Abstract
Intraperitoneally administrated epithelial cellular adhesion molecule (EpCAM) monoclonal antibody is a therapeutic agent in patients with malignant effusion in several types of carcinoma. However, the role of EpCAM in peritoneal metastasis (PM) lesions and primary lesions of gastric cancer (GC) is still unclear. Therefore, in this study, we investigated EpCAM expression in GC patients with PM. We investigated the expression of EpCAM in 35PM lesions and 104 biopsy samples as primary lesions. Immunohistochemical staining was performed using the Ventana Benchmark XT (Roche Diagnostics) system. EpCAM expression was evaluated by calculating the total immunostaining score, which is the product of the proportion score and the intensity score. Overexpression was defined as a total score greater than 4. All PM specimens showed overexpression of EpCAM, and GC cells in both the surface layer and the deep layer of the PM showed a high expression of EpCAM. Meanwhile, in the biopsy sample, the expression of EpCAM ranged from none to strong. The EpCAM score results for PM specimens and biopsy samples were 11.0 ± 2.0 and 6.9 ± 3.9, respectively. The difference between the scores was statistically significant (P < 0.05). The intraperitoneally administrated EpCAM antibody might have a anti-cancer effect in PM lesions of GC. Additionally, it can be assumed that only GC cells which express a high level of EpCAM might metastasize to the peritoneum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the second most common cause of cancer-related death worldwide [1]. Although surgery is the only curative procedure for localized advanced GC, for metastatic or recurrent GC patients, chemotherapy is the only therapeutic approach.
Recently, a number of new drugs to treat GC have become available. Unfortunately, these agents are not particularly effective, resulting in a high recurrence rate, a low survival rate, and a poor prognosis for metastatic or recurrent GC patients [2]. Additionally, GC patients with peritoneal metastasis (PM) have lower survival rates than other GC patients. In a multicenter prospective study, the median survival time was only 3.1 months for GC patients with PM [3]. Thus, another type of treatment for GC patients, particularly those with PM, is required. For example, target therapies that are associated with the expression of a particular gene may open up a new avenue for cancer treatments.
The epithelial cellular adhesion molecule (EpCAM) is a 39–42-kDa, 314-amino-acid type I transmembrane glycoprotein [4]. EpCAM is detected in the basolateral membrane of the majority of epithelial tissues, and overexpression of EpCAM has been demonstrated in a variety of epithelial cancers [5, 6].
EpCAM has been reported to have effects on cell adhesion, signaling, migration, proliferation, and differentiation, each of which are properties related to metastasis of several types of cancer [7]. In addition, an EpCAM monoclonal antibody, catumaxomab, has been licensed for clinical use in the European Union since 2009 for the intraperitoneal treatment of malignant effusion in patients with EpCAM-positive cells where standard therapy is not available or no longer feasible. Heiss et al. have reported that catumaxomab conferred a puncture-free survival in a prospective randomized phase II/ III trial [8]. Furthermore, a subsequent analysis of the report by Heiss et al. revealed that catumaxomab had a significant overall survival benefit to GC patients [9]. However, the expression of EpCAM on the primary lesions and PM lesions of GC is still unclear. Therefore, in this study, we investigated EpCAM expression in GC patients with PM.
Materials and methods
Surgical specimens
Biopsy samples and specimens of PM were obtained from 35 GC patients during upper gastrointestinal endoscopy and staging laparoscopy conducted in our department between 2008 and 2011. All GC patients lacked non-curative factors, such as distant metastasis to liver, lung, or lymph nodes except for PM. In accordance with the Department of Surgery Kinki University Faculty of Medicine policy, written informed consent was obtained from the patients at the time of initial treatment.
Initial treatment
The initial treatment of these patients consisted of single intraperitoneal administration of paclitaxel followed by sequential systemic chemotherapy with S-1 plus paclitaxel. The details of the treatment regimen were described previously [10].
Immunohistochemical study
All sections were placed on the Ventana Benchmark XT (Roche Diagnostics) for detection of the EpCAM oncoprotein. The sections were dewaxed and then subjected to pretreatment with cell conditioning 1 solution (Roche Diagnostics) for 30 min. Sections were then washed with reaction buffer followed by incubation with the mouse monoclonal primary antibody EpCAM (0.1 μg/mL, Vu1D9, Cell Signaling Technology, USA) for 32 min. On-board detection using ultraView Universal DAB kit (Roche Diagnostics), used in accordance with the manufacturer’s instructions, was used to detect the location of the primary antibody EpCAM.
Immunohistochemical analysis
EpCAM expression was evaluated by calculating the total immunostaining score, which was defined as the product of the proportion score and the intensity score. EpCAM expression was evaluated by the following formula [11]: the proportion score described the estimated fraction of positively stained tumor cells (0, none; 1, < 10 %; 2, 10–50 %; 3, 50–80 %; 4, > 80 %). The intensity score represented the estimated staining intensity (0, no staining; 1, weak; 2, moderate; 3, strong). The total score ranged from 0 to 12. EpCAM overexpression was defined as a total score greater than 4 [12].
Statistical analyses
The statistical software GraphPad Prism 5 (GraphPad Software Inc, USA) was used to analyze data by Fisher's exact test. A difference of P < 0.05 was considered as significant.
Results
Patient characteristics
The patients had a median age of 58.6 years (range 22–75 years). There were ten female and 25 male patients. Borrmann III and IV types accounted for the majority. The details of the main clinicopathological features of patients are presented in Table 1. The median survival time of the 35 patients was 23.4 months.
Expression of EpCAM in biopsy samples of gastric cancer
EpCAM expression in 104 biopsy samples from 35 GC patients was determined with immunohistochemical staining. On average, we investigated 2.97 biopsy samples per patient. EpCAM was located on the membrane of GC cells. We observed a diverse range of EpCAM expression intensities. The staining scores of EpCAM ranged from 0 to 12, with an average score of 6.9 ± 3.9. Eighty samples showed overexpression of EpCAM. Figure 1a, b shows representative cases.
EpCAM expression in a biopsy sample of gastric cancer. a Strong reactivity of EpCAM was visible in most gastric cancer cell membranes in biopsy samples. A representative samples with a score of 12 is shown. b Representative sample of gastric cancer cells in a biopsy sample with no reactivity of EpCAM (scored as 0). EpCAM epithelial cellular adhesion molecule
Expression of EpCAM in PM of gastric cancer
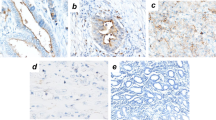
EpCAM expression in 35PM specimens from 35 GC patients was determined with immunohistochemical staining. EpCAM was located not only on the membrane; diffuse staining was also found in the cytoplasm. Strongly positive-staining tumor cells were found in both the surface layer and the deep layer of the peritoneum. The resulting staining scores of EpCAM ranged from 8 to 12, with an average score of 11.0 ± 2.0. All PM specimens were classified as having EpCAM-overexpressing tumors. Figure 2 shows a representative case.
EpCAM expression of gastric cancer cells in a peritoneal metastasis lesion. a High expression of EpCAM is observed in most gastric cancer cells in the peritoneum, scored as 12. b Gastric cancer cells show a high expression of EpCAM in the surface layer of the peritoneum. c Gastric cancer cells also show a high EpCAM expression in the deep layer of the peritoneum. EpCAM reactivity shows the membrane and cytoplasm of tumor cells. EpCAM epithelial cellular adhesion molecule
A significant difference in immunoreactive intensity and average staining score of EpCAM was found between the PM specimens and the biopsy samples (P < 0.05; Table 2).
Discussion
Between 70 and 100 % of tumor cells in malignant effusions from gastric, ovarian, breast, and colorectal cancer have been found to express EpCAM [13–15]. However, the expression of EpCAM in PM lesions has not been defined. In our study, all specimens of PM with GC showed EpCAM overexpression. This is the first report to reveal these results.
In our study, the expression of EpCAM was stronger in the PM lesions than in the primary lesions. The expression of EpCAM in primary lesions was investigated in biopsy samples. The biopsy samples showed a wide range of EpCAM expression. Conversely, in the PM lesions, almost all GC cells showed a strong EpCAM expression. Furthermore, in vitro studies of EpCAM showed enhanced cell proliferation independent of c-myc and cyclin D1/E [16, 17].
Additionally, it was reported that EpCAM negatively modulated cadherin-mediated cell adhesion by disruption of the link between α-catenin and F-actin [18]. Furthermore, EpCAM loosened the tight junctions between cells and modulated proliferation, differentiation, and tissue maintenance [19]. Similar phenomena have already been confirmed in breast and renal cancer [19]. In gastric cancer, overexpression of EpCAM might also disrupt cell–cell contact, enabling the cellular migration that is required for metastasis [19]. Thus, only GC cells whose proliferation was enhanced by EpCAM might metastasize to the peritoneum, as this is one of the most frequent metastatic sites of GC.
GC patients with PM have poorer survival outcomes than other GC patients [3]. To improve the survival rate of GC patients with PM, multidisciplinary methods, including intraperitoneal chemotherapy, hyperthermia, and aggressive surgery, have been used to treat PM [20] [21]. However, these trials did not result in a satisfactory clinical outcome. One of the reasons that PM resists multidisciplinary therapy is due to the stem cell characteristics of the cancer cells. Cancer stem cells are responsible for cancer relapse as they are resistant to conventional cancer therapy, such as chemotherapy and radiation [22, 23]. In our results, all PM specimens showed EpCAM overexpression. EpCAM expression is a biologically and clinically relevant characteristic of cancer stem cells from primary GC tissue [24].Therefore, GC cells in PM lesions may have stem cell-like characteristics. The very poor clinical outcomes in GC patients with PM are consistent with these findings.
To improve treatment outcomes of GC with PM, antibody-based cancer therapies are required. Catumaxomab, which is specific for the EpCAM target antigen, is used to treat cancer patients with malignant ascites in the European Union. The clinical benefit of catumaxomab administered by the intraperitoneal route was demonstrated by prospective randomized phase II/ III trials [8]. The antibody can deliver a deadly signal to the cancer cell only by binding to the surface target. However, it seems that the unsatisfactory antitumor effect of catumaxomab on disseminated lesions in the peritoneum is due to the limited penetration of intraperitoneal catumaxomab into the peritoneal surfaces. Additionally, in our study, GC cells in PMs that expressed EpCAM were present not only in the surface layer but also in the deep layer of the peritoneum. Therefore, intraperitoneally administered catumaxomab may only be effective to treat cancer cells in malignant ascites and in the surface layer of the peritoneum.
To further improve treatment outcomes, the investigation of combination therapies comprising systemic chemotherapy plus intraperitoneal catumaxomab and/or intravenously administered catumaxomab may be necessary. Further investigations are required in the future.
References
Corley DA, Buffler PA (2001) Oesophageal and gastric cardia adenocarcinomas: analysis of regional variation using the Cancer Incidence in Five Continents database. Int J Epidemiol 30(6):1415–1425
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9(3):215–221. doi:10.1016/S1470-2045(08)70035-4
Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, Porcheron J, Peix JL, Francois Y, Vignal J, Gilly FN (2000) Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 88(2):358–363. doi:10.1002/(SICI)1097-0142(20000115)88:2<358::AID-CNCR16>3.0.CO;2-O
Hartung G, Hofheinz RD, Dencausse Y, Sturm J, Kopp-Schneider A, Dietrich G, Fackler-Schwalbe I, Bornbusch D, Gonnermann M, Wojatschek C, Lindemann W, Eschenburg H, Jost K, Edler L, Hochhaus A, Queisser W (2005) Adjuvant therapy with edrecolomab versus observation in stage II colon cancer: a multicenter randomized phase III study. Onkologie 28(6–7):347–350. doi:10.1159/000084595
Went P, Vasei M, Bubendorf L, Terracciano L, Tornillo L, Riede U, Kononen J, Simon R, Sauter G, Baeuerle PA (2006) Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br J Cancer 94(1):128–135. doi:10.1038/sj.bjc.6602924
Varga M, Obrist P, Schneeberger S, Muhlmann G, Felgel-Farnholz C, Fong D, Zitt M, Brunhuber T, Schafer G, Gastl G, Spizzo G (2004) Overexpression of epithelial cell adhesion molecule antigen in gallbladder carcinoma is an independent marker for poor survival. Clin Cancer Res 10(9):3131–3136
Baeuerle PA, Gires O (2007) EpCAM (CD326) finding its role in cancer. Br J Cancer 96(3):417–423. doi:10.1038/sj.bjc.6603494
Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, Dudnichenko AS, Aleknaviciene B, Razbadauskas A, Gore M, Ganea-Motan E, Ciuleanu T, Wimberger P, Schmittel A, Schmalfeldt B, Burges A, Bokemeyer C, Lindhofer H, Lahr A, Parsons SL (2010) The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer 127(9):2209–2221. doi:10.1002/ijc.25423
Linke R, Klein A, Seimetz D (2010) Catumaxomab: clinical development and future directions. MAbs 2(2):129–136
Imano M, Peng YF, Itoh T, Nishikawa M, Satou T, Yasuda A, Inoue K, Kato H, Shinkai M, Tsubaki M, Yasuda T, Imamoto H, Nishida S, Furukawa H, Takeyama Y, Okuno K, Shiozaki H (2012) A preliminary study of single intraperitoneal administration of paclitaxel followed by sequential systemic chemotherapy with S-1 plus paclitaxel for advanced gastric cancer with peritoneal metastasis. Anticancer Res 32(9):4071–4075
Spizzo G, Obrist P, Ensinger C, Theurl I, Dunser M, Ramoni A, Gunsilius E, Eibl G, Mikuz G, Gastl G (2002) Prognostic significance of Ep-CAM AND Her-2/neu overexpression in invasive breast cancer. Int J Cancer 98(6):883–888. doi:10.1002/ijc.10270
Wenqi D, Li W, Shanshan C, Bei C, Yafei Z, Feihu B, Jie L, Daiming F (2009) EpCAM is overexpressed in gastric cancer and its downregulation suppresses proliferation of gastric cancer. J Cancer Res Clin Oncol 135(9):1277–1285. doi:10.1007/s00432-009-0569-5
Passebosc-Faure K, Li G, Lambert C, Cottier M, Gentil-Perret A, Fournel P, Perol M, Genin C (2005) Evaluation of a panel of molecular markers for the diagnosis of malignant serous effusions. Clin Cancer Res 11(19 Pt 1):6862–6867. doi:10.1158/1078-0432.CCR-05-0043
Diaz-Arias AA, Loy TS, Bickel JT, Chapman RK (1993) Utility of BER-EP4 in the diagnosis of adenocarcinoma in effusions: an immunocytochemical study of 232 cases. Diagn Cytopathol 9(5):516–521
De Angelis M, Buley ID, Heryet A, Gray W (1992) Immunocytochemical staining of serous effusions with the monoclonal antibody Ber-EP4. Cytopathology 3(2):111–117
Munz M, Kieu C, Mack B, Schmitt B, Zeidler R, Gires O (2004) The carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferation. Oncogene 23(34):5748–5758. doi:10.1038/sj.onc.1207610
Osta WA, Chen Y, Mikhitarian K, Mitas M, Salem M, Hannun YA, Cole DJ, Gillanders WE (2004) EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res 64(16):5818–5824. doi:10.1158/0008-5472.CAN-04-0754
Winter MJ, Nagelkerken B, Mertens AE, Rees-Bakker HA, Briaire-de Bruijn IH, Litvinov SV (2003) Expression of Ep-CAM shifts the state of cadherin-mediated adhesions from strong to weak. Exp Cell Res 285(1):50–58
Du W, Ji H, Cao S, Wang L, Bai F, Liu J, Fan D (2010) EpCAM: a potential antimetastatic target for gastric cancer. Dig Dis Sci 55(8):2165–2171. doi:10.1007/s10620-009-1033-8
Imano M, Imamoto H, Itoh T, Satou T, Peng YF, Yasuda A, Kato H, Shiraishi O, Shinkai M, Yasuda T, Takeyama Y, Okuno K, Shiozaki H (2012) Safety of intraperitoneal administration of paclitaxel after gastrectomy with en-bloc D2 lymph node dissection. J Surg Oncol 105(1):43–47
Sugarbaker PH, Yonemura Y (2000) Clinical pathway for the management of resectable gastric cancer with peritoneal seeding: best palliation with a ray of hope for cure. Oncology 58(2):96–107
Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS (2006) Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Canc 5:67. doi:10.1186/1476-4598-5-67
Alkatout I, Kabelitz D, Kalthoff H, Tiwari S (2008) Prowling wolves in sheep's clothing: the search for tumor stem cells. Biol Chem 389(7):799–811. doi:10.1515/BC.2008.094
Han ME, Jeon TY, Hwang SH, Lee YS, Kim HJ, Shim HE, Yoon S, Baek SY, Kim BS, Kang CD, Oh SO (2011) Cancer spheres from gastric cancer patients provide an ideal model system for cancer stem cell research. Cell Mol Life Sci 68(21):3589–3605. doi:10.1007/s00018-011-0672-z
Acknowledgments
The authors express their appreciation to Dr. Harumasa Ohyanagi, vice board director of the University of KinDAI Himeji for his expert comments on the manuscript. We also wish to thank Mr. Tadao Uesugi and Miss Fusako Kamada for technical assistance.
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Imano, M., Itoh, T., Satou, T. et al. High expression of epithelial cellular adhesion molecule in peritoneal metastasis of gastric cancer. Targ Oncol 8, 231–235 (2013). https://doi.org/10.1007/s11523-012-0239-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-012-0239-4