstracts 29:TPS154
Platzek I, Zastrow S, Deppe PE, Grimm MO, Wirth M, Laniado M, Stroszczynski C (2010) Whole-body MRI in follow-up of patients with renal cell carcinoma. Acta Radiol 51:581–589. doi:10.3109/02841851003724846
Lyrdal D, Boijsen M, Suurkula M, Lundstam S, Stierner U (2009) Evaluation of sorafenib treatment in metastatic renal cell carcinoma with 2-fluoro-2-deoxyglucose positron emission tomography and computed tomography. Nucl Med Commun 30:519–524
Vercellino L, Bousquet G, Baillet G, Barre E, Mathieu O, Just PA, Desgrandchamps F, Misset JL, Hindie E, Moretti JL (2009) 18 F-FDG PET/CT imaging for an early assessment of response to sunitinib in metastatic renal carcinoma: preliminary study. Cancer Biother Radiopharm 24:137–144. doi:10.1089/cbr.2008.0527
Liu G, Jeraj R, Perlman S, Vanderhoek M, Kolesar J, Eickhoff J, Alberti D, Wilding G (2008) Pharmacodynamic study of FLT-PET imaging in patients treated with sunitinib. ASCO Meet Abstr 26:3515
Beer AJ, Kessler H, Wester HJ, Schwaiger M (2011) PET Imaging of Integrin alphaVbeta3 Expression. Theranostics 1:48–57
Backer MV, Backer JM (2012) Imaging key biomarkers of tumor angiogenesis. Theranostics 2:502–515. doi:10.7150/thno.3623
Beer AJ, Lorenzen S, Metz S, Herrmann K, Watzlowik P, Wester HJ, Peschel C, Lordick F, Schwaiger M (2008) Comparison of integrin alphaVbeta3 expression and glucose metabolism in primary and metastatic lesions in cancer patients: a PET study using 18 F-galacto-RGD and 18 F-FDG. J Nucl Med 49:22–29. doi:10.2967/jnumed.107.045864
Nagengast WB, de Korte MA, Oude Munnink TH, Timmer-Bosscha H, den Dunnen WF, Hollema H, de Jong JR, Jensen MR, Quadt C, Garcia-Echeverria C, van Dongen GA, Lub-de Hooge MN, Schroder CP, de Vries EG (2010) 89Zr-bevacizumab PET of early antiangiogenic tumor response to treatment with HSP90 inhibitor NVP-AUY922. J Nucl Med 51:761–767. doi:10.2967/jnumed.109.071043
Wang JQ, Miller KD, Sledge GW, Zheng QH (2005) Synthesis of [18 F]SU11248, a new potential PET tracer for imaging cancer tyrosine kinase. Bioorg Med Chem Lett 15:4380–4384. doi:10.1016/j.bmcl.2005.06.038
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Acknowledgments
The authors wish to thank all the clinicians participating in the four regional face-to-face working meetings of the EVALUATION project: Jordi Andreu, Hospital Universitari Vall d'Hebron, Barcelona, Spain; José A. Arranz, Hospital Universitario Gregorio Marañón, Madrid, Spain; Isabel Chirivella, Hospital Clínico Universitario de Valencia, Valencia, Spain; Serafín Costilla, Hospital Universitario Central de Asturias, Oviedo, Asturias, Spain; Delfina Dualde, Hospital Clínico Universitario de Valencia, Valencia, Spain; Emilio Esteban, Hospital Universitario Central de Asturias, Oviedo, Asturias, Spain; Enrique Gallardo, Corporaciò Sanitària Parc Taulí, Sabadell, Barcelona, Spain; Belén González, Hospital Son Llàtzer, Palma de Mallorca, Baleares, Spain; Nuria Lainez, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Pedro Lastra, Hospital Universitario Marqués de Valdecilla, Santander, Cantabria, Spain; Alberto Martínez, Hospital Universitario 12 de Octubre, Madrid, Spain; Esther Martínez, Complejo Hospitalario de Jaén, Jaén, Spain; Enrique de Miguel, Hospital Universitario Gregorio Marañón, Madrid, Spain; Álvaro Montesa, Hospital Regional Universitario Carlos Haya, Málaga, Spain; Amelia Muñoz, Hospital Universitario Ntra. Sra. de la Candelaria, Tenerife, Canarias, Spain; Susana Pardo, Hospital Son Llàtzer, Palma de Mallorca, Baleares, Spain; Begoña Pérez, Hospital Universitario Virgen del Rocío, Sevilla, Spain; Álvaro Pinto, Hospital Universitario La Paz, Madrid, Spain; Manuel Ruza, Hospital Universitario Reina Sofía, Córdoba, Spain; Carmen Sánchez, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain; Juan Sepúlveda, Hospital Universitario 12 de Octubre, Madrid, Spain. The authors also wish to thank Ana Martín from HealthCo S.L. (Madrid, Spain) for her help in preparing the first draft of this manuscript. The scientific meetings along with medical writing services were supported financially by Novartis S.A. of Spain. Novartis S.A. of Spain was given the opportunity to comment on the first draft of the manuscript, but all the decisions about its content were taken by the authors. All authors have approved the final version of the submitted manuscript.
Conflict of interest statement
The authors declare that they do not have any conflict of interest that may inappropriately influence this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
León, L., García-Figueras, R., Suárez, C. et al. Recommendations for the clinical and radiological evaluation of response to treatment in metastatic renal cell cancer. Targ Oncol 9, 9–24 (2014). https://doi.org/10.1007/s11523-013-0304-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-013-0304-7