Abstract
In the present work, inclusion complexes of α-terpineol (Terp) and β-cyclodextrin (BCD) were prepared by the coprecipitation method. Phase solubility studies were performed and thermodynamic parameters involved in the complex formation were calculated. The solubility of Terp increased linearly as the concentration of BCD was increased, confirming the 1:1 stoichiometry of the complex. The stability constants decreased along with increasing temperature. The negative value of the enthalpy and of the Gibbs free energy demonstrated that the process is exothermic and spontaneous. Since complexation gives more ordered systems, the negative value obtained for the entropy change evidenced the encapsulation of Terp. Terp was completely encapsulated in BCD at the preparation conditions and studied molar ratios, as confirmed in the freeze-dried samples by differential scanning calorimeter. The presence of Terp greatly modified the BCD water sorption curves, and the amount of adsorbed water was lower for the complexes. The limited water solubility of Terp could be overcome by the formation of BCD inclusion complexes, and the complexes were stable at different storage conditions (relative humidities 11–97% and 25 °C). The obtained phase solubility data are useful for food or pharmaceutical products formulation involving cyclodextrins and stability predictions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Encapsulation in cyclodextrins (CDs) is a method that has been used during the last years in cosmetics and drug and food industries for many purposes. As a result of their molecular structure and shape, cyclodextrins possess the ability to act as molecular containers by entrapping guest molecules in their internal cavity. They are cyclic oligosaccharides composed of 6, 7, or 8 α-D-glucopyranose units with a relatively hydrophobic central cavity and hydrophilic outer surface.1 They are chemically and physically stable molecules obtained by the enzymatic modification of starch.2 The hydrophobic cavity forms inclusion complexes with a wide range of molecules.3–5
No covalent bonds are formed or broken during the ligand/CD complex formation. However, in aqueous solution, the complexes readily dissociate reaching equilibrium between the released (free) molecules and the ones located within the CD.
The physical, chemical, and biological properties of compounds encapsulated by CDs may be drastically modified.6 Encapsulation leads to enhancing dissolution rate, membrane permeability, and bioavailability of nutraceuticals of low solubility. CDs act as flavor carriers, they protect against oxidation, light-induced decompositions, and heat-induced changes. Moreover, they improve shelf life of food products and mask or reduce undesired tastes.7 Possibly one of the most important properties of encapsulation in CDs is the increase of the aqueous solubility of various sparingly soluble compounds.3, 8, 9
Flavors and fragrances are extremely important in food, cosmetic, chemical, and pharmaceutical industries. Flavor plays an important role in consumer satisfaction and influences further consumption of foods. Most of the available flavor compounds are obtained by chemical synthesis or extraction. However, consumers’ negative perception towards chemicals added to food, cosmetics, and other household products has induced the flavor companies to direct their attention towards compounds of biological origin.10 Essential natural oils have emerged as an interesting alternative. Terpineol is a naturally occurring terpenic alcohol constituent of essential oils of many types of plants and flowers. It is usually a mixture of isomers alpha-, beta-, and gamma- with alpha-terpineol as the major constituent. Terpineol is a useful flavor and fragrance compound for perfumes and cosmetics, which is generally employed in formulations at 0.03%.11 Moreover, it could be used as antifungal12 and as ingredient in inhalants/decongestants pharmaceutical products.13 Currently, research has been undertaken considering the antitumor activity of α-terpineol (Terp).14 Manufacturing and storage processes often cause modifications in overall flavor of products by reducing aroma compound intensity or producing off-flavor components.15
In addition, a limiting factor of the use of terpenoids is their very low aqueous solubility.16 The formation of inclusion complexes with CDs may increase both flavor stability by limiting degradation or loss during processing and storage17 and also its solubility and dissolution rate.8, 18, 19
In the practical application of cyclodextrins, attention should be directed towards the dissociation equilibrium and stoichiometry of the inclusion complex. When a CD complex is dissolved in water or introduced into fluids, it dissociates to free components in equilibrium with the complex. The stability constant (K c) is a useful index to estimate the binding strength of the complex and the changes in the physicochemical properties of the ligand or guest molecule.20
In present work, physical properties and stability of freeze-dried inclusion complexes of α-terpineol and β-cyclodextrin were evaluated as a function of water content and storage time. The solubility of Terp was determined by phase solubility studies and the thermodynamic parameters involved in the complex formation were calculated.
Materials and Methods
Materials
β-Cyclodextrin (BCD; containing eight water molecules/molecule of BCD, Mr 1135) was from Sigma Chemical Co. (St Louis, MO, USA). Terp was obtained from Polymetron S.R.L, Germany. All other chemicals were of analytical grade and purchased from Mallinckrodt Chemical Works (St. Louis, MO, USA).
Methods
Phase Solubility Studies
Phase solubility studies were carried out according to the method described by Higuchi and Connors.18 An excess amount of Terp (15 mM) was mixed in an aqueous solution containing increasing amounts of BCD (0–15 mM) using a laboratory shaker at 25 °C, 27 °C, 33 °C, and 47 °C during 24 h. The samples were centrifugated at 15,000 rpm for 15 min and the supernatants were filtered through a 0.45-μm PTFE filter. Then, the amount of Terp in each aqueous solution was determined spectrophotometrically by measuring the absorbance at 240 nm. The experiments were carried out in triplicate at each temperature.
The stability constants, K c, were calculated from the straight-line portion of the phase solubility diagram according to the Higuchi–Connors equation18:
Preparation of the Solid BCD Inclusion Complexes
Inclusion complexes of BCD with α-terpineol as guest molecule (Terp/BCD) were prepared by the coprecipitation method.8 Solutions of BCD (1.85 g/100 ml) were prepared and heated at 50 °C shaking until complete solubilization of the CD. Terp was dispersed in the BCD aqueous solution in suitable proportions for Terp/BCD molar ratios 1:1 and 1:3. Ligands as terpenes21 and flavonones,20 which are completely encapsulated in the cavity of the CDs, form complexes at molar ratio 1:1. Other ligands, as fatty acids, are not completely encapsulated and the stable inclusion complex with CD would be obtained with 1:3 molar ratios.22 We thus chose 1:1 and 1:3 ratios to evaluate the degree of encapsulation.
The systems were stirred at a constant rate for 3 h at 50 °C and for 3 h at room temperature. The obtained solutions were then stored overnight at 3 °C to promote the precipitation of the complexes. The suspensions were filtered (PTFE filters of 0.45 μm), frozen at −26 °C for 24 h and freeze-dried in a Heto Holten A/S freeze-dryer (operating at a condenser plate temperature of −111 °C, chamber pressure of 30 Pa, and shelf temperature of 25 °C). The secondary drying was also performed at 25 °C.
Once dehydrated, the systems were equilibrated to different relative humidities (RH) and the water sorption isotherms, glass transition, and melting events in the samples were studied. Since the employed BCD was obtained as an octahydrate, it was dried in a vacuum oven (at 90 °C for 48 h) up to water content smaller than 3% dry basis (d.b.), before the experiments.
Determination of the Water Sorption Isotherms
Sorption isotherms were determined by the standard isopiestic static-gravimetric method. After freeze-drying, samples of BCD or their complexes with Terp were distributed into glass 5 ml vials (around 200 mg/vial) and placed into vacuum desiccators containing saturated salt solutions which provide different RH: LiCl, KCOOCH3.5H2O, MgCl2.6H2O, K2CO3.2 H2O, NaBr.2H2O, NaCl, KCl, and K2SO4 for 11%, 22%, 33%, 43%, 57%, 75%, 84%, and 97% RH at 25 °C ± 1 °C, respectively.23 The water content of the samples was determined as a function of time of storage until reaching the equilibrium condition (differences in weight smaller than 0.0005 g).
Determination of Water Content
The total water content of the samples was determined by difference in weight before and after drying in a vacuum oven at 96 °C ± 2 °C during 48 h. These drying conditions had been proved to be adequate to assess constant weight after drying and were selected from previous studies. The determinations were performed in duplicate, and the average value was reported. The calculated confidence interval for a 95% certainty was between 3% and 4% of the absolute values. Results were express in % d.b.
Differential Scanning Calorimeter
Differential scanning calorimetry (DSC) was used to verify the formation of complexes, the release of the guest molecule during storage, and to determine the glass transition temperature (T g) of the analyzed systems.
A DSC, Mettler Toledo equipment model 822 (Mettler Toledo AG, Switzerland), with a STARe Thermal Analysis System version 3.1 software (Mettler Toledo AG), was used for all the measurements. The instrument was calibrated with indium and zinc. All measurements were made at 10 °C/min, using hermetically sealed aluminum pans (Mettler, 40 μL capacity), and an empty pan was used as a reference. The confidence interval estimated for temperature values was 1 °C. An average value of two replicate samples was reported for each measurement.
The dynamic method was used to determine melting points (T m) and heats of fusion (ΔH m) of the pure terpineol and of the ligand in the complexes. T m for Terp was 35 °C taken as the onset of the melting peak. Each sample was heated at a rate of 10 °C/min from −20 °C up to 110 °C. The percentage of free Terp (ligand; that which has been released or that which was not encapsulated) in the system after humidification and during storage was determined as the ratio of the fusion enthalpy of terpineol in the system at time t (corrected according to the water content of the samples) and the fusion enthalpy of the pure Terp. Then, the percentage of free ligand was expressed as indicated in the following equation:
where ΔH s is the heat of melting of free Terp in the systems and ΔH o is the heat of melting of pure terpineol. Since the boiling point of Terp is 218 °C24, the losses by evaporation were considered negligible.
Duplicate determinations were carried out for each sample and the average values were reported. The calculated confidence interval for a 95% certainty was between 5% and 7% of the absolute values. The glass transition temperature was recorded as the onset temperature of the endothermal discontinuities in the curves of heat flow versus temperature.
Storage Study
The percent of free ligand in the system was evaluated by DSC as indicated in Eq. 2 after equilibration at different RH and after storage in the vacuum desiccators at 25 °C. Average values of duplicate analysis are reported.
Results
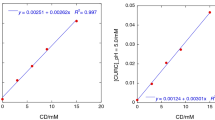
The α-terpineol complexes with β-cyclodextrin were generated in solution as well as in the solid state. Phase solubility studies were carried out in aqueous systems at different temperatures to calculate the stability constants, (K c), and the thermodynamic values for the formation of the complexes Terp/BCD. The phase solubility diagrams made at 27 °C, 33 °C, and 47 °C are shown in Figure 1 (data at 25 °C were similar to those obtained at 27 °C and are not shown). The plots showed that the solubility of Terp increased linearly along with the concentration of CD so they can be consequently considered as AL-type diagrams suggesting the formation of 1:1 complexes.18 It can be seen from the intercept values that the aqueous solubility of terpineol increased with temperature, as well as the total concentration of this compound in the presence of BCD.
The stability constants, K c, of the complexes at the studied temperatures were calculated from the straight-line portion of the phase solubility diagram using Eq. 1 and are shown in Table 1. These stability constants decreased with increasing temperature, as expected for an exothermic process.
A small K c value indicates a weak interaction, whereas a large one indicates the possibility of limited release of ligand. The effect of temperature on the stability constants, with regards to the complex formation of various compounds with BCD, has been referred by several authors.8, 20, 25
The phase solubility data made at different temperatures allowed to obtain additional information such as the thermodynamic parameters involved in the formation of the complex. The integrated form of the Van’t Hoff equation allows the calculation of the enthalpy values and of entropy changes, depending on the variations of the stability constants with temperature20, 26:
The K c values plotted in a Van’t Hoff plot are shown in Figure 2. The complex Terp/BCD showed a linear function between K c and the inverse of the absolute temperature (1/T). The relative thermodynamic parameters were calculated and are given in Table 2 compared with the values obtained by other authors for terpenoids such as thymol and geraniol,27 for the flavonones hesperetin and naringenin,20 and for vanillin.8 The negative value of the enthalpy change (ΔH) indicates that the interaction process of the ligands with BCD, leading to complex formation, is exothermic. The relatively small enthalpy changes were in the range from −68 to −7 kJ mol−1 and are typical of low energy interactions prevailing in related flavor–BDC complexes. They could derive from new interactions, like hydrophobic ones (due to the displacement of the water molecules from the cavity of the BCD by the more hydrophobic ligand), increase of Van der Waals interactions between the molecules, formation of hydrogen bonds, and other low energy interactions.8, 20
The negative values observed for the entropy changes can be explained considering that inclusion moderately hinders the free rotation of the included molecule around its symmetry axis.8, 20, 28 The Gibbs free energy change for the interactions that take place during the inclusion process was calculated using the following equation:
The negative value of ΔG 25 for terpineol, as for the rest of the ligands (Table 2), indicates that the inclusion process in BCD is spontaneous.
DSC was used to verify the formation of the complex in the solid state.8 The total or partial disappearance of thermal events (melting point) corresponding to guest molecules, when they are examined as CD complexes, is generally taken as a proof of complex formation.29, 30 Thermograms obtained by DSC for α-terpineol, BCD and for the freeze-dried Terp/BCD complexes, both in 1 to 1 and 1 to 3 ligand:CD molar ratios, are shown in Figure 3. The thermogram corresponding to pure Terp shows an endothermic peak at nearly 35 °C corresponding to its melting point. As can be seen in Figure 3, the curves Terp/BCD did not show any sharp endothermic peak in the range of the melting point of the pure compound (35 °C), as a consequence of its encapsulation in the host BCD.
When the complexes of CD and a convenient ligand are obtained by freeze-drying, the whole ligand will be in the final system, either free or encapsulated. The complete disappearance of the endothermic DSC signal is a strong evidence of the total inclusion of the ligand inside the cavity of CD.29, 30 If the disappearance of the thermal signal in the thermograms is only partial, the inclusion may not be completed. Therefore, the ratio between this signal and the theoretical value of the melting enthalpy of the ligand is an approximation to quantify the percentage of free ligand in the system.31
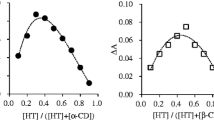
The “driving force” for complexation is not yet completely understood,32 but it seems that it is the result of various effects: substitution of water molecules from the inner cavity, which is energetically favored; the release of CD ring strain when the complex is formed; Van der Waals forces and hydrogen bond interactions, which are established when the complex is formed.20, 33, 34 Considering the high influence of water interactions on complex formation and stability, when the dry formulations of CD containing the active components are stored, the analysis of the water adsorption behavior becomes of fundamental importance to define the appropriate storage conditions.34 Figure 4 shows the effect of complex formation on the water adsorption isotherms of β-cyclodextrin. The presence of terpineol greatly modified the CD water sorption curves. BCD adsorbs more water than the complexes and reaches a plateau from 52% RH due to the formation of a stable crystalline hydrate. The complexes adsorbed less water and did not show a plateau which indicates that no hydrates were formed and the isotherm followed a sigmoid-type behavior with a marked increase of water content at more than 85% relative humidity.
Present results support the idea of substitution of water molecules from the inner cavity by hydrophobic ligands. Furthermore, these results confirmed that the water molecules inside the cavity could be easily removed by compounds of an adequate size and hydrophobicity to occupy the BCD cavity, and thus, forming energetically favored inclusion complexes.
Glass transition temperatures were determined by DSC for the freeze-dried BCD and its complexes with terpineol, the obtained values are shown as a function of RH in Figure 5. The thermograms evidenced a well-defined glass transition, which confirmed the amorphous nature of the systems in all cases. For a certain RH, both water content and T g values were lower for Terp/BCD respect to the CD matrix (Figures 4 and 5, respectively) showing the plasticizing effect of terpineol. In presence of Terp, structural changes take place affecting the physical characteristics of the matrix. The measured T g values were in agreement with the physical appearance of the samples, which were collapsed when their T g was lower than the storage temperature (25 °C). The T g values of BCD were only detectable for the samples equilibrated up to 43%RH, since at higher RHs the stable form of the BCD is the crystalline hydrate.
Not only are the thermodynamic parameters of complex formation of interest, but also kinetics and stability aspects of the complexes as a function of relative humidity. Figure 6 shows the measured free ligand after storage the 1:1 Terp/BCD complexes at different RH at 25 °C during 65 days. Data values determined previously for thymol34, another terpenoid flavor compound, and myristic acid33, were included for comparative purposes. According to calorimetric studies to determine complex stability, different types of behavior could be proposed. In systems like α-terpineol encapsulated in BCD at molar ratio1:1, the ligand was almost completely encapsulated and remained included up to 65 days of storage, even at the higher RH (free ligand concentration did not change). Contrary to Terp, thymol release was observed (free ligand concentration increased) when the RH was higher than 75%. In the case of myristic acid, a non-polar aliphatic acid, its inclusion in CD was initially incomplete, both in 1:1 and 1:3 molar ratios, but it was favored during storage at high relative humidities (Figure 6), thus decreasing the free ligand. It has also been reported that encapsulation of myristic acid increases in presence of an excess of BCD (molar ratio 1:3).33 These results agree with a recently proposed model by other authors who suggest that for fatty acids the stable inclusion complex with CD would be the 1:3 or 1:4 molar ratios.22
Percentage of free ligand in β-cyclodextrin (BCD) systems stored at different RH at 25 °C during 65 days. Data values determined previously for thymol34, another terpenoid flavor compound, and myristic acid 33 were included for comparative purposes. Terp α-terpineol, Myr myristic acid; thymol. All systems were prepared in 1:1 ligand–CD molar ratio
Discussion
Encapsulation improved the solubility of Terp in water. Phase solubility study pointed out the formation of 1:1 stoichiometric complexes between terpineol and β-cyclodextrin, which was influenced by temperature variations. This process was shown to be exothermic and energetically favored. According to this, the complex formation equilibrium was shifted towards free ligand with increasing temperature. Taking into account the size of the BCD cavity (7.80 Å) and the dimension of the terpineol molecule (4.30 × 8.15 Å), this flavor can accommodate inside this host at 1:1 stoichiometric ratio (Figure 7). These results are also in accord to those shown by Astray et al.35 for the encapsulation of different terpenoids and flavonoids. The obtained thermodynamic parameters from the solubility data at different temperatures helped to explain certain phenomena regarding complex formation and BCD interactions with the ligands. Those data describe the dynamic equilibrium which is established between the ligand, the CD and the complex in aqueous media. The obtained entropy change value was negative, probably due to a decrease in translational and rotational degree of freedom for the complex, in comparison to the free molecules.
Once the complex is formed and dehydrated, the thermodynamic parameters obtained for aqueous systems are not enough to describe complex stability regarding relative humidity. Thus, the analysis of the water adsorption behavior becomes of fundamental importance to define the appropriate storage conditions of the dehydrated complexes. The stability of the freeze-dried complex as a function of time was influenced by water content and storage temperature and also by the nature of the ligand. The water adsorption data and glass transition values obtained revealed that complex stability involves the displacement of water molecules from the cavity of BCD by the ligand molecule that is being included.
References
M.E. Brewster, T. Loftsson, Adv. Drug Deliv. Rev. 59, 645–666 (2007)
A. Hedges, Am Chem Soc 98, 2035–2044 (1998)
M.L. Calabrò, S. Tommasini, P. Donato, D. Raneri, R. Stancanelli, P. Ficarra, J. Pharm. Biomed. Anal. 35, 365–377 (2004)
C. Alvariza, R. Usero, F. Mendicuti, Spectrochim Acta A 67, 420–429 (2007)
C. Lucas-Abellán, I. Fortea, J.M. López-Nicolás, E. Núñez-Delicado, Food Chem. 104, 39–44 (2007)
N.E. Polyakov, T. Leshina, T.A. Konovalova, E.O. Hand, L.D. Kispert, Free Radic. Biol. Med. 36, 872–880 (2004)
L. Szente, J. Szejtli, Trends Food Sci. Technol. 15, 137–142 (2004)
V.T. Karathanos, I. Mourtzinos, K. Yannakopoulou, N.K. Andrikopoulos, Food Chem. 101, 652–658 (2007)
Z. Lu, B. Cheng, Y. Hu, Y. Zhang, G. Zou, Food Chem. 113, 17–20 (2009)
A. Adams, J.C.R. Demyttenaere, N. De Kimpe, Food Chem. 80, 525–534 (2003)
S.P. Bhatia, D. McGinty, C.S. Letizia, A.M. Api, Food Chem. Toxicol. 46, 128–130 (2008)
D. Pitarokili, M. Couladis, N. Petsikos-Panayotarou, O. Tzakou, J. Agric. Food Chem. 50, 6688 (2002)
R. Tisserand, T. Balacs, Essential Oil Safety: A Guide for Health Care Professionals (New York, Churchill Livingstone, 1995)
S.B. Hassan, H. Gali-Muhtasib, H. Göransson, R. Larsson, Anticancer Res. 30, 1911–1919 (2010)
M. Arias, M.S. García-Falcón, L. García-Río, J.C. Mejuto, R. Rial-Otero, J. Simal-Gándara, J. Food Eng. 78(1), 69–73 (2007)
I. Fichan, C. Larroche, J.B. Gros, J. Chem. Eng. Data 44(56–62), 109–114 (1999)
B. Bhandari, B. D’Arcy, G. Young, Int. J. Food Sci. Technol. 36, 453–461 (2001)
T. Higuchi, K. Connors, Adv Anal Chem Instrum 4, 117–212 (1965)
T. Loftsson, M.E. Brewster, J. Pharm. Sci. 85, 1017–1025 (1996)
S. Tommasini, D. Raneri, R. Ficarra, M.L. Calabrò, R. Stancanelli, P. Ficarra, J. Pharm. Biomed. Anal. 35, 379–387 (2004)
M.J. Choi, A. Soottitantawat, O. Nuchuchua, S.G. Min, U. Ruktanonchai, Food Res. Int. 42, 148–156 (2009)
M. Regiert, J Incl Phenomenon Macrocycl Chem 57, 471–474 (2007)
L. Greenspan, J. Res. Natl Bur. Stand. 81A, 89–96 (1977)
M.J. O’Neil (ed.), Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 12th edn. (Merck, Whitehouse Station, 2006). 9103
M.V. Rekharsky, Y. Inoue, Chem. Rev. 98, 1875–1917 (1998)
T. Hoshino, K. Uekama, J. Pitha, Int. J. Pharm. 98, 239–242 (1993)
I. Mourtzinos, N. Kalogeropoulos, S.E. Papadakis, K. Konstantinou, V.T. Karathanos, J. Food Sci. 73(1), 89–94 (2008)
A. Cooper, D.D. MacNicol, J Chem Soc Perkin Trans 2, 761–763 (1978)
R.O. Williams, V. Mahaguna, M. Sriwongjanya, Eur. J. Pharm. Biopharm. 46, 355–360 (1998)
T. Pralhad, K. Rajendrakumar, J. Pharm. Biomed. Anal. 34, 333–339 (2004)
C. dos Santos, M.F. Mazzobre, B. Elizalde, M.P. Buera, Alimentos Cienc. Ingeniería Alimentos 16(2), 71–73 (2007)
G. Astray, C. Gonzalez-Barreiro, J.C. Mejuto, R. Rial-Otero, J. Simal-Gándara, Food Hydrocolloids 23, 1631–1640 (2009)
M.F. Mazzobre, B. Elizalde, C. dos Santos, P. Ponce Cevallos, M.P. Buera, Nanoencapsulation of Food Ingredients in Cyclodextrins: Effect of Water Interactions and Ligand Structure, in Functional Food Product Development. Part I: New Technologies For Functional Food Manufacture, ed. by J. Smith, E. Charter (Wiley, Blackwell, 2010), pp. 25–38
P.A. Ponce Cevallos, M.P. Buera, B.E. Elizalde, J. Food Eng. 99, 70–75 (2010)
G. Astray, J.C. Mejuto, J. Morales, R. Rial-Otero, J. Simal-Gándara, Food Res. Int. 43, 1212–1218 (2010)
Acknowledgments
The research described in this paper is financially supported by CONICET (PIP 100468, 100846), UBA (Project UBACyT X-024) and ANPCYT (PICT 2008 02928). MFM and MPB are members of CONICET.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mazzobre, M.F., dos Santos, C.I. & Buera, M. Solubility and Stability of β-Cyclodextrin–Terpineol Inclusion Complex as Affected by Water. Food Biophysics 6, 274–280 (2011). https://doi.org/10.1007/s11483-011-9208-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-011-9208-1