Abstract
Recent advances in genetics and oncology have led to development of a wide array of molecular therapeutics in the management of patients with metastatic renal cell carcinoma. These drugs have revolutionized the treatment of advanced disease by significantly improving patient outcomes. State-of-the-art cross-sectional imaging techniques play a seminal role in the evaluation of treatment response by providing reproducible, objective data, thereby permitting accurate quantification of tumor burden. Evolving functional imaging techniques such as perfusion and diffusion studies continue to advance the technology beyond assessing changes in tumor size and morphology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
About one third of the patients with renal cell carcinoma (RCC) present either with locally advanced disease or with metastases [1]. In addition, about 30% of patients may develop metastatic disease after initial successful treatment of localized RCC. RCC is remarkably resistant to conventional chemotherapy and radiotherapy; metastatic RCC (mRCC) thus carries an extremely poor prognosis, with a 5-year survival of 10% and a median survival of 7 to 11 months [2].
Recent advances in cytogenetics and molecular biology of RCCs have unraveled characteristic oncological pathways that are activated due to specific “driver” mutations. Clear cell RCC is the most common histological subtype, accounting for 70% to 80% of all RCCs and comprising 90% to 95 % of all mRCCs. It is now well established that most sporadic clear cell RCCs are characterized by genetic or epigenetic silencing of the von-Hippel Lindau gene (VHL). Thus, many “small” molecules that act at molecular targets in various downstream and related pathways of VHL inactivation have been developed.
In oncological trials involving cytotoxic chemotherapy, imaging studies such as computed tomography (CT) and magnetic resonance imaging (MRI) have traditionally provided reproducible and readily measurable objective response criteria, including quantification of disease burden and changes in tumor size/morphology. However, morphological criteria may not provide meaningful data in trials where targeted therapeutics that modify tumor perfusion or metabolism are being employed. Accurate and rapid assessment of treatment response of mRCC to targeted drugs is extremely important in optimal patient management. State-of-the-art imaging techniques such as dynamic contrast-enhanced ultrasonography (DCE-US), dynamic contrast-enhanced computed tomography (DCE-CT), and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) may provide unique physiological data on changes in tumor perfusion that can be correlated well with advanced histopathological findings. In addition, positron emission tomography (PET) also may provide functional information about the tumor and help in early prediction of treatment response. Newer functional parameters may serve as better surrogate imaging markers that may correlate with clinical end points such as patient survival times. This article discusses new imaging techniques and response evaluation criteria for mRCCs treated with antiangiogenic drugs (Tables 1 and 2).
Implications of Genetics of Metastatic Renal Cell Carcinoma on Patient Management
Approximately 60% of sporadic clear cell RCCs, the most common histological subtype of mRCCs, develops secondary to spontaneous inactivating mutations of VHL [1]. VHL, located on the short arm of chromosome 3, functions as a tumor suppressor gene and encodes for the VHL protein (pVHL) [3]. Malfunctioning of this protein results in uncontrolled activation of hypoxic response even in the absence of tissue hypoxia, leading to elevated levels of hypoxia-inducible factor (HIF) and upregulation of downstream hypoxia response genes and proteins, including vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) [3]. Related metabolic pathways, such as mammalian target of rapamycin (mTOR), are also activated [4]. Thus, overexpression of growth factors is believed to promote tumor cell growth, survival, and angiogenesis [5]. The development of targeted therapies for mRCCs is based on the discovery of small molecules that can interrupt the VHL downstream pathways and downregulate the associated growth factors. In addition, a number of potential targets have been vigorously studied in patients with mRCC with non–clear cell histology.
Over the past 15 years, a number of drugs have been approved by the US Food and Drug Administration (FDA) for the management of mRCC (clear cell histology). Currently, targeted therapy with tyrosine kinase inhibitors (TKIs), VEGF inhibitors, and mTOR inhibitors (either alone or in combination) is the standard of care for patients with mRCCs [6]. Sunitinib, pazopanib, and sorafenib are FDA-approved TKIs that act on several tyrosine kinases including VEGF receptors, PDGF receptors, stem cell factor receptors, and fibroblast growth factor receptors. Bevacizumab, a humanized monoclonal antibody that inhibits the action of VEGF, is always used along with interferon-α. Temsirolimus and everolimus inhibit mTOR kinase pathway and downregulate HIF activity, resulting in decreased levels of VEGF and PDGF.
Evolution of Imaging Criteria for Assessing Tumor Response
The Response Evaluation Criteria in Solid Tumors (RECIST), the most commonly used international guidelines for evaluation of treatment response in solid tumors, is primarily based on assessing changes in tumor size [7]. A modified version of RECIST guidelines (RECIST 1.1) has been published recently [8]. RECIST methodology is simple, easily quantifiable, and serves as a simple objective end point for tumor response evaluation. However, new targeted therapies may cause early/extensive tumor necrosis without a marked decrease in size, and thus, a good clinical response may be underestimated by RECIST criteria [9]. Because targeted chemotherapy initially causes disease stabilization with associated morphological changes rather than substantial tumor regression in these patients, best response as per the RECIST criteria may take up to 10 months after initial drug administration [10••, 11]. In their study, van der Veldt et al. [10••] showed that at first response, 13% of patients with mRCC treated with sunitinib had partial response (PR), 69% had stable disease (SD), and 18% had progressive disease (PD) as per the RECIST criteria; however, 18% of patients with initial SD reached a PR at a later time. Another recent study by Abel et al. [12] concluded that decrease in primary tumor diameter above 30% is rare in patients with mRCC treated with targeted therapy, with most patients demonstrating minimal or no decrease in primary tumor diameter. These studies emphasize the importance of development of new evaluation criteria in early assessment of treatment response in mRCC patients, which includes size as well as tumor morphology. Thiam et al. [13] have attempted to determine the size threshold for CT evaluation of mRCC, which may best reflect treatment response; they concluded that a relative reduction of 10% size of the sum of the largest diameters of the targeted lesions appears to be a reliable threshold for identifying responders instead of the 30% threshold used in RECIST for PR. This view has been corroborated by the findings of a recent retrospective study by Krajewski et al. [14], who found that a 10% reduction in sum of the longest diameters on the first follow-up CT is an optimal early predictor of positive outcome in patients with mRCC receiving VEGF inhibitors.
Choi et al. [15] developed new criteria to assess treatment response in patients with gastrointestinal stromal tumors receiving imatinib, which includes changes in size or changes in tumor attenuation expressed as Hounsfield units (HU) on CECT [15]. Van der Veldt et al. [10••] concluded that Choi’s criteria were useful for early prediction of clinical outcome in mRCC treated with sunitinib. Another study by Hittenger et al. [16] showed that Choi’s criteria defines more mRCC patients as partial responders at early stages of therapy than with RECIST; however, this was not an effective selection for patients with prolonged median survival. Nathan et al. [17] have evaluated modified Choi’s criteria in which changes of both size and attenuation of target lesions have to be considered to define an objective response. In their study, they concluded that both size and arterial phase density of metastatic RCC lesions treated with TKIs correlate well with the median time to progression, while RECIST and standard Choi criteria appear inferior [17].
Size and attenuation CT (SACT) criteria have been developed recently to improve treatment response assessment in mRCC patients on targeted therapies [18]. According to these criteria, a favorable response is defined as appearance of no new lesions and any of the following: 1) 20% or greater decrease in tumor size; 2) 10% or greater decrease in tumor size half or more of the non-lung target lesions with 20 HU or greater decreased mean attenuation; and 3) one or more non-lung target lesions with 40 HU or greater decreased mean attenuation. An unfavorable response is identified with any of the following: 1) 20% or greater Increase in tumor size; and 2) new metastases, marked contrast-fill in of the target lesions, or new enhancement in a previously homogenously hypoattenuating nonenhancing mass [18]. Mean attenuation measurements are based on three-dimensional volumetric measurements and lung lesions are not eligible as target lesions because of inconsistent mean attenuation results from averaging between soft tissues and air; this is a potential limitation of this criteria because lung is a common site of involvement in mRCC patients. In their study, Smith et al. [18] showed that a favorable response based on SACT criteria had a sensitivity of 75% and specificity of 100% for identifying patients with progression-free survival (PFS) of over 250 days, versus 16% and 100% for RECIST, and 93% and 44% for modified Choi criteria. They concluded that use of SACT criteria had markedly improved therapeutic response assessment in metastatic RCC patients [18].
Morphology, Attenuation, Size, and Structure (MASS) criteria have been developed to correct the deficiencies in SACT criteria [19••]. Target lesions are assessed on routine contrast-enhanced CT images obtained during the portal venous phase and there is incorporation of morphology and structure of target lesions in addition to size and attenuation. According to MASS criteria, a favorable response is defined as appearance of no new lesions and any of the following: 1) 20% or greater decrease in tumor size; and 2) one or more predominantly solid enhancing lesions with marked central necrosis or marked decreased attenuation (≥ 40 HU). An unfavorable response is defined as any of the following: 1) 20% or greater increase in tumor size in the absence of marked central necrosis and marked decreased attenuation; and 2) new metastases, marked central fill-in, or new enhancement of a previously homogenously hypoattenuating mass [19••]. Smith et al. [19••] showed that a favorable response according to MASS criteria had a sensitivity of 86% and specificity of 100% in identifying patients with a PFS of over 250 days versus 17% and 100% respectively for RECIST PR. In addition, different categories of response according to these criteria differed significantly from one another with respect to time to progression and disease-specific survival. They concluded that response assessment by MASS criteria is more accurate than SACT, RECIST, or modified Choi criteria in predicting disease outcome and PFS in patients with mRCC.
Recently, Goh et al. [20] tried to assess changes in tumor CT texture as a predictive biomarker of treatment response in mRCC treated with TKIs. Changes in tumor entropy (reflecting texture irregularity) and uniformity were derived with a software algorithm at baseline and after two treatment cycles. In their study, tumor entropy decreased by 3% to 45% and uniformity increased by 5% to 21% after administration of TKIs. They concluded that CT texture analysis reflecting tumor heterogeneity is an independent factor associated with time to progression, and the addition of this to standard response assessment may improve the prediction of response to TKIs in mRCC.
State-of-the-Art Imaging techniques for Quantifying Treatment Response
Dynamic Contrast-Enhanced Computed Tomography
The antiangiogenic effects of targeted therapies are expected to cause reduction in tumor vascularity earlier than morphological changes. Accordingly, a technique capable of quantifying tumor vascularity would be most appropriate for treatment response monitoring. Also known as perfusion CT, DCE-CT is now available for functional assessment of tumor vascularity. An important advantage of DCE-CT is that contrast enhancement is linearly proportional to the iodine concentration; thus, quantitative analysis of tumor vascular parameters is relatively straightforward [21]. In addition, the excellent temporal and spatial resolution of CT provides a method for monitoring the detailed time course change of tissue enhancement. However, the high radiation dose associated with perfusion CT exams is the main disadvantage.
The basic principle of perfusion CT is that the temporal change of tumor enhancement depends on the tissue iodine concentration and is an indirect reflection of tissue vascularity [21]. Blood flow (BF), blood volume (BV), mean transit time (MTT), and permeability surface area product (PS) are the commonly used tissue perfusion parameters calculated using the perfusion CT data. A baseline unenhanced CT is acquired first to select a target lesion for the dynamic acquisition, which is performed in two phases using a cine acquisition. A faster image acquisition scheme is used during initial phase of the bolus to ensure accurate characterization of the rapid delivery of contrast (ie, first pass); typically, one image is obtained every second for a total of about 40 to 60 seconds during this initial phase. A second phase of imaging is performed for calculating permeability measurements by obtaining images every 10 seconds for 2 minutes after the first pass study [21]. A less frequent sampling during the second phase decreases the overall radiation dose of the CT perfusion examination without compromising the quality of the data for kinetic assessment of tumor perfusion. Postprocessing of perfusion CT images is performed on a dedicated workstation using special perfusion software that generates color-coded images based on estimated BV, BF, MTT, and PS (Fig. 1). Tumor blood flow (TBF) is defined as flow rate through the tumor and is measured as milliliters per minute per 100 mL (per 100 g of tissue) and may be a reflection of tumor vascularity and grade. Tumor blood volume (TBV) is measured in milliliters per 100 mL that is defined as volume of flowing blood within the tumor tissue and denotes tumor vascularity. MTT is the average time taken to travel from artery to vein and is related to perfusion pressure. PS is defined as total flux from plasma to interstitial space, and may be an indirect measure of intratumoral immature leaky vessels, and measured in milliliters per minute per 100 mL [21].
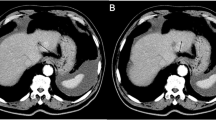
Dynamic contrast-enhanced computed tomography (DCE-CT) for monitoring response to interferon therapy in a patient with metastatic clear cell renal cell carcinoma to the left adrenal gland. Color perfusion CT maps a before and b one cycle after treatment show decreased blood volume within the metastatic deposit
Fournier and colleagues [22••] studied the usefulness of CT-based tumor perfusion parameters in prediction and early detection of antiangiogenic therapy response for mRCC. They found that TBF and TBV in RCC metastases show a 50% decrease after a single cycle of treatment with antiangiogenic drugs, and these values are significantly higher in treatment responders than in nonresponders. They concluded that tumor perfusion parameters determined with DCE-CT can help in predicting biological response to targeted therapy before initiation of the treatment and help in detecting an early response after a single cycle of treatment. However, in their study, there was no significant correlation between patients’ progression-free or overall survival and tumor baseline parameters or early change in parameters after treatment. Another study by Chen et al. [23] showed that TBV, TBF, and PS of RCC correlate positively with MVD and may reflect the tumoral angiogenesis. Ng and colleagues [24], in their study, concluded that highly vascularized metastatic RCC depicts high baseline TBF and shows a worse prognosis; and tumoral perfusion parameters may be used as biomarkers of disease progression.
Dynamic Contrast-Enhanced Magnetic Resonance Imaging
DCE-MRI has the capability of predicting tissue vasculature noninvasively, including tumor angiogenesis and early effects of antiangiogenic therapy [25••]. DCE-MRI is performed by acquisition of sequential MRI images before, during, and after intravenous administration of contrast agent, which should diffuse freely into extracellular space. Contrary to routine contrast-enhanced MRI that provides a snapshot of enhancement at one point of time, DCE-MRI measures variation in vessel enhancement over time and is related to tissue perfusion [26]. DCE-MRI has been emerging as a promising method for monitoring tumor response to targeted therapy [27]. Lack of ionizing radiation and the improved sensitivity of MRI to detect small amounts of intravenous contrast are the potential advantages of DCE-MRI over DCE-CT [28]. Although no standardized DCE-MRI imaging protocols are available currently, three sets of imaging data are usually acquired: 1) localizer sequence; 2) sequence to calculate the baseline T1 values in the tumor; and 3) dynamic imaging data for about 5 to 10 minutes during and after contrast administration [29]. Analysis of imaging data allows the generation of “time-signal” curves, which provide peak enhancement, rate of peak enhancement, and enhancement gradient or signal enhancement ratio [27]. Although these values are valuable and easy to apply, they provide no insight into the underlying physiology and are highly dependent on imaging protocol and scanner [26]. To overcome this problem, a “pharmacokinetic model” has been proposed, which can provide contrast-enhancement parameters that relate to underlying vascular anatomy and physiology [26]. A “two-compartment model” is commonly used for this purpose, which assumes that contrast resides in and exchanges between two compartments in the tumor: the vascular space and extravascular extracellular space (EES) [26]. Based on dynamic images three physiological parameters are calculated, including the volume transfer constant, Ktrans (min-1), between the plasma and the EES; the volume of EES per unit volume of tissue; and the flux rate constant between EES and plasma (Kep) [26]. A change in Ktrans between the pre- and post-treatment DCE-MRI examinations of over 40% is usually considered as a true difference caused by the drug effect [30]. Malignant tumors tend to exhibit a rapid and high level of enhancement (ie, wash-in) followed by a relatively rapid wash-out, whereas benign tumors tend to show slow enhancement and washout [26].
Flaherty et al. [31] conducted a pilot study to assess the role of DCE-MRI in predicting PFS with sorafenib treatment in mRCC patients. They found that Ktrans decreased significantly (60%) after sorafenib treatment. The percent decline in Ktrans as measured by DCE-MRI and change in tumor size by CT scan were significantly associated with PFS. High baseline Ktrans before treatment also was associated with improved PFS [31]. Another study by Hahn et al. [32] showed that DCE-MRI was a good pharmacokinetic marker and that mRCC patients with high baseline Ktrans had a prolonged PFS [32]. A recent study by Hillman et al. [33] assessed DCE-MRI of sunitinib-induced vascular changes to schedule chemotherapy in RCC xenograft tumors. They concluded that DCE-MRI has clinical potential to select the dose and schedule of antiangiogenic therapy to schedule chemotherapy and improve its efficacy.
Arterial Spin Labeling Magnetic Resonance Imaging
Arterial spin labeling (ASL) is an MRI-based technique that can be used to measure tissue blood flow without the administration of exogenous contrast agents [34]. “Magnetic labeling” of nuclear spins of endogenous water in the arterial blood is the basic principle of ASL-MRI that can provide both qualitative and quantitative images of tissue blood flow [34]. Labeling is usually done with either saturating or inverting water protons by using radiofrequency pulses, before they enter the ROI. When labeled arterial blood enters the tissue, the net magnetization of tissue will be reduced compared to controlled condition in which the arterial spins are not labeled. The difference of the magnetic signal between the control image and the labeled image gives the measurement of tissue blood flow [35]. The major advantages of ASL- MRI include 1) lack of intravenous contrast administration, which gives the option of performing multiple measurements at different time points during the treatment; 2) water molecules are freely diffusible into the extracellular space, so that signal intensity on ASL-MRI is directly proportional to blood flow; and 3) lack of ionizing radiation [28]. Depending on the anatomic location of the tumor (eg, abdomen), the ASL perfusion acquisitions may require optimization of the techniques like background suppression and respiratory motion synchronization schemes. Using dedicated software, perfusion images can be generated and blood flow measurements are calculated (Fig. 2) [28].
Arterial spin labeling (ASL) for monitoring response to antiangiogenic therapy in a patient with metastatic clear cell renal cell carcinoma to the liver. a Axial T2-weighted single-shot fast spin echo image demonstrates a large metastatic lesion in the liver measuring 12.8 cm in longest dimension. b ASL image at the same level demonstrates high levels of tumor perfusion. c Axial T2-weighted single-shot fast spin echo image obtained after completion of two cycles of pazopanib therapy re-demonstrates the mass with a longest diameter of 10.4 cm, which represents a 19% decrease in size (ie, stable disease). d ASL image obtained at the same time as c demonstrates absence of tumor blood flow indicating a favorable response to therapy
The feasibility of ASL MRI in estimating blood flow in RCC was assessed by de Bazelaire et al. [36]. In their study, they concluded that with background suppression, the ASL technique is helpful in quantifying TBF in RCC and may be helpful for evaluating tumor response to antiangiogenic drugs. In another study, de Bazelaire and colleagues [37••] used ASL-MRI to assess the response of patients with mRCC to vatalanib. TBF and size by RECIST criteria were evaluated by ASL-MRI immediately before and 1 month after initiation of treatment and these values were compared with bidimensional tumor response at 4 months. The authors concluded that decrease in TBF with vatalanib therapy as shown by ASL-MRI at 1 month was predictive of a favorable clinical outcome, whereas tumor size changes at 1 month were not. Based on these data, the authors concluded that ASL may serve as an early predictor of clinical response to targeted therapy in mRCC [37••]. Recently, Schor-Bardach et al. [38] assessed the response rate to sorafenib therapy for three different human RCC xenografts implanted in nude mice and found excellent correlation between viable tumor at histopathology and the presence of blood flow in the tumor on ASL-MRI [38]. Furthermore, the authors concluded that baseline ASL-MRI was predictive of response to sorafenib [38].
Diffusion-Weighted Magnetic Resonance Imaging
Diffusion-weighted MRI (DW-MRI) is an MRI technique that can help in noninvasive characterization of biological tissues on the basis of water diffusion properties. Based on motion of water molecules within the tissues, DW-MRI can differentiate highly cellular tissues from acellular tissues, solid masses from cystic masses, and can monitor change in tumoral cellularity during treatment [27]. DW-MRI sequences are acquired after application of two additional symmetrical gradients around 180° refocusing pulse in conventional spin-echo imaging, which allows measurement of the microscopic movement of water molecules [27]. Apparent diffusion coefficient (ADC) values can be calculated in the ROI by acquiring multiple images with different amplitudes or duration of different gradients (b values). Tissues with high cellularity or decreased interstitial spaces (such as tumors) show restricted diffusion that results in low ADC values compared to tissues with low cellularity or increased interstitial space [28].
The capability of DW-MRI to act as a potential biomarker in predicting and monitoring tumor response to targeted therapy has been described in literature [39, 40]. However, to our knowledge, the role of DW-MRI in assessing response to antiangiogenic drugs in patients with mRCC has not been reported. Several studies have concluded that ADC values can clearly differentiate benign from malignant tumors and may be helpful in characterization of different histological subtypes of RCCs [41, 42]. Treatment-induced tumor cell lysis and necrosis can cause increase in ADC values that may predict changes in tumor volume. This makes DW-MRI a potential biomarker for early response to treatment. In addition, a positive association between therapy-induced elevation in ADC values and better clinical outcomes has been described in certain treatments [43, 44]. A recent pilot study by Morgan et al. [45] showed that DW-MRI has potential for monitoring patients with early prostate cancer who opt for active surveillance. Further studies are warranted to evaluate the role of DW-MRI as a biomarker for response to targeted therapy in mRCC.
Dynamic Contrast Enhanced-Ultrasonography
DCE-US permits mapping of tumor macro- and microcirculation and can be performed using two different approaches. The first method involves the bolus injection of microbubbles (ultrasound contrast agent [UCA]) and time intensity curve (TIC) analysis; most clinical studies are based on this method. A single-plane imaging is usually performed with 4 frames per second over the duration of the UCA enhancement. The average intensity using the raw linear data within an ROI can be displayed as a function of time(ie, a TIC that describes the wash-in and wash-out of the contrast agent in the ROI) [46]. The other method consists of intravenous infusion drip using a pump over 5 to 20 minutes and disruption-replenishment analysis. UCA is first imaged without being disrupted at a low mechanical index (MI), then the MI is increased for a few frames causing bubble disruption; immediately after that, the MI is returned to the nondisrupting level to observe the replenishment of the microbubbles into the ROI (Fig. 3) [47].
Dynamic contrast-enhanced ultrasound (DCE-US) for early treatment response assessment to sunitinib in a patient with metastatic renal cell carcinoma to the abdominal wall. a B-mode, DCE-US, and corresponding CT performed before treatment show high vascularity within the tumor. b DCE-US performed at day 7 demonstrates the beginning of necrosis within the tumor. c Contrast uptake curves with a strong decrease in AUC with a CT scan performed at 3 months. AUC—area under the curve; CT—computed tomography
Initial monitoring for tumor response with contrast-enhanced US relied on qualitative analyses in mRCC [48, 49], gastrointestinal stromal tumor (GIST) [50], and sarcoma [51], but new methodologies using the raw linear data have been developed to produce more robust and semiquantitative indices. Analyses including wash-in and wash-out times can be performed with curve fitting to determine functional parameters. The main parameters include peak intensity, AUC, area under the wash-out (AUWO), and area under the wash-in (all corresponding to blood volume); time to peak intensity and slope of the wash-in (both corresponding to blood flow); and MTT. No permeability information can be obtained because of the pure blood pool nature of microbubbles [52]. More recently, studies using semiquantitative techniques with bolus injection in RCC, hepatocellular carcinoma (HCC) [53], and GIST [54] have been published. These studies showed that two parameters representing blood volume (AUC and AUWO) correlated with the RECIST response. For the study focus on RCC, Lassau et al. [55••] demonstrated a correlation with PFS and overall survival [55••]. A French multicenter study performed in 19 centers with 65 radiologists and involving many tumors, including mRCC, GIST, colorectal metastases, melanoma, breast cancer, and HCC, treated with antiangiogenic therapies has been ongoing. The preliminary results (400 patients) found that the AUC is one of the parameters that correlated to response at 6 months in patients characterized as good responders or poor responders [56]. There is now emerging evidence with these standardized studies including more than 600 patients that indicate DCE-US may be used with appropriate tools to differentiate between responders and nonresponders at an earlier stage than conventional methods, and this allows tailoring of the treatment regimen, particularly changing treatment of nonresponders or adapting dose according the toxicity [57]. DCE-US has been endorsed by the European Medical Oncology Society to assess response under biological therapy for GIST [58] and is now implemented in the European Federation of Societies for Ultrasound in Medicine and Biology 2011 guidelines for the monitoring of treatments, with a level A;1b recommendation according the Oxford recommendations for this indication [59].
Positron Emission Tomography
Positron emission tomography (PET) imaging has been used as an important functional imaging technique that can assess tissue metabolism by using radiolabeled molecules. 18-F fluorodeoxyglucose (FDG) is a glucose analogue that is commonly used as a radiotracer in PET studies. 18-F FDG shows an increased uptake in metabolically active cells that utilize glucose. Given the fact that most malignant cells have high glucose consumption, they show higher uptake of FDG on PET images and uptake is proportional to the proliferation and number of viable cells in the tumor. The standardized uptake value is based on a ratio between tracer uptake in the tumor and heterogenous distribution of tracer within the patient; it represents quantitative assessment of the tracer uptake. The major limitation of PET is the identification of anatomical landmarks. The development of PET-CT scanners is mainly to overcome this limitation and to integrate the anatomical data from CT with the functional images obtained from PET that can enhance the speed and quality of images obtained.
Because the FDG uptake is limited in RCC metastases, FDG PET has no major role in imaging of mRCC [60]. However, when mRCC shows an increased uptake of FDG, PET may play an important role in the response evaluation of antiangiogenic therapy [61]. Minamimoto et al. [62] evaluated the usefulness of FDG PET-CT for mRCC in evaluating early response to TKIs and in predicting PFS. The authors concluded that PET-CT has potential value for evaluating early treatment response to TKIs in mRCC and in predicting PFS; however, further studies are needed to verify these findings [62]. In a recent study, Revheim et al. [63] evaluated the clinical benefit of PET-CT in mRCC treated with sunitinib and concluded that high baseline FDG uptake indicates aggressive disease and that the degree of reduction in FDG uptake after sunitinib treatment adds valuable prognostic information.
In addition to the commonly used radiotracer 18-F FDG, other PET traces are being evaluated in patients with mRCC [64, 65]. Middendorp et al. [64] evaluated fluoroethyl choline (FEC) PET-CT in mRCC treated with TKIs and concluded that treatment monitoring may be feasible with FEC PET. Hugonnet and colleagues [65] studied the usefulness of fluoromisonidazole PET-CT in mRCC treated with sunitinib. They concluded that sunitinib induced hypoxia in initially hypoxic target metastasis but did not induce significant hypoxia in nonhypoxic target lesions. Patients with initially hypoxic targets have stronger PFS than others [65].
Conclusions
New response evaluation criteria and functional imaging techniques are being developed for early, reliable, and accurate assessment of treatment response to molecularly targeted therapy in patients with mRCC. While RECIST criteria is based on change in tumor size, newer criteria such as SACT and MASS incorporate size as well as treatment-induced morphological changes of the tumor that allow early prediction of disease outcome in these patients. Functional imaging techniques that may have a great potential to be used as biomarkers of early tumor response in mRCC, such as DCE-CT, DCE-MRI, DCE-US, ASL-MRI, DW-MRI, and PET, are being investigated. Although these imaging modalities are not widely available for clinical use at present, in the near future, they are going to play a seminal role in the evaluation and surveillance of treatment response in mRCC patients treated with molecularly targeted therapies.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–90.
Ather MH, Masood N, Siddiqui T. Current management of advanced and metastatic renal cell carcinoma. Urol J. 2010;7:1–9.
Brugarolas J. Renal-cell carcinoma–molecular pathways and therapies. N Engl J Med. 2007;356:185–7.
Cho D, Signoretti S, Regan M, et al. The role of mammalian target of rapamycin inhibitors in the treatment of advanced renal cancer. Clin Cancer Res. 2007;13:758s–63s.
Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–49.
Herrmann E, Bierer S, Wulfing C. Update on systemic therapies of metastatic renal cell carcinoma. World J Urol. 2010;28:303–9.
Shanbhogue AK, Karnad AB, Prasad SR. Tumor response evaluation in oncology: current update. J Comput Assist Tomogr. 2010;34:479–84.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised recist guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
van der Veldt AA, Meijerink MR, van den Eertwegh AJ, et al. Targeted therapies in renal cell cancer: recent developments in imaging. Target Oncol. 2010;5:95–112.
•• van der Veldt AA, Meijerink MR, van den Eertwegh AJ, et al.: Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Br J Cancer 2010;102:803–809. This study demonstrated the drawbacks of RECIST criteria in response evaluation assessment of metastatic RCC to antiangiogenic therapy.
Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase iii trial. Lancet. 2008;372:449–56.
Abel EJ, Culp SH, Tannir NM, et al. Primary tumor response to targeted agents in patients with metastatic renal cell carcinoma. Eur Urol. 2011;59:10–5.
Thiam R, Fournier LS, Trinquart L, et al. Optimizing the size variation threshold for the CT evaluation of response in metastatic renal cell carcinoma treated with sunitinib. Ann Oncol. 2010;21:936–41.
Krajewski KM, Guo M, Van den Abbeele AD, et al. Comparison of four early posttherapy imaging changes (Eptic; RECIST 1.0, tumor shrinkage, computed tomography tumor density, Choi criteria) in assessing outcome to vascular endothelial growth factor-targeted therapy in patients with advanced renal cell carcinoma. Eur Urol. 2011;59:856–62.
Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–9.
Hittinger M, Staehler M, Schramm N, et al.: Course of size and density of metastatic renal cell carcinoma lesions in the early follow-up of molecular targeted therapy. Urol Oncol 2011.
Nathan PD, Vinayan A, Stott D, et al. CT response assessment combining reduction in both size and arterial phase density correlates with time to progression in metastatic renal cancer patients treated with targeted therapies. Cancer Biol Ther. 2010;9:15–9.
Smith AD, Lieber ML, Shah SN. Assessing tumor response and detecting recurrence in metastatic renal cell carcinoma on targeted therapy: importance of size and attenuation on contrast-enhanced CT. AJR Am J Roentgenol. 2010;194:157–65.
•• Smith AD, Shah SN, Rini BI, et al.: Morphology, attenuation, size, and structure (MASS) criteria: Assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. AJR Am J Roentgenol 2010;194:1470–1478. This reference proposed the new response evaluation criteria (MASS) for metastatic RCC patients receiving targeted therapies.
Goh V, Ganeshan B, Nathan P, et al. Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology. 2011;261:165–71.
Kambadakone AR, Sahani DV. Body perfusion CT: technique, clinical applications, and advances. Radiol Clin North Am. 2009;47:161–78.
•• Fournier LS, Oudard S, Thiam R, et al.: Metastatic renal carcinoma: Evaluation of antiangiogenic therapy with dynamic contrast-enhanced CT. Radiology 2010;256:511–518. The authors evaluated the role of perfusion CT in early response assessment of metastatic RCC to antiangiogenic therapy.
Chen Y, Zhang J, Dai J, et al. Angiogenesis of renal cell carcinoma: perfusion CT findings. Abdom Imaging. 2010;35:622–8.
Ng CS, Wang X, Faria SC, et al. Perfusion CT in patients with metastatic renal cell carcinoma treated with interferon. AJR Am J Roentgenol. 2010;194:166–71.
•• Rosen MA, Schnall MD: Dynamic contrast-enhanced magnetic resonance imaging for assessing tumor vascularity and vascular effects of targeted therapies in renal cell carcinoma. Clin Cancer Res 2007;13:770 s-776s. This reference showed the importance of DCE-MRI in assessing RCC vascularity and vascular effects of targeted therapies.
Choyke PL, Dwyer AJ, Knopp MV. Functional tumor imaging with dynamic contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging. 2003;17:509–20.
Harry VN, Semple SI, Parkin DE, et al. Use of new imaging techniques to predict tumour response to therapy. Lancet Oncol. 2010;11:92–102.
Pedrosa I, Alsop DC, Rofsky NM. Magnetic resonance imaging as a biomarker in renal cell carcinoma. Cancer. 2009;115:2334–45.
O'Connor JP, Jackson A, Parker GJ, et al. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer. 2007;96:189–95.
Jackson A, O'Connor JP, Parker GJ, et al. Imaging tumor vascular heterogeneity and angiogenesis using dynamic contrast-enhanced magnetic resonance imaging. Clin Cancer Res. 2007;13:3449–59.
Flaherty KT, Rosen MA, Heitjan DF, et al. Pilot study of DCE-MRI to predict progression-free survival with sorafenib therapy in renal cell carcinoma. Cancer Biol Ther. 2008;7:496–501.
Hahn OM, Yang C, Medved M, et al. Dynamic contrast-enhanced magnetic resonance imaging pharmacodynamic biomarker study of sorafenib in metastatic renal carcinoma. J Clin Oncol. 2008;26:4572–8.
Hillman GG, Singh-Gupta V, Al-Bashir AK, et al. Monitoring sunitinib-induced vascular effects to optimize radiotherapy combined with soy isoflavones in murine xenograft tumor. Transl Oncol. 2011;4:110–21.
Martirosian P, Boss A, Schraml C, et al. Magnetic resonance perfusion imaging without contrast media. Eur J Nucl Med Mol Imaging. 2010;37 Suppl 1:S52–64.
Pedrosa I, Rafatzand K, Robson P, Wagner AA, Atkins MB, Rofsky NM, Alsop DC: Arterial spin labeling MR imaging for characterisation of renal masses in patients with impaired renal function: initial experience. Eur Radiol 2011.
De Bazelaire C, Rofsky NM, Duhamel G, et al. Arterial spin labeling blood flow magnetic resonance imaging for the characterization of metastatic renal cell carcinoma(1). Acad Radiol. 2005;12:347–57.
•• de Bazelaire C, Alsop DC, George D, et al.: Magnetic resonance imaging-measured blood flow change after antiangiogenic therapy with PTK 787/zk 222584 correlates with clinical outcome in metastatic renal cell carcinoma. Clin Cancer Res 2008;14:5548–5554. The authors evaluated ASL-MRI in evaluating blood flow change after antiangiogenic therapy to metastatic RCC.
Schor-Bardach R, Alsop DC, Pedrosa I, et al. Does arterial spin-labeling MR imaging-measured tumor perfusion correlate with renal cell cancer response to antiangiogenic therapy in a mouse model? Radiology. 2009;251:731–42.
Patterson DM, Padhani AR, Collins DJ. Technology insight: water diffusion MRI–a potential new biomarker of response to cancer therapy. Nat Clin Pract Oncol. 2008;5:220–33.
Byun WM, Shin SO, Chang Y, et al. Diffusion-weighted MR imaging of metastatic disease of the spine: assessment of response to therapy. AJNR Am J Neuroradiol. 2002;23:906–12.
Wang H, Cheng L, Zhang X, et al. Renal cell carcinoma: diffusion-weighted MR imaging for subtype differentiation at 3.0 t. Radiology. 2010;257:135–43.
Razek AA, Farouk A, Mousa A, et al. Role of diffusion-weighted magnetic resonance imaging in characterization of renal tumors. J Comput Assist Tomogr. 2011;35:332–6.
Huang CF, Chiou SY, Wu MF, et al. Apparent diffusion coefficients for evaluation of the response of brain tumors treated by gamma knife surgery. J Neurosurg. 2010;113(Suppl):97–104.
Roth Y, Tichler T, Kostenich G, et al. High-b-value diffusion-weighted MR imaging for pretreatment prediction and early monitoring of tumor response to therapy in mice. Radiology. 2004;232:685–92.
Morgan VA, Riches SF, Thomas K, et al. Diffusion-weighted magnetic resonance imaging for monitoring prostate cancer progression in patients managed by active surveillance. Br J Radiol. 2011;84:31–7.
Peronneau P, Lassau N, Leguerney I, et al. Contrast ultrasonography: necessity of linear data processing for the quantification of tumor vascularization. Ultraschall Med. 2010;31:370–8.
Williams R, Hudson JM, Lloyd BA, et al. Dynamic microbubble contrast-enhanced US to measure tumor response to targeted therapy: a proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapy. Radiology. 2011;260:581–90.
Lamuraglia M, Escudier B, Chami L, et al. To predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib: pilot study using dynamic contrast-enhanced doppler ultrasound. Eur J Cancer. 2006;42:2472–9.
Escudier B, Lassau N, Angevin E, et al. Phase i trial of sorafenib in combination with ifn alpha-2a in patients with unresectable and/or metastatic renal cell carcinoma or malignant melanoma. Clin Cancer Res. 2007;13:1801–9.
Lassau N, Lamuraglia M, Chami L, et al. Gastrointestinal stromal tumors treated with imatinib: monitoring response with contrast-enhanced sonography. AJR Am J Roentgenol. 2006;187:1267–73.
Lassau N, Lamuraglia M, Vanel D, et al. Doppler US with perfusion software and contrast medium injection in the early evaluation of isolated limb perfusion of limb sarcomas: prospective study of 49 cases. Ann Oncol. 2005;16:1054–60.
Cosgrove D, Lassau N. Imaging of perfusion using ultrasound. Eur J Nucl Med Mol Imaging. 2010;37 Suppl 1:S65–85.
Lassau N, Koscielny S, Chami L, et al. Advanced hepatocellular carcinoma: early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification–preliminary results. Radiology. 2011;258:291–300.
Lassau N, Chami L, Koscielny S, et al.: Quantitative functional imaging by dynamic contrast enhanced ultrasonography (DCE-US) in gist patients treated with masatinib. Invest New Drugs 2010.
•• Lassau N, Koscielny S, Albiges L, et al.: Metastatic renal cell carcinoma treated with sunitinib: early evaluation of treatment response using dynamic contrast-enhanced ultrasonography. Clin Cancer Res 2010;16:1216–1225. They evaluated DCE-US in early evaluation of treatment response after sunitinib therapy to metastatic RCC.
Lassau N, Chami L, Chebil M, et al. Dynamic contrast-enhanced ultrasonography (DCE-US) and anti-angiogenic treatments. Discov Med. 2011;11:18–24.
Michels J, Lassau N, Gross-Goupil M, et al. Sunitinib inducing tumor lysis syndrome in a patient treated for renal carcinoma. Invest New Drugs. 2010;28:690–3.
Casali PG, Blay JY. Soft tissue sarcomas: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v198–203.
Piscaglia F, Nolsoe C, Dietrich CF, et al.: The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 2011.
Majhail NS, Urbain JL, Albani JM, et al. F-18 fluorodeoxyglucose positron emission tomography in the evaluation of distant metastases from renal cell carcinoma. J Clin Oncol. 2003;21:3995–4000.
Lyrdal D, Boijsen M, Suurkula M, et al. Evaluation of sorafenib treatment in metastatic renal cell carcinoma with 2-fluoro-2-deoxyglucose positron emission tomography and computed tomography. Nucl Med Commun. 2009;30:519–24.
Minamimoto R, Nakaigawa N, Tateishi U, et al. Evaluation of response to multikinase inhibitor in metastatic renal cell carcinoma by fdg pet/contrast-enhanced CT. Clin Nucl Med. 2010;35:918–23.
Revheim ME, Winge-Main AK, Hagen G, et al. Combined positron emission tomography/computed tomography in sunitinib therapy assessment of patients with metastatic renal cell carcinoma. Clin Oncol (R Coll Radiol). 2011;23:339–43.
Middendorp M, Maute L, Sauter B, et al. Initial experience with 18f-fluoroethylcholine PET/CT in staging and monitoring therapy response of advanced renal cell carcinoma. Ann Nucl Med. 2010;24:441–6.
Hugonnet F, Fournier L, Medioni J, et al. Metastatic renal cell carcinoma: relationship between initial metastasis hypoxia, change after 1 month’s sunitinib, and therapeutic response: An 18f-fluoromisonidazole PET/CT study. J Nucl Med. 2011;52:1048–55.
Disclosures
V. S. Katabathina: none. Dr. Nathalie Lassau has received honoraria from Pfizer, Bracco Imaging, Toshiba, Novartis, and Hoffman-La Roche. Dr. Ivan Pedrosa has received grants from Pfizer, GlaxoSmithKline, and Amgen. C. S. Ng: none; S. R. Prasad: none.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katabathina, V.S., Lassau, N., Pedrosa, I. et al. Evaluation of Treatment Response in Patients with Metastatic Renal Cell Carcinoma: Role of State-of-the-Art Cross-Sectional Imaging. Curr Urol Rep 13, 70–81 (2012). https://doi.org/10.1007/s11934-011-0233-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11934-011-0233-x