Abstract
In this study, the antifungal compound natamycin was encapsulated in methyl-β-cyclodextrin (heptakis(2,6-di-O-methyl)-β-cyclodextrin, Me-β-CD) to improve its aqueous solubility and stability. The aqueous solubilities of natamycin in the presence of β-CD, 2-hydroxypropyl-β-CD, 6-O-α-maltosyl-β-CD, and Me-β-CD were compared. The Me-β-CD showed the best result to increase the solubility of natamycin in aqueous. The pH stability of natamycin was improved by the formation of inclusion complex with Me-β-CD, especially at acidic conditions. The degradation of natamycin under UV-light exposure followed first-order kinetics with half-life times (t1/2) of 59.2 and 157.5 min in pure form and Me-β-CD inclusion complex, respectively. The in vitro antifungal activities of natamycin/Me-β-CD complex against Aspergillus niger food pathogen were evaluated. The results demonstrated that the natamycin/Me-β-CD complex could effectively improve the aqueous solubility and photostability of natamycin without compromising in antifungal activities. Finally, the molecular inclusion mechanisms and geometrical configurations of the natamycin/Me-β-CD complex were studied using molecular dynamics simulations. This research may lead to the development of more effective inclusion-based delivery systems to encapsulate and protect lipophilic antimicrobial agents for food applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Natamycin is an effective food preservative with a broad range of activities against fungal pathogens and has wide regulatory status throughout the world. It inhibits the growth of fungal pathogens at a very low concentration and the production of fungi mycotoxins which may create a public health hazard [1]. However, the very low aqueous solubility (about 30~50 mg/L) and stability of natamycin limit its applications. Developing an effective natamycin carrier with improved solubility and stability is of utmost importance [2, 3].
An important technology in the development of natural ingredients or food additives is the design of effective encapsulated formulations to improve their solubility and stability [4, 5]. In this context, molecular encapsulation in cyclodextrins (CDs) that provide a stabilizing environment for hydrophobic compounds is considered as one of the most relevant techniques [6,7,8]. Pu et al. [7] found that the solubility and stability of tertiary butylhydroquinone could be largely improved by the complexation with CDs. It was shown that the solubility of natamycin could be improved by the formation of natamycin/CDs inclusion complexes in aqueous solution [9]. The natamycin/β-CD and natamycin/γ-CD complexes were also found with a significantly improved photostability than natamycin in its pure form [2]. Recently, the inclusion complex of natamycin with β-CD-acrylamide copolymer was characterized and showed improvement in water solubility and antifungal activity [10].
It has been demonstrated that methylated β-CD has a much lower hygroscopicity and an aqueous solubility up to 50 fold higher than unsubstituted β-CD [11]. In addition, the methylation of β-CD makes the inner surface of the torus more hydrophobic and less steric hindrances that consequently improves the encapsulation efficiency of lipophilic molecules [12,13,14]. Other glycoside β-CD derivatives with better water solubility such as glucosyl-β-CD, and 6-O-α-maltosyl-β-CD were also studied and demonstrated for their high encapsulation efficiency [15, 16]. According to our best knowledge, the types of β-CD derivative used as encapsulating agents for natamycin are limited, and the suitability of natamycin/β-CD derivatives such as methyl-β-CD and the inclusion mechanisms have not been explored.
Besides experimental methods, computational approaches are valuable tools for understanding interaction mechanisms at an atomic level [17,18,19]. Molecular dynamics (MD) simulations have been used to investigate the preferred binding mode of molecules and CDs–guest interactions [19]. It is known that natamycin is a rather bulky molecule with a mycosamine group and a macrocyclic lactone ring, and only a portion of its molecule is encapsulated into the CDs cavity. It is important to keep the mycosamine group intact and freely accessible in the inclusion structures since its binding with fungi ergosterol represents the first and decisive step required for antifungal action [20]. Yet, details on the host-guest interactions and the inclusion modes of natamycin/β-CDs complex are still fragmentary.
The aim of this work was to improve the water solubility, stability and antifungal activity of natamycin by inclusion with β-CD derivatives, and study the inclusion mechanisms from a molecular level. Firstly, the abilities to increase the aqueous solubility of natamycin by four β-CD derivatives including β-CD, 2-hydroxypropyl-β-CD (HP-β-CD), 6-O-α-maltosyl-β-CD (M-β-CD) and heptakis(2,6-di-O-methyl)-β-cyclodextrin (Me-β-CD) were compared. Secondary, the aqueous solubilities of natamycin in the presence of different Me-β-CD concentrations were determined. Then, the pH stability, photostability and the in vitro antifungal activity against Aspergillus niger of natamycin and natamycin/Me-β-CD inclusion complex were tested and compared. Finally, the geometrical configurations of the natamycin/Me-β-CD complex and the inclusion mechanisms were studied using molecular dynamics simulations.
Materials and Methods
Materials
Natamycin was supplied by Zhejiang Sliver-Elephant Bio-engineering (Zhejiang, China). The β-cyclodextrin, HP-β-CD (average degree of substitution is 0.5–1.3 unit of 2-hydroxypropyl per glucose unit) and Me-β-CD (Mw. 1331.36) were purchased from Sigma Aldrich (Shanghai, China). The M-β-CD was purchased from Aladdin (Shanghai, China). Aspergillus niger was conserved by our laboratory. All other reagents used were of analytical grade.
Aqueous Solubilities
A standard curve of natamycin in methanol was prepared beforehand. Excess amounts of natamycin were added to 10 mL of aqueous solutions of β-CD, HP-β-CD, Me-β-CD and M-β-CD. The concentration of different CDs in water was fixed at 8 mM (9.08 × 10−3 g/mL for β-CD, 11.17 × 10−3 g/mL for HP-β-CD, 11.67 × 10−3 g/mL for M-β-CD, and 10.65 × 10−3 g/mL for Me-β-CD). The solutions were then incubated by a laboratory shaker at 200 rpm for 24 h at 20 °C to achieve equilibrium [21]. Finally, the solutions were filtered with a 0.45 μm filter and the quantity of natamycin was measured at 303 nm using a UV-visible spectrophotometer (UV-2600, Shimadzu, Japan).
A similar protocol was used for the determination of natamycin solubility in a high concentration of Me-β-CD. Five Me-β-CD solutions (0.025, 0.075, 0.125, 0.150, and 0.175 g/mL, molecular weight 1331.36) were prepared carefully. Excess amounts of natamycin were added to the solutions and then incubated at 200 rpm for 24 h to achieve equilibrium at 20 °C. The solution was filtered, diluted and subjected to spectrophotometric analysis as described above. All experiments were performed three times and the data were shown as the mean ± standard deviation (SD).
Stabilities
A certain amount of natamycin was diluted in water and 8 mM (10.65 mg/mL) Me-β-CD solution to prepare final concentrations of natamycin of 50 mg/L and 500 mg/L in free and complex states, respectively. The solutions (10 mL) were adjusted to pH values of 1~9 using buffer solutions and storage under dark for 12 h at 20 °C. The solution was filtered, diluted and measured as above by UV spectrophotometer.
The photostability of the natamycin/Me-β-CD complex was assessed using methods from the literature with a minor modification [22]. The free natamycin and complex solution were prepared as above. Equal amounts of the solution (40 mL) were taken into plastic Petri dishes and put under a UV light (365 nm, 50 W) with a vertical distance of 30 cm in a Biological Safety Cabinet (HFsafe-1500, Shanghai, China). The exploration area for each solution is 55 cm2. After exposure for a certain time, an aliquot of each sample (3 mL) was collected, and the concentration of natamycin was measured as above. All the samples were performed in triplicate.
The following Eq. (1) was used to calculate the retention rate of natamycin
where R was retention ratio of natamycin, C0 and Ct was the content of natamycin in solution initially and with irradiation time t, respectively.
Bioassay of the Fungicidal Activities
The fungicidal activities of natamycin and natamycin/Me-β-CD complex against Aspergillus niger were determined by the inhibition zone method. Excess amounts of natamycin were added to 10 mL aqueous solutions containing different concentrations of Me-β-CD (0, 2.67, 5.33, 7.99, and 10.65 mg/mL). The solutions were then agitated at 20 °C to achieve equilibrium as above and filtered. The clear solutions (5.0 mL) were put into a Petri dish (9 cm) and under UV-irradiated in the cabinet (HFsafe-1500) for 6 h. Another five solutions were stored under dark at the same conditions for comparison.
Potato Dextrose Agar (PDA) plates were prepared with a concentration of 39 g/L, and every plate contained 20 mL of PDA medium. A 200 μL of Aspergillus niger (>10 7 CFU/mL) solution was equally spread on each plate. Round filter papers with a diameter of 60 mm were soaked into the solutions of natamycin/Me-β-CD complex including UV-irradiated or not, and then placed in the center of the plates. After that, the plates were incubated in a 34 °C incubator (MJX-160B-Z, Shanghai, China) over 5 days. Finally, the photos of the plates were obtained and analyzed.
Molecular Dynamics Simulations
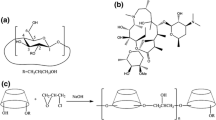
The initial coordinates of natamycin (pimaricin) and Me-β-CD were based on crystal structures from references [19, 23]. The structure of Me-β-CD was rebuilt with methyl substituents at O2 and O6 positions of each glucose unit based on the crystal structure. The number of substituents for constructing Me-β-CD molecule is fourteen. There are two possible orientations as the side of the primary (head, H) or the secondary (tail, T) hydroxyl groups of CD facing the molecule of natamycin. These two orientations were considered which yielding altogether four possible inclusion poses. Four initial configurations (1, 2, 3, and 4) were constructed as shown in Fig. 1. These molecular assemblies were subsequently immersed in a periodic box of TIP3P25 water using the VMD package, with a margin of at least 8 Å from each edge of the box to any atom of the host:guest complex.
All the molecular dynamics simulations were carried out using GROMACS [24], and the GROMOS96 force field was chosen for all MD simulations [25, 26]. MD simulations were conducted for 30 ns. The systems were simulated at a constant temperature of 300 K using V-rescale method [27] and at a constant pressure of 1.0 bar using Parrinello-Rahman method [28] with the time constant 0.1 and 2.0 ps. The trajectories were analyzed using the GROMACS package after MD simulations.
Results and Discussion
Water Solubilities of Natamycin in Complexes
The water solubilities of natamycin in the presence of β-CD, HP-β-CD, M-β-CD and Me-β-CD are shown in Fig. 2a. The solubility of natamycin in pure water at 20 °C is determined as 54.9 ± 1.4 μg/mL. The concentrations of natamycin in water are all significantly increased in the presence of CDs. The aqueous solubilities of natamycin increase by about 6.5, 9.6, 10.3 and 13.7 times in the presence of β-CD, HP-β-CD, M-β-CD and Me-β-CD, respectively. The M-β-CD and HP-β-CD are hydrophilic CD derivatives and show similar results. The Me-β-CD with lipophilic methyl groups gives the best results. Similar results are obtained for the inclusion of other lipophilic compounds by methylated β-CD [29, 30]. It can be attributed to the fact that the methylation of the CD ring extends its hydrophobic cavity and makes the inner surface more hydrophobic [13]. On the other hand, the water solubility of Me-β-CD at room temperature (about 200~250 mg/mL) is by far larger than β-CD (about 18.5 mg/mL). So, the Me-β-CD was selected and tested for the encapsulation of natamycin to improve its aqueous solubility and stability.
a The solubility of natamycin in water at 293 K in the presence of different cyclodextrins including β-cyclodextrin (β-CD), 2-hydroxypropyl-β-cyclodextrin (HP-β-CD), 6-O-α-Maltosyl-β-cyclodextrin (M-β-CD) and heptakis(2,6-di-O-methyl)-β-cyclodextrin (Me-β-CD) (8 mM CDs in aqueous); b The maximum aqueous solubility of natamycin in the presence of different concentration of Me-β-CD at 293 K
The aqueous solubility of a lipophilic compound in the presence of different concentrations of the enhancer is an important property for its applications. The aqueous solubilities of natamycin in the presence of Me-β-CD (0~175 mg/mL) at 20 °C were studied and shown in Fig. 2b. Although the water solubility of Me-β-CD at room temperature is about 200~250 mg/mL, it is not recommended to use at such high concentrations because of the high viscosity. The solubility of natamycin increases linearly with the increasing of Me-β-CD concentrations. The solubility is increased by about 180 times (10.3 ± 0.2 mg/mL) in the presence of 175 mg/mL of Me-β-CD. The minimum inhibitory concentrations (MICs) of natamycin for Aspergillus species and Fusarium species were reported as 2~64 μg/mL and 2~32 μg/mL, respectively [31]. High concentrations of natamycin stock solution which are by far greater than its MICs can be prepared with the help of Me-β-CD. The relationship between the aqueous solubility of natamycin (S, mg/mL) and the concentration of Me-β-CD (C, mg/mL) is obtained as S = −0.000082 × C2 + 0.0646 × C + 0.1574 with R2 of 0.9867. The complexation efficiency defined by Loftsson [32] is calculated as 0.11. That is, about one molecule of natamycin is encapsulated in a complex per ten Me-β-CD molecules [32]. On the other hand, the solubility diagram has a small negative deviation from the linearity indicating that Me-β-CD is less effective at high concentrations. It is supposed that the enhancer or inclusion complex will form aggregates in aqueous, especially at high concentrations. These aggregation behaviors will decrease the chemical potentials of Me-β-CD at high concentrations, which led to negative deviations.
The pH and Photo-Stability of Natamycin
The effects of pH on the stability of natamycin in the presence and absence of Me-β-CD under dark condition are shown in Fig. 3a. The results show that the retention of free natamycin at pH 6 is higher than 95%. The retentions of natamycin in acidic pH condition (1~3) are quite low. It has already been demonstrated that polyene antibiotics including nystatin, amphotericin B and natamycin are unstable and lose their antifungal activity at extremes of pH and light [2, 33]. The retention of natamycin in the inclusion complex was higher than its free state, especially at acidic pH conditions. The results demonstrate that the inclusion complex can retard the degradation of natamycin at harsh pH conditions. In real applications, some juice systems are in low acidic pH range, such as apple juice at about pH 3~4. The natamycin encapsulated in the inclusion complex can increase its chemical stability in these systems.
a The retention ratio of natamycin in aqueous at different pH in free (50 mg/mL) and inclusion (500 mg/mL in presence of 8 mM of Me-β-CD) conditions under dark for 12 h at 293 K; b Natamycin retention ratio vs irradiation time under a UV light (365 nm, 50 W) at 293 K in free and complex conditions and their degradation kinetics
Under UV-light exposure, the natamycin exhibited quite low stability as shown in Fig. 3b. After 3 h irradiation, almost 90% of natamycin was decomposed in the absence of Me-β-CD. However, the natamycin/Me-β-CD complex exhibited better retention ratio. It can be seen that the retention ratio of natamycin after 2 h’ exposure is 20.5 ± 0.9% in pure form and 47.3 ± 1.6% in the complex, respectively. This improvement is due not only to the physical protection barrier but also to the molecular inclusion effects. Similar results were found that the photostabilities of lipophilic compounds were largely improved by the molecular inclusion in CDs [2, 5, 8]. The kinetic parameters (k) and coefficients of determination (R2) for natamycin degradation under UV-light exposure were obtained by fitting to the first-order kinetic model as shown in Fig. 3b. It shows that the degradation of natamycin follows first-order kinetics. The values of half-life time (t1/2) are calculated at 59.2 and 157.5 min for natamycin in pure and complex forms, respectively. The results again demonstrate the protective effect of Me-β-CD inclusion on natamycin degradation against UV irradiation.
Bioassay of the Improvements of Solubility and Photostability
The in vitro antifungal test against Aspergillus niger was performed to verify the solubility increase and activities of the natamycin/Me-β-CD complex. Aspergillus species existed in foods as spoilage fungi. The inhibitions of Aspergillus niger by natamycin are shown in Fig. 4. The diameter of inhibition zones of the natamycin/Me-β-CD complex (Fig. 4b–e) is larger than that of the free natamycin (Fig. 4a), and increases with the increasing of Me-β-CD concentration. This in vitro bioassay also suggests the increasing solubility of natamycin in the presence of Me-β-CD. The results also indicate that the complexes keep the antifungal activities of natamycin. The binding of its mycosamine group with fungi ergosterol represents the first and decisive step required for antifungal action of natamycin [20]. We suppose that only a portion of the macrocyclic lactone ring in natamycin structure is encapsulated into Me-β-CD cavity, and the mycosamine group is intact and freely accessible. However, the complex may also exert its antifungal activity via the disassociated molecules.
Improvement of antifungicidal activity of natamycin against Aspergillus niger with the increase of Me-β-CD concentration in complex solution. Free natamycin was (a), the Me-β-CD concentration of natamycin/Me-β-CD complex solution were (b) 1.0 mM, c 2.0 mM, d 3.0 mM, and (e) 4.0 mM. The (a’-e’) were (a-e) under UV-irradiated
The protective effects of Me-β-CD inclusion on natamycin under UV irradiation were also tested by in vitro bioassay. The antifungal activities of the natamycin/Me-β-CD complexes after irradiation are shown in Fig. 4aʹ–eʹ. As expected, natamycin is inactivated by UV light without Me-β-CD. The natamycin/Me-β-CD complexes under the same UV condition still maintain their antifungal activities against Aspergillus niger. However, the inhibition zones of the natamycin/Me-β-CD complex with UV radiation (Fig. 3bʹ–eʹ) were smaller than that without radiation which indicated that there were some extents of degradation under UV radiation even in the inclusion complexes. Overall, these results demonstrate that the molecular inclusion of natamycin by Me-β-CD can effectively improve its aqueous solubility and photostability without compromising in vitro antifungal activities.
The Inclusion Mechanism by Molecular Dynamics Simulations
Four orientations of the relative positions of two molecules as shown in Fig. 1 were simulated. The root mean square deviation (RMSD) of the backbone of the complex was calculated to estimate the stability of all the conformations [34]. It showed that all the conformations had reached a steady state after 30 ns MD simulation (data not shown).
The centroid distances between the molecule of natamycin and Me-β-CD with time are shown in Fig. 5a. It is found that the complex is formed for all four orientations, and the conformations 1 and 4 show more stable and compact structures. However, the results showed that the natamycin molecule didn’t insert into the cavity of Me-β-CD according to the geometry result (data were not shown) for conformations 2 and 3. There were only conformations 1 and 4 with the complex of natamycin inserted into the cavity of Me-β-CD. So, the potential of mean force (PMF) of conformations 1 and 4 was calculated in order to compare the binding energy (ΔG) of natamycin and Me-β-CD (Fig. 5b). The value of ΔG is −25 kcal/mol for the conformation 4 while it is −15 kcal/mol for the conformation 1. It indicates that the conformation 4 is more feasible than conformation 1. So, as shown in Fig. 1, the natamycin is inserted into the Me-β-CD cavity from head to tail with its hydrophobic section.
a The distances (nm) between natamycin and Me-β-CD with time (ns); b The potential of mean force (PMF) of conformations 1 and 4 with position (nm); c The number of hydrogen bonding of conformations 1 and 4 with simulation time (ns); d The hydrophobic and hydrophilic solvent-accessible surface area (SASA) values of the conformation 4 with simulation time (ns)
Hydrogen bond and hydrophobic interactions are considered as crucial factors for the interaction of complex. The numbers of hydrogen-bond between natamycin and Me-β-CD of the conformations 1 and 4 are shown in Fig. 5c. The average numbers of hydrogen bond of conformation 4 is higher than those of conformation 1. Representation of hydrogen bonds of conformation 1 and 4 are shown in Fig. 6. The hydrogen bonds between natamycin and the inside and outside atoms of Me-β-CD probably increase the stability of the conformation 4 in comparison with the conformation 1.
The hydrophobic and hydrophilic solvent-accessible surface area (SASA) values of the most feasible interaction model (conformation 4) are shown in Fig. 5d. It is observed that the decrease of the hydrophilic SASA occurs more slowly than that of the hydrophobic SASA. It suggests that the binding of natamycin and Me-β-CD is driven by hydrophobic force [34]. It is reasonable that the hydrophobic section of natamycin is included in the hydrophobic cavity of CDs [22] to decrease both the contacting surface to water molecules. These results show that with more feasible hydrogen bonds and hydrophobic interactions, the conformation 4 (the molecular configuration can be seen in Fig. 6b) with natamycin inserted into the hydrophobic cavity of Me-β-CD from head to tail is predominant.
Conclusions
The encapsulation efficiencies of natamycin by β-CD, HP-β-CD, Me-β-CD, and M-β-CD were compared and the natamycin/Me-β-CD complex gave the best results. The relationship between the aqueous solubility of natamycin (S, mg/mL) and the concentration of Me-β-CD (C, mg/mL) at 20 °C was determined as S = −0.000082 × C2 + 0.0646 × C + 0.1574 with R2 of 0.9867. The pH stability of natamycin was improved by the formation of inclusion complex with Me-β-CD, especially at acidic conditions. Photoprotective effect of the Me-β-CD inclusion complex on natamycin was studied. The degradation of natamycin under UV-light irradiation followed the first-order kinetics and the degradation half-life times (t1/2) were calculated as 59.2 and 157.5 min for natamycin in the free and inclusion conditions, respectively. The molecular inclusion of natamycin by Me-β-CD could effectively improve its aqueous solubility and photostability without compromising in vitro antifungal activities. Molecular dynamics simulations showed that the conformation 4 with the structure of natamycin inserted into the hydrophobic cavity of Me-β-CD from head to tail were predominantly formed due to the more feasible hydrogen bonds formation and hydrophobic stacking interactions.
References
C.P.O. Resa, R.J. Jagusb, L.N. Gerschenson, Food Control 35, 101–108 (2014)
J.L. Koontz, J.E. Marcy, J. Agric. Food Chem. 57, 7106–7110 (2003)
E. Cevher, D. Şensoy, M. Zloh, L. Mülazımoğlu, J. Pharm. Sci. 97, 4319–4335 (2008)
S. Ho, Y.Y. Thoo, D.J. Young, L.F. Siow, LWT–Food Sci. Technol. 86, 555–565 (2017)
E.J. Pérez-Monterroza, A.M. Chaux-Gutiérrez, C.M.L. Franco, V.R. Nicoletti, Food Biophys. 13, 343–352 (2018)
S. Ho, Y.Y. Thoo, D.J. Young, L.F. Siow, LWT–Food Sci. Technol. 100, 368–373 (2019)
H. Pu, Q. Sun, P. Tang, L. Zhao, Q. Li, Y. Liu, Food Chem. 260, 183–192 (2018)
A. Celebioglu, Z.I. Yildiz, T. Uyar, J. Agric. Food Chem. 66, 457–466 (2018)
J.L. Koontz, J.E. Marcy, W.E. Barbeau, S.E. Duncan, J. Agric. Food Chem. 51, 7111–7114 (2003)
Y.F. Li, J. Jin, Q. Guo, Y.M. Ha, Q.P. Li, Carbohydr. Polym. 125, 288–300 (2015)
L. Szente, J. Szejtli, Adv. Drug Deliv. Rev. 36, 17–28 (1999)
L. Angiolini, M. Agnes, B. Cohen, K. Yannakopoulou, A. Douhal, Int. J. Pharm. 531(2), 668–675 (2017)
A. Duarte, A. Martinho, A. Luis, A. Figueiras, M. Oleastro, F.C. Domingues, LWT–Food Sci. Technol. 63, 1254–1260 (2015)
L.L. Wang, S.S. Li, P.X. Tang, J. Yan, K.L. Xu, H. Li, Carbohydr. Polym. 129, 9–16 (2015)
B.G. Liu, Y. Li, H.C. Xiao, Y.L. Liu, H.Z. Mo, H.J. Ma, J. Food Sci. 80(6), C1156–C1161 (2015)
B.G. Liu, W. Li, J. Zhao, Y. Liu, X.A. Zhu, G.Z. Liang, Food Chem. 141(2), 900–906 (2013)
Q. Ding, X. Cui, G.H. Xu, C.H. He, K.J. Wu, AICHE J. 64, 4080–4088 (2018)
S. Fang, H.J. Xie, H.Y. Chen, L. Wang, S.Y. Tian, J. Chem. Thermodyn. 113, 144–150 (2017)
J. He, C. Christophe, X.G. Shao, W.S. Cai, J. Phys. Chem. C 118, 24173–24180 (2014)
Y.M. Te Welscher, H.H. Ten Napel, M.M. Balagué, C.M. Souza, H. Riezman, B. De Kruijff, E. Breukink, J. Biol. Chem. 283, 6393–6401 (2008)
J.P. Fan, T.T. Yuan, J.X. Yu, X.H. Zhang, Y.H. Cao, J. Chem. Eng. Data 63, 642–650 (2018)
W. Khuntawe, M. Karttunend, J. Wong-Ekkabut, Phys. Chem. Chem. Phys. 19, 24219–24229 (2017)
P.M. Kells, H. Ouellet, J. Santos-Aberturas, J.F. Aparicio, L.M. Podust, Chem. Biol. 17(8), 841–851 (2010)
M.J. Abraham, T. Murtola, R. Schulz, S. Páll, J.C. Smith, B. Hess, SoftwareX 1(2), 19–25 (2015)
X. Daura, A.E. Mark, W.F. van Gunsteren, J. Comput. Chem. 19, 535–547 (1998)
B. Hess, C. Kutzner, D. van der Spoel, E. Lindahl, J. Chem. Theory Comput. 4, 435–447 (2008)
G. Bussi, D. Donadio, M. Parrinello, J. Chem. Phys. 126, 014101 (2007)
M. Parrinello, A. Rahman, J. Appl. Phys. 52, 7182–7190 (1981)
A.R. Green, J.K. Guillory, J. Pharm. Sci. 78, 427–431 (1989)
G.J. You, L.L. Sun, X.X. Cao, H.H. Li, M. Wang, Y.N. Liu, X.L. Ren, LWT–Food Sci. Technol. 94, 172–177 (2018)
C.Q. Sun, P. Lalitha, N.V. Prajna, R. Karpagam, M. Geetha, K.S. O'Brien, Ophthalmology 121, 1495–1500 (2014)
T. Loftsson, M.E. Brewster, J. Pharm. Sci. 101, 3019–3032 (2012)
J.M. Hamilton-Miller, J. Pharm. Pharmacol. 25, 401–407 (1973)
Z. Shao, S. Fang, Y. Li, J. Chen, Y. Meng, Int. J. Biol. Macromol. 118(Pt B), 2208–2215 (2018)
Acknowledgments
The authors acknowledge support from the Key Research and Development Project of Zhejiang Province, China (2019C02088).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fang, S., Peng, X., Liang, X. et al. Enhancing Water Solubility and Stability of Natamycin by Molecular Encapsulation in Methyl-β-Cyclodextrin and its Mechanisms by Molecular Dynamics Simulations. Food Biophysics 15, 188–195 (2020). https://doi.org/10.1007/s11483-019-09620-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-019-09620-z