Abstract
The rate of almond breakdown during gastric digestion may be influenced by structural changes that occur during roasting. The primary objective of this study was to investigate in vivo physical property changes of raw and roasted almonds during gastric digestion, using the growing pig as a model for an adult human. Seventy two male pigs were fed a meal of raw or roasted almonds and digested samples were taken 20, 60, 180, 300, 480, and 720 min after meal consumption from the proximal and distal stomach regions. Particle size distribution, rheological flow behavior, and textural attributes of gastric digesta were measured. Particle size distributions were fit to the Rosin-Rammler function to determine the median particle diameter (x50) and distribution spread (b) parameters. Median particle diameter was statistically influenced by stomach region (p < 0.0001). Evidence of gastric sieving was observed by an increased median particle diameter and narrower distribution spread in the distal region. To elucidate on textural changes of diced almonds during digestion, an in vitro study was conducted in a static gastric environment. Results indicated that a majority of textural changes occurred during the first hour of digestion, a trend unobserved in the in vivo trial. No significant differences in physical property changes were observed between raw and roasted almonds during gastric digestion in vivo as measured by particle size distribution, textural attributes, and rheological flow behavior. This suggests that raw and roasted almonds break down at a similar rate in the gastric environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Almonds (Prunis dulcis) are a type of tree nut that is consumed worldwide. Central California produced 1.4 billion pounds of almonds in the 2009–2010 growing season, which constituted about 80 % of the global almond production (Kamil & Chen, 2012). Almonds are a good source of bioactive compounds such as monounsaturated oleic acid (ω-9 fatty acid), vitamin E, sterols, and flavonoids [1, 2]. Due to their positive health benefits, almonds have been the subject of many research studies on nutrient availability, release, and absorption during digestion, both in vivo and in vitro [3–8]. These studies have demonstrated the importance of almond cell wall structure on the accessibility of the nutrients present in almonds. The level of physical disruption of cell walls, either by mastication or processing, will clearly influence the digestion process of almonds in the stomach and intestines [6, 8].
Almonds are sold commercially as either raw or roasted almonds. Almonds may be hot-air roasted or oil-roasted [9]. During roasting, the almond physical structure may be altered due to high temperature conditions. Recent studies have used imaging and microscopic techniques to demonstrate that roasting of almonds causes microstructural changes in the almond kernel. Specifically, the cell walls of the inner parenchyma are disrupted [9, 10]. Such physical changes may impact the overall breakdown of almonds during digestion, as well as the rate of release and subsequent bioavailability of nutrients and bioactive compounds.
Physical breakdown of foods during gastric digestion will play an important role in the release of nutritional components inside of the food matrix. However, information on the physical property changes in foods during digestion is limited. The objective of this study was to measure the changes in physical properties of a meal of raw or roasted almonds during gastric digestion. The growing pig was accepted here as a model for an adult human stomach [11, 12]. The rheological properties, textural attributes, and particle size distribution were quantified to determine if almond roasting influenced the physical breakdown process. In addition, an in vitro study was conducted on the textural changes in raw and roasted almonds to clarify the mechanisms of almond textural changes during digestion. This work presents the first extensive quantitation of certain physical properties of a rigid food material (i.e. raw and roasted almonds) during gastric digestion in vivo.
Materials and Methods
Food Material
Medium diced raw and hot-air roasted almonds were kindly provided by the Almond Board of California (Blue Diamond Growers, Sacramento, CA). Both types of almonds were of commercial medium diced size (size range from 3.2 to 8.7 mm).
Almond Composition Analysis
Almond total lipid content was determined using AOAC official method 2003.05. Fiber content was measured using AOAC official method 991.43. Moisture content was measured after heating in a vacuum oven at 100 °C until constant weight (AOAC official method 925.40). Total nitrogen was measured by LECO total combustion [13]. Crude protein was calculated by multiplying the total nitrogen content by a factor of 5.18. Bomb calorimetry was used to determine the gross energy, and ash was determined by heating overnight at 500 °C.
In Vivo Trial Parameters
All animal handling protocols were approved by the Massey University Ethics Committee (Protocol 11/30). Seventy two male pigs (mean bodyweight 23.0 ± 0.2 kg) were housed in individual metabolism crates in a temperature controlled room (23 ± 1 °C) at Massey University, Palmerston North, New Zealand. The pigs were fed according to Table 1 during the test period and were given 30 min to consume each meal before any uneaten portion was removed. Meals were fed daily at 9:00 and 16:00. Meal composition (Table 1) was formulated to meet the dietary requirements of the growing pig, and allow for the pigs to become accustomed to eating almonds, which are not part of a normal pig diet. The quantity of almonds was limited during a majority of the test period to ensure the pigs were not receiving an excess of lipids in their diet. Each pig was randomly assigned to an experimental diet such that half of the pigs received raw almonds (36 total pigs) and the remaining pigs received roasted almonds (36 total pigs). Each pig received only raw or roasted almonds during the entire test period, depending on their experimental diet assignment. Unless otherwise noted in Table 1, water was available ad libitum. On the final day of the study, water was removed at least 2 h prior to feeding. Each pig was fed half of their daily food portion (normal meal) of almonds only, with the addition of 12.5 % meal weight of water. After 30 min, all uneaten food was removed and weighed.
20, 60, 180, 300, 480, or 720 min after the final meal was finished, the pigs were sedated and euthanized as previously described [14]. Each pig was randomly assigned to a digestion time, such that there was an average of six pigs (range: 5–7 pigs) for each digestion time. Gastric chyme samples were taken from the proximal and distal stomach regions as previously described [14]. All samples were kept on ice after sampling and analyzed within 6 h for textural and rheological properties. Samples were stored at 4 °C until particle size distribution measurements.
In Vivo Trial Measurements
Particle Size Distribution
The almond gastric digesta samples were wet sieved to determine the particle size distribution. The following sieve sizes were used: 0.075, 0.15, 0.30, 0.50, 0.71, 1.00, and 2.00 mm (Endecotts, London, UK). Sieves were stacked and approximately 10 g (dry matter) of almond gastric digesta was placed on the top sieve. Samples were rinsed for 5 min with tap water at a flow rate of 185 mL/s. Samples from each sieve were dried for 1 h at 60 °C in a convection oven to remove surface water. Preliminary investigation showed that these drying conditions were sufficient to remove any surface water due to the wet sieving process, but not cause additional drying of the almond pieces. Dried samples collected from each sieve were weighed.
The particle size distribution of the almond diet (raw and roasted, diced almonds) was measured by placing 10 g of almonds on the same sieve stack as used for the almond gastric digesta. The almonds were shaken for 10 min on a sieve shaker (EMS-8, Electrolab, Mumbai, India) on intermittent setting, power = 20. The mass of almonds remaining on each sieve was weighed.
The cumulative percentage of particle mass was fit to the Rosin-Rammler equation [15]:
where Cmass is the cumulative mass percentage (varies from 0 to 100 %) of particles of a size x (mm), x50 is the median particle diameter (mm), and b is a dimensionless constant representing the distribution spread. The parameter b varies from 0 to infinity, where a larger value of b equates to a narrower distribution spread. This expression has been previously used to describe particle breakdown during mastication and industrial processing [15–21].
Rheological Property Measurement
Rheological properties of almond gastric digesta were measured using mixer viscometry techniques. Such methods have proven successful to measure rheological properties of materials containing large particles [22, 23]. All measurements were completed on an ARG2 Rheometer (TA Instruments, New Castle, DE, USA) using a vane rotor geometry. Prior to measurement, samples were equilibrated for 2 min at 37 °C inside of the mixer cup. After equilibration, a shear rate sweep was conducted between 0.01 and 1 s−1, according to the protocol of previous studies on gastric digesta rheological properties [24].
Texture Analysis
A bulk compression method was chosen to determine the textural attributes of the almond gastric digesta, due to the inhomogeneity of the digesta and the desire for an overall measure of textural changes. A similar bulk compression method was previously used to quantify textural changes in walnuts due to roasting [25]. All analyses were completed on a TA.XT2 Texture Analyzer (Texture Technologies Corp., Scarsdale, NY) using a TA-94 bulk compression cell with a 45 mm diameter plunger. Prior to analysis, the bulk compression cylinder was filled to 15 mm height with digesta, and the top of the sample was gently flattened to ensure uniform surface area during compression. The pre-test plunger speed was 2 mm/s, the test speed (during compression) was 1 mm/s, and the post-test speed was 4 mm/s. The compression test began once the plunger came into contact with the sample. Samples were compressed 5 mm (approximately 1/3 of total sample height). Three replicates were completed per sample from each treatment.
In Vitro Trial Parameters
The in vitro static soaking trial was conducted to elucidate key factors that may have attributed to the changes (or lack thereof) of almond digesta textural properties during gastric digestion in vivo. The same raw and roasted medium diced almonds were used in this portion of the study as were used in the in vivo trial. In each experiment, 15 g of almonds were placed into a 200 mL beaker and mixed with 5 mL of simulated saliva (recipe as described by Bornhorst and Singh [26]) for approximately 30 s. This was followed by the addition of 40 mL of simulated gastric juice prepared according to Bornhorst and Singh [26], with the addition of 3.33 g/L lipase according to Roman et al. [27]. Almonds were incubated in a water bath preheated to 37 °C for 5, 15, 30, 45, 60, 120, 240, 480, 720, and 1,440 min.
In Vitro Trial Measurements
Texture Analysis
After soaking, almond samples were passed through a strainer to separate the particles from the unabsorbed simulated gastric juice and saliva. Preliminary trials showed that removal of the unabsorbed digestion fluids gave more uniform textural results. After straining, samples were immediately measured for textural properties following the same procedure as described above for the in vivo samples. All texture measurements were done on 8 replicate samples for each treatment.
Moisture Content Determination
Moisture content was determined according to AOAC Official Method 925.40 [13]. Six replicate samples for each treatment were measured.
Statistical Analysis
Statistical analysis of variance of the in vivo trial data was performed as previously described [14] using SAS Enterprise 4.3 (SAS, Cary, NC, USA). Statistical analysis of the in vitro texture and moisture data was completed using a 2-factor factorial design, with almond type and soaking time as the main effects and a significance level of p < 0.05.
Results & Discussion
Almond Composition Analysis
Raw and roasted almond composition is given in Table 2, and was similar to previously reported values [2]. Raw almonds had a higher moisture content and total dietary fiber content compared to roasted almonds. Roasted almonds had greater lipid content. Both raw and roasted almonds had similar protein and ash contents.
In Vivo Trial
Particle Size Distribution
The breakdown of food during mastication in pigs has not been extensively documented, as the most common method of measurement in humans is the “chew and spit” test. In human in vivo trials, subjects masticate a test food for a certain chewing time (or until a swallow is triggered), then expectorate the chewed food to allow for particle size determination [28, 29]. Due to the practical difficulties of conducting such a study in pigs, knowledge of the breakdown of food during mastication in pigs is quite limited. Pig mastication has been monitored by electromyography, allowing for measurement of the movement of the jaw muscles [30] as well as CT scanning and MR imaging [31], but these techniques have only allowed for the masticatorybiomechanics to be studied, not the chemical and physical breakdown of food that occurs during the mastication process.
Prior to consumption, a majority of the raw and roasted almond diets (undigested almonds) had sizes of greater than 2 mm. To get an estimation of the breakdown of almond particles during mastication, the initial almond diets were compared to the gastric chyme contents after 20 min of digestion (the shortest experimental time point). The percent of particle mass found on each sieve is shown in Fig. 1. It can be observed that after mastication and only a limited amount of time in the stomach, notable structural breakdown occurred. 97 to 100 % of the raw and roasted almond diets had a size greater than 2 mm. After 20 min of digestion, only 34–54 % of the almond gastric digesta still had a size greater than 2 mm, indicating the breakdown to the large almond particles that occurred during mastication and this 20 min period.
The cumulative mass percent of almond digesta on each sieve was fit to the Rosin-Rammler distribution function (Eq. 1) to give a quantitative description of the particle size distribution. The Rosin-Rammler function parameters x50 and b give a measure of the median particle diameter and distribution spread, respectively. The Rosin-Rammler model proved to be a good fit for the particle size data with R2 values ranging from 0.97 to 1.00.
Median particle diameter (x50) was statistically significant across stomach regions (p < 0.0001), with the distal stomach having a larger calculated x50 compared to the proximal stomach (Table 3). The average x50 across all digestion times in the proximal stomach was 1.59 mm for raw almonds and 1.69 for roasted almonds, whereas the average x50 value in the distal stomach was 1.98 mm for raw almonds and 2.21 mm for roasted almonds. Since gastric emptying occurs in the distal region of the stomach, it is hypothesized that the larger median particle size in the distal region was due to the emptying of smaller particles through the pylorus into the small intestine.
The distribution spread (b), was statistically significant with stomach region, almond type, digestion time, stomach region x almond type interaction, and stomach region x digestion time interaction (Table 3). A larger b value indicates a narrower particle size distribution spread. Overall, the distribution spread of raw almonds was less (larger b) compared to roasted almonds. In both raw and roasted almonds, the particle size distribution spread in the distal stomach region was narrower compared to the proximal region. Across all digestion times, raw almonds had a spread parameter (b) of 1.38 in the proximal stomach region and 1.68 in the distal region compared to 1.21 and 1.38 in the proximal and distal stomach regions, respectively, for roasted almonds.
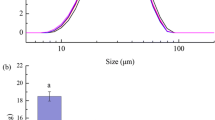
Figure 2 shows an example of the observed cumulative mass percent values and the Rosin-Rammler model predictions for raw and roasted almonds after 720 min of digestion. From this figure, it is clear that after 720 min of digestion, the proximal and distal stomach regions have distinct particle size distributions. The distal region had a larger median particle diameter and smaller distribution spread (larger b value) in both raw and roasted almonds. On average (in raw and roasted almonds), the x50 in the proximal region after 720 min of digestion was 1.72 mm compared to 2.22 mm in the distal region. The distribution spread, or b, was 1.32 in the proximal region and 1.73 in the distal region. The narrower spread in the distal region may be attributed to the emptying of smaller particles from the pylorus to the small intestine. It is also interesting to note that while the spread of the distal region becomes narrower as the digestion process progresses, the spread in the proximal region remains nearly constant.
The particle size distribution data showed clear evidence of gastric sieving of almond particles in the gastric antrum. Gastric sieving is the process by which the gastric antrum retains particles greater in size than 1–2 mm, allowing smaller particles and aqueous solutions to exit the stomach through the pylorus [32]. Gastric sieving has been shown to occur in both human and dog stomachs [33, 34]. Evidence of gastric sieving is demonstrated in the current study by the increase in median particle diameter and narrowing particle size spread in the distal region with increasing digestion time. Although this may be counterintuitive, it must be taken into consideration that as the almond particles are broken down in the gastric antrum, the smallest particles are emptied through the pylorus, leaving only the larger particles in the distal stomach region. However, any small particles in the proximal stomach must wait until they reach the pylorus to be emptied from the stomach. This observation is clearly seen in Fig. 3, which shows an example of almond gastric chyme after 480 and 720 min of digestion. In the distal stomach, there appears to be an accumulation of very large almond particles, but few small particles. This observation was supported by the particle size distribution data described above. The occurrence of gastric sieving was observed similarly in both raw and roasted almonds.
Rheological Properties
Rheological properties of almond gastric chyme were measured using mixer viscometry techniques due to the presence of large almond particles and a limited amount of accessory liquid [22, 23]. Previous studies have demonstrated that gastric chyme for chicken intestinal contents [35] and pig gastric contents behave as Herschel-Bulkely fluids. In order to provide a comparison to previous studies, the shear stress-shear rate data was fit to the Herschel-Bulkely model as previously described [36]. The model parameters and R2 values are given in Table 4. The Herschel-Bulkely model provided a good fit for the gastric chyme from the proximal stomach region, with an average R2 value of 0.80. However, the model did not provide a good fit for the data from the distal region, with an average R2 of 0.42. This discrepancy may be caused by the presence of many large particles in the distal region chyme. Although mixer viscometry was chosen as the measurement method due to the ability to provide accurate data about materials with large particles, there may have been an excess of particles and not enough remaining gastric secretions to provide accurate measurements in the distal region.
Yield stress (σo) is the finite stress necessary to cause a material to flow [23]. The only factor that significantly influenced yield stress in this study was stomach region. In all cases, the almond gastric digesta exhibited a yield stress, with values ranging from 42 to 421 Pa during the 720 min digestion period (Table 4). The gastric chyme from the distal region had a higher yield stress overall than the proximal region, although differences between raw and roasted almonds were not significant, suggesting they follow similar breakdown patterns. The average yield stress (across all digestion times) for raw almonds was 296 Pa in the distal region and 193 Pa in the proximal region. Similarly, the average yield stress for roasted almonds was 268 Pa in the distal region compared to 180 Pa in the proximal region. The larger yield stress measured in the distal chyme may have been caused by the larger median particle size in the distal stomach. In the study of concrete rheology, it has been hypothesized that the yield stress indicates the amount of friction between solid particles [37]. This could mean that the distal region samples which had a larger yield stress encountered a greater amount of friction between the large almond particles.
The almond chyme n values were statistically significant in terms of the stomach region (p = 0.011), with the proximal region having lower n values compared to the distal region (Table 4). The almond chyme exhibited both shear thinning (n < 1) and shear thickening (n > 1) behavior [23], depending on the specific digestion time and stomach region. In raw almond gastric chyme, all samples from the proximal region except after 180 min of digestion and the distal chyme samples from 20 to 300 min of digestion were shear thinning. Their n values ranged from 0.01 to 0.86 (average n = 0.33). In roasted almond gastric chyme, all proximal region samples as well as distal region samples after 20 and 300 min of digestion were shear thinning. Their n values ranged from 0.19 to 0.76 (average n = 0.45). These values are similar in magnitude to n values reported in two previous studies of brown and white rice gastric chyme rheological properties [14, 36].
The almond chyme exhibited shear thickening behavior in the raw almond samples from the proximal region after 180 min digestion and in both the raw and roasted almond chyme samples from the distal region after 60, 180, 480, and 720 min of digestion, with n values ranging from 1.24 to 30.81 (average n = 15.96). The shear thickening behavior of these almond chyme samples may be explained by the high volume fraction of large particles that were present. Previous studies have reported (reversible) shear thickening behavior in concentrated solutions of solid particles. The critical shear rate where the solutions will become shear thickening is governed by a multitude of factors, such as the volume percent of solids, the specific particle size distribution of the solid particles, the particle-particle interactions, and the particle shape [38, 39]. The increase in median particle diameter observed in the distal region from the particle size distribution analysis (Table 3) may help to explain the occurrence of this shear thickening phenomenon in the distal chyme samples. In addition, shear-thickening behavior (n >1) has been previously observed in concrete rheological properties described by the Herschel-Bulkely model [37].
The K value, or chyme consistency index, was statistically significant in terms of stomach region (p < 0.0001) and digestion time (p = 0.0164). K values were greater in the proximal region compared to the distal region, and decreased over time. The average K value (of raw and roasted almonds) in the proximal region decreased from 536 to 447 Pa · sn from 20 to 720 min of digestion, compared with the distal region that decreased from 211 to 102 Pa · sn. The K values observed in the current study are similar to K values reported for rice gastric chyme [14, 36].
To compare the shear stress-shear rate data without taking into consideration the Herschel-Bulkely model parameters, the shear stress value at one shear rate (0.10 s−1) was selected from each data set and compared statistically. Neither almond type, digestion time, nor stomach region were statistically significant (p > 0.05), but the stomach region x digestion time interaction term was significant (p = 0.027). The shear stress at 0.10 s−1 was greater in the proximal region compared to the distal region and decreased to a greater extent in the distal region during the digestion period. An example of a typical shear stress-shear rate curve is given in Fig. 4. The shear stress values (at 0.01 s−1) were greater in the proximal region compared to the distal region at the beginning of the digestion process (20–60 min). At longer digestion times, the distal region had a greater shear stress compared to the proximal region. For example, the shear stress of the raw almond meals was 458 Pa in the proximal region and 384 Pa in the distal region after 20 min of digestion. The shear stress of the roasted almond meals was 534 Pa in the proximal region and 437 Pa in the distal region after 20 min of digestion. After 300 min of digestion, the shear stress in the proximal region was 341 Pa for raw almonds and 333 Pa for roasted almonds. In contrast, the shear stress in the distal region was 487 Pa for raw almonds and 409 Pa for roasted almonds. The observed changes in rheological properties may have been caused by a combination of factors including increased gastric moisture content, changes in particle size distribution, and the presence of other gastric secretions, such as mucins.
Texture Analysis
Textural properties of raw and roasted diced almonds were measured during gastric digestion using a bulk compression method [25]. This method was chosen to obtain an overall understanding of the time dependent textural properties of gastric chyme during digestion. Individual digested almond pieces were not examined because the initial diced almonds were not uniform in shape or size. If an almond fragment was selected from the gastric chyme, it would have been very difficult to determine its initial particle size and the extent of physical breakdown it may already have experienced, making individual measurements less meaningful compared to measurements on the bulk gastric chyme.
The measured textural parameters were the peak force (N) and the compression work, or the positive area under the compression curve (N · s), which are given in Table 5. The peak force was not significantly different between almond type, digestion time, or stomach region (p > 0.05). Compression work was significantly different across stomach regions (p = 0.0003). Compression work was generally larger in the proximal stomach compared to the distal stomach, with values ranging from 165 to 286 N · s in the proximal region compared to values of 116 to 223 N · s in the distal region.
Overall, the texture measurements (Table 5) did not allow for a comprehensive analysis of almond breakdown during gastric digestion due to the high variability across treatments, with an average coefficient of variation of 21.3 % for peak force and 19.2 % for compression work. This large variation may suggest that the real changes in textural properties were masked due to other uncontrollable in vivo parameters or highly variable measurement techniques. A follow-up in vitro study was conducted (see next section) to help understand possible causes of bulk textural changes of diced almonds during digestion.
In Vitro Trial
Texture Analysis and Moisture Content Determination
To further elucidate upon the mechanisms and trends of bulk textural changes in diced raw and roasted almonds during digestion, an in vitro study was conducted in a static gastric environment. The in vitro study did not attempt to mimic the physical breakdown that occurs in the stomach, but instead to determine the influence of immersion into the acidic and enzymatic (pepsin and lipase) environment of gastric secretions. The textural parameters analyzed from the in vitro study were the same as the in vivo study; the peak force (N) during compression as well as the compression work (N · s). In addition, the moisture content of the almonds was measured to allow for a comparison of the liquid absorption in relation to the textural changes in the almond pieces.
Peak force during compression was significantly influenced by digestion time (p < 0.0001) and almond type x digestion time interaction (p = 0.0011). Peak force decreased from 48 N after 5 min of static digestion to 18 N after 1,440 min in raw almonds and from 77 to 26 N over the same time period in roasted almonds. Roasted almonds had a larger initial peak force compared to raw almonds (340 N for roasted and 259 N for raw almonds). Over the 1,440 min digestion period, roasted almonds showed a more rapid decrease in peak force with increased digestion time than raw almonds. However, at the longest soaking times (720 and 1,440 mins), raw and roasted almonds had a similar average peak force (20 N for raw and 22 N for roasted almonds). Compression work was significantly influenced by digestion time (p < 0.0001) and the almond type x digestion time interaction (p = 0.0048). Similar trends in compression work were seen. One contrasting observation is that the compression work for raw and roasted almonds did not become similar after 1,440 mins of soaking, with average compression work values of 37 N for roasted almonds and 27 N for raw almonds.
Almond roasting has been reported to result in cell wall disintegration and damage to the cytoplasmic network [9], which may alter diffusion patterns of liquids into the solid food matrix. These microstructural changes could account for the differences seen between almond textural properties during most of the in vitro digestion period. However, the results from the current study demonstrate that the long-time peak force (after 1,440 mins) is similar between raw and roasted almonds, but the compression work is greater in roasted almonds compared to raw almonds after 1,440 mins. These differences suggest that further investigation into the role of microstructure in the breakdown of almonds during the digestion process is warranted. In addition, it has been shown that the almond cell walls (i.e. dietary fiber) play a key role in influencing the release of nutrients from the almond kernel [4, 7]. Without some type of physical disruption, the cell walls will remain largely intact suggesting that the static in vitro digestion model does not accurately mimic the actual digestion process because it does not provide any physical damage to the almond pieces by mastication or peristaltic movement. However, the in vitro static model was able to show that there are large textural changes in diced almonds simply due to the absorption of gastric juice without any additional physical breakdown.
When compared to the in vivo data, the in vitro textural parameters are of similar order of magnitude, but smaller in value. For example, after 60 min of digestion the average peak force value for raw almonds was 83 N (in vivo) compared to 30 N (in vitro). The in vitro texture data (Table 6) indicated that a majority of the textural changes occurred within the first hour of in vitro digestion when only liquid absorption is concerned. On average, 97 % of the changes in peak force and compression work with respect to the undigested almonds occurred during the first hour of in vitro digestion. In addition, the in vitro study also demonstrated that during the first 15 min, the peak force decreased 84 % from its initial (undigested almonds) value. This suggests that only having one experimental time point shorter than 60 min in the in vivo trials (20 min) left many of the key textural changes occurring due to the absorption of gastric juice unobserved in the in vivo study. This experimental time point limitation in the in vivo study was due to logistical constraints. However, this leaves many opportunities for future studies of texture changes in almonds during digestion. These studies should keep in mind that many changes may occur during the first hour of gastric digestion.
As shown in Table 6, peak force and compression work decreased in both raw and roasted almonds and the moisture content increased with increasing digestion time. Moisture content was statistically significant in terms of digestion time, almond type, and time x almond type interaction (p < 0.0001). The initial moisture content of raw almonds was greater than that of roasted almonds (4 % vs. 1.2 % for raw and roasted almonds, respectively see Table 2). Throughout the entire digestion period studied, raw almonds maintained a greater moisture content than roasted almonds, increasing from 23.0 to 46.9 % over 1,440 mins compared to an increase of 17.6 to 44.5 % in roasted almonds. The absorption of gastric secretions may have played a role in the changes in textural properties observed during this in vitro study. The specific interactions between moisture absorption, acid/enzymatic hydrolysis, and almond textural changes during digestion are an interesting topic that merits future study.
Conclusions
This study has presented the first extensive quantitation of selected physical properties of a rigid food matrix (raw and roasted almonds) during gastric digestion in vivo. The observed particle size distributions in the stomach fit the Rosin-Rammler distribution function. Particle size distributions changed with digestion time, showing evidence of gastric sieving in both raw and roasted almonds, as demonstrated by increased median particle diameter and a narrower distribution spread in the distal stomach over increased digestion time. Observed changes in textural (peak force and compression work during bulk compression) and rheological properties of the in vivo gastric chyme did not show consistent trends over the 720 min digestion period. However, in vitro static soaking experiments demonstrated that a majority of the texture and moisture content changes in almonds occurred during the first hour of static soaking. This suggests that due to the experimental sampling points in the in vivo study, initial changes in almond textural and rheological properties may not have been measured. For a future in vivo study, it is recommended that a larger number of time points should be selected, if logistically feasible, during the beginning portion of the digestion process, as this seems to be the period when the largest changes in texture and rheology occur.
References
C.-Y. Chen, K. Lapsley, J. Blumberg, J. Sci, Food Agric. 86(14), 2245–2250 (2006)
S. Yada, K. Lapsley, G. Huang, J. Food Compos. Anal. 24(4–5), 469–480 (2011)
G. Mandalari, G. Bisignano, M.S.J. Wickham, Clin. Transl. Allergy 1, 20 (2011)
G. Mandalari, R. Faulks, G.T. Rich et al., J. Agric. Food Chem. 56, 3409–3416 (2008)
G. Mandalari, A. Tomaino, G.T. Rich et al., Food Chem. 122(4), 1083–1088 (2010)
B.A. Cassady, J.H. Hollis, A.D. Fulford, R.V. Considine, R.D. Mattes, Am. J. Clin. Nutr. 89(3), 794–800 (2009)
P.R. Ellis, C.W.C. Kendall, Y. Ren et al., Am. J. Clin. Nutr. 80, 604–613 (2004)
J.A. Novotny, S.K. Gebauer, D.J. Baer, Am. J. Clin. Nutr. 96(2), 296–301 (2012)
A. Altan, K.L. McCarthy, R. Tikekar, M.J. McCarthy, N. Nitin, J. Food Sci. 76(2), E212–E221 (2011)
P. Varela, J.M. Aguilera, S. Fiszman, LWT Food Sci. Technol. 41(1), 10–17 (2008)
P. Moughan and A. Rowan, presented at the Proceedings of the Nutrition Society of New Zealand, 1989 (unpublished)
E. Miller, D. Ullrey, Annu. Rev. Nutr. 7(1), 361–382 (1987)
A. o. O. A. C. AOAC, Official methods of analysis. (Association of Official Analytical Chemists, Washington DC, 2000)
G.M. Bornhorst, M.J. Ferrua, S.M. Rutherfurd, D.R. Heldman, R.P. Singh, Food Biophysics 8(2), 137–150 (2013)
P. Rosin, E. Rammler, Zement 31, 427–433 (1933)
L.W. Olthoff, A. Van Der Bilt, F. Bosman, H.H. Kleizen, Arch. Oral Biol. 29(11), 899–903 (1984)
L.W. Olthoff, A. van der Bilt, A. de Boer, F. Bosman, J. Texture Stud. 17(3), 275–289 (1986)
A. van der Bilt, L.W. Olthoff, H.W. van der Glas, K. van der Weelen, F. Bosman, Arch. Oral Biol. 32(8), 579–586 (1987)
A. van der Bilt, H.W. van der Glas, L.W. Olthoff, F. Bosman, J. Dent, Res. 70(5), 931–937 (1991)
H.W. van der Glas, A. van der Bilt, L.W. Olthoff, F. Bosman, J. Dent, Res. 66(10), 1547–1550 (1987)
S.C. Hutchings, K.D. Foster, J.E. Bronlund, R.G. Lentle, J.R. Jones, M.P. Morgenstern, Food Qual. Prefer. 22(4), 332–339 (2011)
C.L. Dikeman, K.A. Barry, M.R. Murphy, J.G.C. Fahey, Nutr. Res. 27(1), 56–65 (2007)
J.F. Steffe, Rheological Methods in Food Process Engineering (Freeman Press, East Lansing, MI, 1996)
G. Bornhorst, N. Ströbinger, S. M. Rutherfurd, R. P. Singh, P. Moughan, Food Biophys. 1–12 (2012)
A. Kita, A. Figiel, Acta Agrophysic 7(1), 87–97 (2006)
G. Bornhorst and R. P. Singh, Food Biophys. 1–10 (2013)
M.J. Roman, B.J. Burri, R.P. Singh, J. Agric, Food Chem. 60(38), 9659–9666 (2012)
M.-L. Jalabert-Malbos, A. Mishellany-Dutour, A. Woda, M.-A. Peyron, Food Qual. Prefer. 18(5), 803–812 (2007)
M.-A. Peyron, A. Mishellany, A. Woda, J. Dent. Res. 83(7), 578–582 (2004)
S.W. Herring, Arch. Oral Biol. 21(8), 473–480 (1976)
G.E.J. Langenbach, F. Zhang, S.W. Herring, A.G. Hannam, J. Anat. 201(5), 383–393 (2002)
K. Schulze, in Neurogastroenterol. Motil. (Blackwell Publishing Limited, 2006), Vol. 18, pp. 172–183
J.M. Becker, K.A. Kelly, Am. J. Physiol. Gastrointest. Liver Physiol. 245(3), G334–G338 (1983)
L. Marciani, P.A. Gowland, A. Fillery-Travis et al., Am. J. Physiol. Gastrointest. Liver Physiol. 280(5), G844–G849 (2001)
T. Takahashi, M. Goto, T. Sakata, Br. J. Nutr. 91(06), 867–872 (2004)
G.M. Bornhorst, N. Ströbinger, S.M. Rutherfurd, R.P. Singh, P.J. Moughan, Food Biophys. 8(1), 12–23 (2013)
C. F. Ferraris and F. de Larrard, Testing and modelling of fresh concrete rheology. (US Department of Commerce, Technology Administration, National Institute of Standards and Technology, 1998)
H.A. Barnes, J. Rheol. 33(2), 329–366 (1989)
A. Fall, A. Lemaître, F. Bertrand, D. Bonn, G. Ovarlez, Phys. Rev. Lett. 105(26), 268303 (2010)
Acknowledgements
The authors would like to acknowledge Shane Rutherfurd, Sharon Henare, Carlos Montoya, Francisco Perez, Stuart Saigeman, and Trent Olson (Riddet Institute, Massey University, New Zealand) for their assistance in animal care and procurement of chyme samples. This project was supported by the Agriculture and Food Research Initiative grant 2009-35503-05195 from the USDA – National Institute of Food and Agriculture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bornhorst, G.M., Roman, M.J., Dreschler, K.C. et al. Physical Property Changes in Raw and Roasted Almonds during Gastric Digestion In vivo and In vitro. Food Biophysics 9, 39–48 (2014). https://doi.org/10.1007/s11483-013-9315-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-013-9315-2