Abstract
Laser induced breakdown spectroscopy (LIBS) has been used to perform in situ analysis of major and minor elements present in the different parts of the Bermuda grass (Cynodon dactylon). In situ, point detection/analysis of the elements in plants without any sample preparation has been demonstrated. LIBS spectra of the different parts (leaf blade, leaf sheath and stem) of fresh C. dactylon plant have been recorded to study the pattern of silica deposition in its different parts. Atomic lines of Si, Mg, Ca, C, Al, Zn, N, Sr, etc. have been observed in the LIBS spectra of the C. dactylon. A close observation of LIBS spectra of the different parts of the plants shows that silica concentration is greater in leaf blades than leaf sheaths and stems. The results obtained with LIBS analysis are also compared with the number density of phytoliths deposited in different parts of C. dactylon. It is observed that the highest silicified cell frequency is present in leaf blades followed by leaf sheaths and stems which is in close agreement with LIBS analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Silicon is the second most abundant element after the oxygen in the earth's crust where it is predominantly found in the form of silica or silicon dioxide. Its applications are numerous and include the silicon industry, biological sciences, nanotechnology, soil science, ecology, agriculture, archaeology, and palaeoenvironment study1–8. Chemistry of silicon is similar to carbon and belongs to the same group of periodic table. It is a promising organic alternative to carbon, the basic unit of life. Many scientists have shown that silicon may have had a part to play in the origin of life. The great majority of researchers entirely ignore the benefits of this element in plants, human and animals; however, in human body, it is required for optimal bone and connective tissue development9. Silicon protects the plants from various bacterial and fungal diseases like rice blast, powdery mildew, and sheath blight10–13 and also enhances the capacity to tolerate the attack of insects and pests,14 as well as various biotic and abiotic stresses giving strength to plants by increasing heavy metal tolerance15–18. Si is also essential element for the growth and development of higher plants19 and its deficiency causes various abnormalities. It improves nutrient balance in wide variety of plant species20–22. In 1996, Harrison23 emphasized that silica in plants is closely connected to cell wall proteins and suggested the involvement of combination of biomolecules in the formation of biogenic silica. During the absorption of water through their roots, it is absorbed in the form of monosilicic acid and deposited in various plant cells such as bulliform cells, parallelepipedal bulliform cells, silica short cells, sinuous walled epidermal long cells, and prickle hairs. It takes on the shape of the cells. These silicified cells are called as phytoliths. Silicon which is taken up by the plant from the soil is beneficial as it improves pest and pathogen resistance, drought, heavy-metal tolerance, and crop quality and yield of the plants. In grasses, silica is mainly deposited in silica short cells, epidermal long cells, bulliform cells, and prickle hairs in the form of opal phytoliths. Thus, it is desirable to perform in situ elemental analysis of different parts of the plants to know the pattern of silica deposition in leaf sheath, leaf blade, and stem of the plants. To perform such type of analysis, an analytical technique which has point detection capability is very much essential.
Various techniques are being utilized for elemental analysis of materials such as ion mobility spectrometry (IMS), mass spectroscopy, inductively coupled plasma atomic emission spectrometry (ICP-AES), graphite furnace atomic absorption spectrometry (GFAAS), but all these techniques are expensive and require lengthy and time-consuming sample preparation and not suitable for in situ and point detection analysis. Recently, laser induced breakdown spectroscopy (LIBS) has emerged as a valuable tool for in situ and in vivo analysis of any type of materials including biomaterials24–26. It does not require any sample preparation; it is a very sensitive, reliable, and quick analytical technique as well as having point detection capability27–29.
Therefore, in the present manuscript, we have used the LIBS technique to study the distribution pattern of silicon and other elements in the leaf blade, leaf sheath, and stem of Cynodon dactylon. We have also used the leaf clearing and phytoliths extraction technique for the determination and arrangement of silicified cells in various parts of C. dactylon (leaf blade, leaf sheath, and stem). C. dactylon is known by a variety of common names, including Bermuda grass, Durva grass, Indian doab grass, Dubo grass, Dog's Tooth grass, Bahama grass, Devil's grass, Couch grass, Arugampul grass, Grama grass, and Scutch grass.
Materials and Methods
Sample Collection and Preparation for Transparency
The leaf blades, stem, and leaf sheath of C. dactylon were collected from the Botany Department, University of Allahabad, Allahabad, Uttar Pradesh, India. Small pieces of leaves were washed in distilled water and made transparent using the technique of Stebbins30 to see the deposition of silica in different cells at different places of the leaves.
Phytolith Extraction
Phytolith extraction from the leaves, leaf sheaths and stems of C. dactylon was made using dry ash technique31. Small pieces of leaves, leaf sheaths and stems from mature plants were washed in distilled water and dilute HCl to remove any mineral particles on the surface and to soften the mineralized tissue. The leaves, leaf sheaths and stems were then placed in a ceramic crucible and ashed for at least 6 h at 400°C in muffle furnace. The ash was treated with HNO3 and KClO3 mixture to remove the organic material. The washed and dehydrated residue was permanently mounted in Canada balsam on glass slides for light microscopy.
We have taken 0.001 g ash for the preparation of glass slides and mounted in Canada balsam. Five slides of leaf blade, leaf sheath, and stem of the C. dactylon for data analysis were taken. The numbers of silicified cells in an area of 1.24 mm2 were counted.
LIBS Experiment
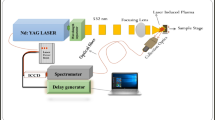
The experimental set-up, described elsewhere32 for recording the LIBS spectra of different parts of C. dactylon is shown in Figure 1. LIBS experimental set-up consists mainly of three parts viz. Laser source (Nd:YAG laser), Spectrometer (Ocean Optics,USA,model LIBS 2000+) with laser focusing and emission collecting optics and target sample. The laser delivers maximum pulse energy of 425 mJ at 532 nm with pulse width of 4 ns and maximum 10 Hz pulse repetition rate. The laser beam is focused using a convex lens onto the surface of the fresh plant leaves of the C. dactylon to create the plasma. The characteristic light emitted from the plasma is collected using a lens fixed at the tip of fiber bundle and finally fed into the entrance slit of spectrometer, equipped with a charge-coupled device (CCD) detector. LIBS spectra of leaf blade, leaf sheath, and stem of the plants were recorded by focusing laser beam at different parts of its surface (Figure 2).
Results and Discussion
Identification and Distribution of Phytoliths
Silica is deposited in inter- and intracellular spaces of the different parts of the C. dactylon. It takes very characteristic shapes like bulliform, parallelepipedal bulliform, saddle shape, prickle hairs, and epidermal cells which are called phytoliths. The transparency of the C. dactylon leaf blade (Figure 3) shows the trend of silica deposition in the cells of midrib and margin areas of the leaf blade. Among all phytoliths of the leaf blade, saddle-shaped phytoliths are highest in frequency along with the prickle hairs which are mostly deposited near the margins of the leaf blade (Figure 3A). Bulliform cells along with parallelepipedal cell phytoliths are mostly deposited near the midrib region of the leaf blade (Figure 3E–F).Various other types of phytoliths like epidermal long cells and sinuous walled long cells are deposited near the vein areas of the leaf blade (Figure 3G). A diversity of phytoliths is present in the different parts of this plant. However, the shape and size of these phytoliths are different (Figure 4).
Phytolith analysis in leaf sheaths suggests that saddle-shaped phytoliths are present in highest amount followed by the prickle hairs and trapezoids (Figure 5B–F). In the stem, very thin layer of silica is deposited below the cuticle of epidermal cells. After counting of various types of phytoliths from the same observed area of slides of leaf blade, leaf sheath, and stem, we find that the highest silicified cell frequency is present in leaf blade of this plant followed by the leaf sheath and stem (Table 1; Figure 6).
LIBS Analysis
A systematic diagram of the LIBS experimental set-up for recording the spectra of different parts of the C. dactylon plant is shown in Figure 1. LIBS spectra of fresh plant of C. dactylon have been recorded under optimized experimental conditions to identify the spatial distribution pattern and deposition of micro- and macroelements in different parts and portions of leaf blade, leaf sheath, and stem. A number of single shot LIBS spectra were recorded using 10 mJ laser energy by translating the target to avoid the crater formation on the leaf blade, leaf sheath, and stem surface. For the 200–500 nm wavelength region, typical LIBS spectra of various parts of C. dactylon are shown in Figures 7, 8, and 9, which undoubtedly shows the presence of atomic lines of Si (288.15 and 251.6 nm ), Mg (279.7, 280.2, 285.2, and 532 nm), Ca (393.36, 396.8, and 422.6 nm), C (247.8 and 229.6 nm), Al (308.2 and 309.2 nm), Zn (202.5 nm), N (463.54 nm), and Sr (407.7 and 460.7 nm). The existence of these persistent lines confirms the presence of these elements in different parts of C. dactylon. These elements play important roles for the development and growth of plants. Magnesium, which is mostly present in chlorophyll, combines with ATP and activates many enzymes needed in photosynthesis, respiration, and formation of DNA and RNA. Calcium is essential for normal membrane functions in all cells; it acts as a binder of phospholipids to each other or to membrane proteins as well as some enzyme activated by Ca32. LIBS spectra of stem (Figure 9) demonstrate the presence of strontium and aluminum in stem which are not present in other parts of the Cynodon plants. Likewise, zinc is present (Figure 7) only in leaf blade. Strontium is nonessential trace mineral which increases the retention of calcium in the stem of C. dactylon, while silica alleviates the toxicity of aluminum in the plants33.
In the present study, we have focused attention to study the distribution pattern of silicon with the help of LIBS and the arrangement of silicified cells with the help of phytolith analysis in different parts of leaf blade, leaf sheath, and stem. The distribution pattern of silica deposition in upper and lower surface of the leaf is shown in the LIBS spectrum (Figure 10) of different portion of C. dactylon leaf (midrib and margins). Figure 10 clearly shows that the intensity of atomic line of Si (288.15 nm, and transition 3s 2 3p2 (1D2)–3s 2 3p4s (1P1)] is highest in the LIBS spectra when the laser beam is focused at midrib area of the leaf, and it is less when the laser beam is focused in the margin area. According to Boltzmann law, the intensity of spectral line is directly proportional to the concentration of the elements34. Thus, Figure 10 clearly reveals that the Si concentration is higher in the midrib area of the leaf and lower in the margin areas. We have recorded the LIBS spectra of five different leaves of C. dactylon and have measured the intensity of the atomic line of Si (288.1 nm) in each spectrum at different locations on the surface of leaf blade and average of these intensities is plotted in Figure 11. Such type of variation in intensity is also performed for leaf sheath and stem and is shown in Figure 12. Comparative frequency of silicified cells of different parts of the plant is shown in Figure 6. Figure 12 clearly demonstrates that the intensity/concentration of Si deposition is different in different parts of the plants, and highest concentration of Si is found in leaf blade followed by the leaf sheath and stem. This statement is verified from Figure 6 which also reveals the variation of silicified cells in various parts of the plants and strongly suggests that the frequency of silicified cells is more in the leaf blade followed by the leaf sheath and stem of C. dactylon.
The transparency of the leaf blade (Figure 3) also shows the deposition of silica in the lumen of large bulliform and parallelepipedal cells near the midrib area. It shows high intensity of Si and silica deposition in the short saddle-shaped cells at the margin areas of the leaf blade. The bulliform and parallelepipedal cells have much more depth than the ordinary epidermal cells and are arranged in rows throughout the length of the upper surface of leaf blade. These silica bodies play very essential role in the rolling of leaves and act like hinges for the rolling of the leaves in dry weather. A number of studies demonstrate that silicon reduces the cuticular transpiration and significantly reduces the loss of water in plants35–37. Silicon deficiency of plants is closely related to excessive transpiration, and at the same time, transpiration rate is responsible for the deposition of Si in plants38. Raven39 suggested that the highest silica deposition in plants is found in major transpiration parts like leaf blade.
The above results suggest that LIBS and phytolith technique can be useful for determination of Si deposition pattern in different parts of plants. LIBS spectra showed highest silica concentration in leaf blade followed by the leaf sheath and stem. This is confirmed by counting of silicified cells from the observed area.
Conclusion
The present study clearly demonstrates that LIBS is suitable technique to perform in situ analysis of even trace element in fresh plants. Its point detection capability plays an important role to know the presence and deposition pattern of major and minor elements in different parts of the plant sample. Phytolith analysis also provides the information about the deposition pattern of silica in different cells of some plant groups. It is quite interesting that both techniques show the same results. Thus, LIBS technique is a quick, simple, and inexpensive alternative means of phytoliths extraction technique for the study of the distribution of the silicon in different parts of the plants. The LIBS spectra showed high intensity of silica where big silicified cells of leaf were present.
References
P.C. Twiss, E. Suess, R.M. Smith, J Soi Sci Soci America 33, 109–115 (1969)
I. Rovner, Quarter Resea 1, 345–359 (1971)
I. Rovner, in Adva in Archae Meth and Theo, 6, ed. by M Schiffer (Academic Press, New York, 1983)
P.C. Twiss, in Quate Envir of Kans Geolo Surv Guid book, 5, ed. by W.C. Johnson (1987), pp. 179–188
D. M. Pearsall, (Academic Press, San Diego 1989)
F. Runge, Z Geomo N F 99, 53–64 (1995)
D.R. Piperno, D.M. Pearsall, Smithsonian Institution Press. (1998)
D.K. Chauhan, D.K. Tripathi, P. Sinha, S.P. Tiwari, Bionature 29(1), 1–9 (2009)
S. Sripanyakorn, R. Jugdaohsingh, R.P.H. Thompson, J.J. Powell, Nutr Bulle 30, 222–230 (2005)
C.F. Lanning, J Agric Foo Chem 4, 350–352 (1966)
F. Fauteux, W. Re'mus-Borel, M.G. James, R.R. Belanger, FEMS Micro Lett 259, 1–6 (2005)
F. Fauteux, F. Chain, F. Belzile, J.G. Menzies, R.R. B’elanger, Proc Nati Acad Sci USA 103, 17554–17559 (2006)
N.K. Savant, G.H. Synder, L.E. Datnoff, Adva in Agro 58, 151–199 (1997)
F.J. Ma, N. Yamaji, Cellul and Molecl Life Scie 65, 3049–3057 (2008)
J.D. Birchall, C. Exley, J.S. Chappell, M.J. Phillips, Nature 338, 146–148 (1989)
D. Neumann, U.Z. Nieden, Phytoc 56, 685–692 (2001)
Y. Liang, J.W.C. Wang, L. Wei, Chemo 58, 475–483 (2005)
V.P.K. da Cunha, A.W.C. Do Nascimento, Water Air Soi Pollut 197, 323–330 (2009)
G.H. Korndorfer, I. Lepsch, in Silicon in agriculture, ed. by L. Datonoff, G. Korndorfer, G. Synder (Elsevier, New York, 2001), pp. 133–147
F.J. Ma, E. Takahasi, (Elsevier Science, Amsterdam, 2002)
F.J. Ma, N. Yamaji, Tren in Plan Scie 11, 8 (2006)
E. Epstein, Ann of appli Biol 155, 155–160 (2009)
C.C. Harrison, Phytoche 41, 37–42 (1996)
A.K. Pathak, A.K. Rai, Asi Jour of Spectr 147–151 (2010)
A.K. Pathak, V.K. Singh, N.K. Rai, A. K. Rai, Pradeep K. Rai, Pramod K. Rai, S. Rai, G.D. Baruah, Lase in Medi Sci. (2010). doi:10.1007/s10103-011-0886-1
V.K. Singh, V. Singh, A.K. Rai, S.N. Thakur, P.K. Rai, J.P. Singh, Applie Opt 47, G38–G47 (2008)
A.K. Rai, H. Zhang, F.U. Yueh, J.P. Singh, A. Weisburg, Spectrochim Acta B At Spectrosc 56, 2371–2383 (2001)
A.K. Rai, V.N. Rai, F.U. Yueh, J.P. Singh, Tren Appl Spect 4, 165–169 (2003)
A.K. Rai, V.N. Rai, D.K. Rai, S.N. Thakur, F.U. Yueh, J.P. Singh, (Elsev Scie, The Netherlands 2007)
G.L. Stebbins, Science 87, 21–22 (1938)
D.R. Piperno, (Academic Press, The University of California, 1988), p. 58
V.K. Singh, V. Singh, A.K. Rai, S.N. Thakur, P.K. Rai, J.P. Singh, Appl Opt 47, G38–G47 (2008)
K.M. Cocker, D.E. Evans, M.J. Hodson, Physio Planta 104, 608–614 (1998)
S. Pandhija, N.K. Rai, A.K. Rai, S.N. Thakur, Appli phys B 98, 231–241 (2010)
J. Seckback, J of Plan Nutri 5, 201–205 (1982)
Y Wong, You Cheong, A. Heitz, J. Deville, In Proceeding 14th Congress of Int Soci of Sug Cane Tech (1971), pp. 777–785
S. Yoshida, Technical Bulletin no. 25. Foo and Ferti Tech Cen Taipei, Taiwan (1975)
E. Epstein, Plan Phys Plan Mole Biol 50, 641–664 (1999)
J.A. Raven, New Phytol 158, 419–436 (2003)
Acknowledgements
Financial assistance from the BRNS, BARC, and Mumbai (no. 2009/37/30/BRNS/2063) is gratefully acknowledged. Mr. Durgesh Kumar Tripathi is also grateful to Allahabad University for providing D.Phil. Fellowship under UGC scheme. Dr. Nilesh Kumar Rai is grateful to CSIR for providing RA Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chauhan, D.K., Tripathi, D.K., Rai, N.K. et al. Detection of Biogenic Silica in Leaf Blade, Leaf Sheath, and Stem of Bermuda Grass (Cynodon dactylon) Using LIBS and Phytolith Analysis. Food Biophysics 6, 416–423 (2011). https://doi.org/10.1007/s11483-011-9219-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-011-9219-y