Abstract
The bioavailability of dietary lipophilic components may be either increased or decreased by manipulating the microstructure and/or physicochemical properties of the foods that contain them. This article stresses how knowledge of the molecular, physicochemical, and physiological processes that occur during lipid ingestion, digestion, and absorption can be used to rationally design food structures to control these processes and therefore impact the rate or extent of lipid digestion and/or absorption. These approaches include controlling the molecular characteristics of the lipid molecules, altering lipid droplet size or interfacial properties, and manipulating food matrix structure and composition. Improved knowledge of the molecular, physicochemical, and physiological processes that occur during lipid ingestion, digestion, and absorption will facilitate the rational design and fabrication of functional foods for improved health and wellness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An improved understanding of the relationship between food properties, digestion, and absorption would facilitate the rational design and fabrication of foods with improved nutritional properties.1,2 This knowledge could be used to design food matrices that encapsulate and protect bioactive components during storage, transport, and utilization but then release them at specific sites within the gastrointestinal (GI) tract or that can either increase or decrease the bioavailability of specific nutrients.1,3–7 Improving our understanding of the influence of food structure on the bioavailability of nutraceuticals has also been stressed as an important part of establishing health claims since bioactive compounds might have reduced bioavailability in some food matrices.8 The purpose of this article is to briefly review the physicochemical and physiological processes that occur during the ingestion, digestion, and absorption of lipids and then to show how this knowledge could be used to rationally design food structures to alter lipid bioavailability. In particular, we focus on structured delivery systems based on emulsion science and technology. Detailed information about lipid digestion and absorption in general can be found in recent review articles.3–5,7,9
Lipid Ingestion, Digestion, and Absorption
In this section, we provide a brief overview of the basic physicochemical and physiological processes that occur during the ingestion, digestion, and absorption of lipids from foods.10,11
Ingestion
The composition, structure, and properties of foods change appreciably during ingestion, mastication, and swallowing due to the complex series of physicochemical, physiological, and biochemical events that occur starting in the human mouth and throat.12–14 Food is mixed with saliva, changes its pH, ionic strength, and temperature, is acted upon by digestive enzymes, interacts with the surfaces of the tongue, mouth, and throat, experiences a complex flow profile, and may be physically broken down into smaller pieces by chewing.15–21 The nature and extent of the changes of the lipid phase of a food in the mouth and throat depends on the original composition and properties of the lipid phase, its structural organization within the food, and the nature of the food matrix.22 Typically, a food or beverage only spends a relatively short time (5 to 20 s) in the mouth before being swallowed.14 The material that is swallowed after ingestion and mastication of a food is usually referred to as the “bolus.”10 The structural organization and properties of the lipids within the bolus depends on their initial structural organization within the food, as well as the duration and intensity of mastication. In many cases, the lipids in the bolus are present as oil droplets, which may vary in size from less than a micrometer (for some food emulsions) to more than a millimeter (for some bulk fats). These oil droplets may have been present in the original food, or they may have been formed within the mouth due to the breakdown of a bulk fat phase.23 In general, there is still a relatively poor understanding of the physicochemical and structural changes that occur within the mouth when fatty foods are consumed, although considerable progress has been made for certain food categories, such as emulsions and gels.15–17,22
Stomach
After the bolus is swallowed, it rapidly passes down the esophagus and into the stomach (Figure 1), where it is mixed with acidic digestive juices (pH ≈ 1–3) containing gastric enzymes, minerals, and various surface-active compounds and is also subjected to mechanical agitation due to movements of the stomach.24,25 Typically, there is an appreciable increase in the pH of the stomach contents after ingestion of a food, followed by a gradual decrease to around pH 2 over the next hour or so. The time taken for the stomach to return to pH 2 depends on the initial pH, buffering capacity, composition, and quantity of the food ingested.26 After being ingested, a food component may remain in the stomach for a period ranging from a few minutes to a few hours depending on its quantity, physical state, structure, and location.24,25 During this time, there may be a breakdown of the food matrix structure, lipid droplet coalescence/disruption processes, and changes in the interfacial composition of the lipid phase due to adsorption/desorption of surface-active substances to the lipid droplet surfaces. In addition, gastric lipase binds to lipid droplet surfaces where it hydrolyzes the emulsified triacylglycerols (TAGs) to diacylglycerols (DAGs), monoacylglycerols (MAG), and free fatty acids (FFAs).27–29 Typically, lipid hydrolysis stops when 10–30% of the fatty acids have been released from the TAGs.
Small Intestine
The partially digested food that leaves the stomach and enters the small intestine is usually referred to as the “chyme” (Figure 1). The emulsified lipids within the chyme are transferred from the stomach to the duodenum where they are mixed with sodium bicarbonate, bile salts, phospholipids, and enzymes secreted by the liver, pancreas, and gall bladder.10 The sodium bicarbonate secreted into the small intestine causes the pH to increase from highly acidic (pH 1 to 3) in the stomach toward neutrality (pH 5.8–6.5) in the duodenum, where the pancreatic enzymes work most efficiently.3 The bile salts and phospholipids originating from the liver (via the gall bladder) are surface active and can facilitate emulsification of the lipids by adsorbing to the droplet surfaces, as well as facilitating lipase absorption.10 In addition, the chyme is subjected to shear flow patterns in the small intestine that promote mixing and emulsification. Lipid hydrolysis continues within the duodenum through the actions of lipases and other enzymes (such as cholesterol esterase) originating from the pancreas.27,30 A pancreatic lipase/colipase complex binds to the lipid droplet surfaces, where it hydrolyzes the TAG to DAG, MAG, and FFA.27–29 Cholesterol esterase hydrolyzes the conversion of cholesteryl ester to free cholesterol (which is the only form absorbed), as well as being able to hydrolyze various other types of lipid esters, such as acylglycerols, phospholglycerides, and esters of vitamins A and D.10 Lipids and lipid digestion products (e.g., FFA, sn-2 MAG, cholesterol, phospholipids, and fat-soluble vitamins) are transported from the surface of the lipid droplets to the intestinal mucosa via mixed micelles and vesicles, where they are absorbed.22 The composition, structure, and concentration of micelles/vesicles present in the small intestine that solubilize and transport digested lipids and other functional lipophilic components depend on the amount and type of lipids ingested.22
Summary of Key Physicochemical Processes and Changes
The properties of the lipid phase may change considerably during their passage through the human digestive tract because of specific changes in solution composition (e.g., pH, ionic strength, biopolymers, surface-active substances), enzyme activities (e.g., lipases, proteases, amylases), and forces/flow profiles (e.g., disruption, mixing, transport) associated with the mouth, stomach, and small intestine.2,22,31,32 Some of the major physicochemical changes that may occur in the lipid phase as a result of these processes are summarized below:
-
Lipid accessibility. Before food lipids can be digested and absorbed, they have to be accessible to attack by the lipases and other enzymes originating from the stomach and small intestine. In principle, the lipids in a food may not be initially accessible to these enzymes because they are trapped in an impermeable matrix, e.g., of protein, sugars, starch, and/or dietary fiber. It may be necessary for this matrix to be disrupted, dissolved, or digested before the lipids are released. Consequently, the disruption, dissolution, or digestion of food matrices within the mouth, stomach, and small intestine may play an important role in determining the extent and/or rate of lipid digestion and absorption. Food components vary considerably in their disruption, dissolution, and digestion properties within the GI tract and therefore may impact lipid accessibility differently. For example, dietary fibers will not be digested by enzymes in the mouth, stomach, or small intestine, whereas proteins (proteases) and starch (amylases) will.33 This may offer a route for food scientists to rationally design foods that control lipid bioavailability.
-
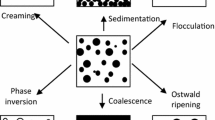
Droplet size. The size of the lipid droplets determines the specific surface area of lipids exposed to the aqueous phase, which should impact the rate of lipid digestion by lipase and other enzymes.34 The initial size of the lipid droplets in a food prior to ingestion can often be controlled during the food manufacturing process, e.g., by selecting appropriate emulsifier type and homogenization conditions.35 Typically, the mean droplet diameter of a food emulsion can be controlled within the range of about 0.1 to 100 μm.35 Nevertheless, there are normally changes in the size of the lipid droplets during passage of food through the human GI tract.32,34,36 The droplet size may either increase or decrease depending on the relative importance of matrix breakdown, droplet coalescence, droplet breakup, and lipid digestion events.3,4,31,32
-
Interfacial characteristics. In principle, the properties of the interfacial layer surrounding the lipid droplets will affect the rate and extent of lipid digestion because lipase has to adsorb to the droplet surfaces before it can hydrolyze the lipids.37 The initial composition, thickness, and charge of the interfacial layers surrounding the lipid droplets is determined by the type of ingredients and unit operations used to produce the food and can often be controlled by the food manufacturer.35 Once the food is ingested, there are normally appreciable changes in these interfacial properties due to a number of different physicochemical processes that occur in the GI tract. First, there may be changes in the total area of the oil–water interface in the system, which may promote either adsorption or desorption of surface-active substances. Second, there may be competitive adsorption processes between emulsifiers originally located at the surfaces of the lipid droplets in the food and various other surface-active substances arising from the food or the human body, e.g., FFAs, phospholipids, proteins, peptides, polysaccharides, and their complexes.26 Third, there may be alterations in interfacial composition due to the adsorption of charged substances, such as biopolymers or multivalent mineral ions, to the droplet surfaces. Fourth, there may be alterations in the chemistry of the molecules present at the interface due to enzyme hydrolysis (see below).
-
Lipid composition. The initial composition of the lipid phase in a food prior to ingestion depends on the type of food oils used to prepare it.10 After ingestion, the type and concentration of lipids present within a food may change as it passes through the GI tract due to the action of lipases and other enzymes upon them.10,33 For example, nonpolar TAGs are converted into more polar DAGs, MAGs, and FFAs by the action of gastric and pancreatic lipase in the stomach and small intestine. Previous studies have shown that there are differences in the absorption pathway of digested lipids after they enter the enterocytes. Short- and medium-chain fatty acids tend to be absorbed directly into the portal vein and pass through the liver before entering the systemic circulation, whereas long-chain fatty acids are assembled into lipoproteins (such as chylomicrons) within the enterocytes and are then transported into the systemic circulation via the lymph system.7
-
Solubilization. A key event in the absorption of digested lipids is their solubilization within mixed bile salt/phospholipid micelles and vesicles and their subsequent transport to the stomach intestinal walls.7 Thus, any food component that can interfere with micelle formation, solubilization, or transport may impact the rate of lipid digestion and absorption.38–40
It should be stressed that foods are highly complex systems that contain a wide variety of other components in addition to lipids, including proteins, polysaccharides, sugars, salts, and vitamins. Many of these components could potentially affect the characteristics of the lipid phase within the stomach GI tract, e.g., by interfering with the various processes mentioned above.
Defining Lipid Bioavailability
It is useful to define the term “lipid bioavailability” since this will help understand how lipid digestion and absorption processes can be controlled using physicochemical and structural approaches. Prior to ingestion, food is comprised of precise concentrations of specific lipid components, which can be given in terms of the grams of lipid per gram of food. As the lipids pass through the GI tract, some of these components are chemically modified (e.g., hydrolysis or oxidation) prior to absorption, so that the absorbed species is different from the ingested one. It is therefore important to be aware of any chemical changes that might occur to a lipid, the rate at which these changes occur, and the factors that impact them, since this will affect its subsequent digestion and absorption behavior.8
The term bioavailability has been defined as the fraction of an ingested component (or its products) that eventually ends up in the systemic circulation.41 For lipids, the bioavailability (F) can be defined as41:
In this equation, F B is defined as the bioaccessibility coefficient or fraction of the lipid that is released from the food matrix into the juices of the GI tract, F T is defined as the transport coefficient or the fraction of the released lipid component that is transported across the intestinal epithelium, and F M is the fraction of the lipid component that reaches the systemic circulation without being metabolized. The value of F M depends on the pathway that the lipid digestion products follow to reach the systemic circulation.10 Long-chain fatty acids are transported into enterocyte cells where they are converted back into triglycerides, repackaged into lipoproteins (chylomicrons and very-low-density lipoproteins) that enter the lymph system (thereby bypassing the liver), and then enter the blood stream near the heart.7 On the other hand, medium- and short-chain fatty acids do not follow this pathway. Instead, they diffuse through the enterocytes and pass directly into the bloodstream via the portal vein, which leads to the liver (where they may be metabolized) and then into the systemic circulation.7 After the component reaches the systemic circulation, it may be distributed between different tissues, where it may be stored, utilized, or excreted.8 The relative rates of these various processes determine the time dependence of the concentrations of the lipid and its metabolites at specific locations within the body. The concentration–time profile of a specific lipid component at a particular site of action will determine its beneficial or adverse affects on human health and wellness. Consequently, it is usually important to measure the concentration of a lipid component at a particular location to establish its efficacy.8
The time dependence of the concentration of a lipid component at a specific site of action in the human body after ingestion of a food often looks something like Figure 2. There is usually a lag period between the ingestion of a food and the first appearance of an increased concentration of the lipid component within the tissue of interest. The duration of this lag period depends on the time taken for the lipid component to be released from the food matrix and then digested, absorbed, and transported to the site where the sample was collected from. The overall shape of the curve depends on where the concentration of the component is measured (e.g., blood, liver, muscle, adipose tissue, feces), as well as the relative rates of release, digestion, absorption, transport, utilization, and excretion of the component.8
When considering factors that can impact the bioavailability of food components, it is also important to distinguish between their influence on the rate and extent of lipid digestion or absorption. Some factors may affect the rate at which a lipid component is digested or absorbed, without affecting the final amount (e.g., a fiber that increases viscosity but does not bind lipids), whereas other factors may affect the final amount of lipid that is digested or absorbed (e.g., a fiber that irreversibly binds lipids). If a food component spends sufficient time within the GI tract, then the rate of digestion and absorption may be less important than their total extent. On the other hand, if the rate of digestion or absorption can be slowed down sufficiently, it may reduce the bioavailability of the component.
Emulsion-based Structural Approaches to Controlling Lipid Bioavailability
Knowledge of the major factors influencing the mastication, digestion, and absorption of lipids by the human body can be used to develop rational strategies for controlling lipid bioavailability. In some foods, it may be desirable to increase lipid bioavailability, e.g., for bioactive lipids that normally have low digestibility/absorption2 or for creating foods for humans that have diseases that prevent efficient lipid digestion/absorption.4 In other foods, it may be important to decrease the bioavailability of (particular) lipids, e.g., in humans that are at risk of cardiovascular disease.3,4 In this section, we provide an overview of some of the major emulsion-based structural approaches that could be used to control lipid digestion and absorption.
Designing the Food Matrix
Ingested lipid droplets may be embedded within food matrices consisting of proteins, carbohydrates, and other natural or manufactured substances (Figure 3). In these systems, the rate and extent of lipid digestion within the stomach and small intestine may depend on how fast the GI tract can breakdown the matrix surrounding them since the lipids may have to be exposed before they can be digested by lipases.42 The food matrix may be broken down through a variety of different processes, such as physical fragmentation, erosion, or dissolution, which may be initiated by changes in pH, ionic strength, or enzyme activity. Alternatively, the digestion rate may depend on how quickly digestive enzymes can penetrate into the matrix and interact with encapsulated lipid substrates, without the matrix first having to be broken down. The rate of matrix disruption, dissolution, and/or digestion will depend on factors such as: the dimensions and exposed surface area of the matrix, the type and concentration of molecules present within the matrix (e.g., specific proteins, polysaccharides, and mineral ions), the nature of the bonds holding the molecules together within the matrix (e.g., electrostatic, hydrophobic, hydrogen bonding, or covalent), and the permeability of the matrix to the transport of small molecules (e.g., enzymes, acids and digestion products). The formation of food matrices with different degradation rates, dissolution rates, or permeabilities may therefore be a useful means of controlling lipid bioavailability or of targeting the release of lipid-based components to specific sites within the digestive tract.2,42
There are a large number of different ways that can be used by food manufacturers to embed lipid droplets within either microscopic or macroscopic matrices. For example, matrices could be formed by: controlled gelation of single or mixed biopolymer systems around lipid droplets,42–46 dehydration of oil-in-water emulsions containing biopolymers or other wall materials,47,48 or thermal treatment or extrusion of starch matrices containing lipid droplets.49–51 Nevertheless, there have been few systematic studies of the impact of food matrix design on the digestibility of lipids using either in vitro or in vivo digestion models, and the authors strongly encourage further research in this area.
Controlling Droplet Size
The rate of lipid hydrolysis in the stomach and small intestine should increase as the mean lipid droplet size decreases, because there is then a larger surface area of exposed lipid available for gastric or pancreatic lipases to attach to.34 The specific surface area of the lipids in an oil-in-water emulsion is given by the expression: A N = 6ϕ/d 32, where A N is the surface area of lipids exposed to the aqueous phase per unit volume of emulsion, d 32 is the surface-weighted mean diameter, and ϕ is the disperse phase volume fraction.35 In vitro experiments of lipase-catalyzed digestion of emulsified lipids have shown that the rate of lipid hydrolysis does increase with decreasing droplet size (increasing surface area).34,52 In principle, it may therefore be possible to control the rate and extent of lipid digestion by controlling the size of the lipid droplets in the stomach and small intestine. This could be done by changing the nature of the emulsifier and homogenization conditions used to prepare the emulsion.35 Nevertheless, in vivo studies where oil-in-water emulsions were injected into the stomach (rather than consumed) have shown that the overall extent of lipid hydrolysis is unaffected by the initial size of the lipid droplets (0.7 versus 10 μm).31 One of the reasons for the differences between in vitro and in vivo experiments is that the size of the lipid droplets often changes after ingestion (see below). In addition, even if there is a change in the rate of lipid hydrolysis, there may still be sufficient time within the human GI tract for all of the lipids to eventually be broken down. Hence, differences in the initial rate of lipid digestion are unimportant over the relatively long time scales that foods remain in the digestive tract since all the lipids are eventually digested.
As a food passes from mouth to stomach to the small intestine, there may be appreciable changes in lipid droplet size because of coalescence, disruption, and/or dissolution processes associated with the chemical, enzymatic, and physical mechanisms occurring in the GI tract. The lipid droplet size may increase, decrease, or remain approximately the same during passage through the stomach and small intestine depending on the initial droplet size and interfacial composition.3,4 The final mean diameter of ingested lipid droplets initially stabilized by phospholipids have been shown to be between 10 and 20 μm in humans, irrespective of the initial particle size.3,4,31,32 When the initial lipid droplets were larger than this range, they tended to be broken down in the stomach, but when they were smaller, they tended to coalesce. Presumably, a steady-state situation was reached where the droplet disruption rate in the stomach was balanced by the droplet coalescence rate. On the other hand, studies using some protein-stabilized lipid droplets (from milk) showed that the droplets remained small throughout the digestion process.53,54 This clearly indicates that the type of molecules at the oil–water interface surrounding the lipid droplets influences their stability to droplet coalescence and disruption and therefore influences the exposed lipid surface area. Consequently, it seems that one must control both droplet size and interfacial composition to control lipid digestibility.
Controlling Interfacial Composition
As mentioned above, the nature of the interfacial layer surrounding the lipid droplets can influence lipid digestion and absorption. Firstly, interfacial properties may affect the stability of the lipid droplets to breakup or coalescence within the mouth, stomach, and small intestine, which will influence the surface area of lipid exposed to enzymes.3,4 Second, the interfacial properties may affect the ability of gastric or pancreatic lipases (as well as colipases, phospholipids, and bile salts) to adsorb to their surfaces and act upon the nonpolar substrates contained within the droplets.55,56 If the lipase cannot adsorb to the droplet surfaces and come into close proximity to the lipids, then the lipids will not be digested. The initial composition and structure of the interfacial layers surrounding the lipid droplets in a food can be controlled by selecting specific emulsifier(s) and homogenization conditions to prepare an emulsion.35,57 Controlling the interfacial characteristics of the droplets entering the mouth (e.g., composition, electrical charge, thickness, surface tension, and rheology) should influence the tendency of the droplets to become disrupted or coalesced, as well as the ability of enzymes and other surface-active substances to adsorb to the droplet surfaces. After the emulsified lipids have entered the mouth, stomach, and small intestine, there may be appreciable changes in the interfacial composition over time due to competitive adsorption between the surface-active molecules present within the aqueous phase and those initially attached to the lipid droplet surfaces.56,58 This process depends on the relative concentrations and surface activities of the various surface-active substances present: the ones with the highest surface activity and concentration tending to dominate at the interface.59,60 One would expect that if the concentration of natural surface-active substances secreted by the human body was much greater than the concentration of surface-active substances arising from the food itself, then the interfacial composition would be dominated by the natural substances, e.g., phospholipids, fatty acids, bile salts, and enzymes. Nevertheless, it may be possible to control the extent of competitive adsorption that occurs within the system by carefully designing and engineering the interfacial characteristics of the lipid droplets, e.g., by using highly surface-active emulsifiers, by cross-linking the interfacial layers, or by forming enzyme-resistant interfacial layers.
Studies with rats have shown that casein-stabilized emulsion droplets are digested more slowly than phospholipid-stabilized droplets, which highlights the importance of interfacial composition on lipid digestion.54 In vitro studies in our laboratory found that the digestion rate of emulsified lipids by lipase depends strongly on interfacial composition.61 The extent of lipid hydrolysis was much greater for emulsions stabilized by proteins (caseinate or whey protein) than for those stabilized by small molecule surfactants (Tween 20 or lecithin). In another in vitro study, we observed that the rate of fatty acid release from emulsified lipids due to digestion with lipase decreased when they were coated with a layer of the cationic biopolymer chitosan using an electrostatic deposition method (Figure 4).62 It was proposed that the relatively thick cationic chitosan layer surrounding the lipid droplets prevented the lipase/colipase complex from coming into close proximity to the emulsified triacylglycerides, thereby slowing down the digestion rate. Nevertheless, animal-feeding studies using a mouse model found that there was no difference between body weights, major organ weights, or fecal fat contents of mice fed chitosan-coated or uncoated lipid droplets, with more than 90% of the fat been digested in all samples.63 This highlights the importance of backing up in vitro studies with in vivo studies to elucidate the efficacy of a proposed strategy. There are a number of possible reasons for the discrepancy between our in vitro and in vivo studies: (1) the passage of the food through the animals’ digestive tract is so long that even though the digestion rate is decreased, the overall amount of digested lipids released is the same for coated and uncoated lipids; (2) there may be a change in the microstructure of the emulsified lipids within an animal that does not occur in the in vitro experiments; for example, the chitosan coating may be displaced from the lipid droplet surfaces in an animal, which makes it ineffective. On the other hand, this result may have important implications for the utilization of chitosan as a material to encapsulate functional bioactive ingredients that need to be released in the human body.
A particularly powerful means of controlling the interfacial characteristics of lipid droplets in foods is the layer-by-layer (LBL) electrostatic deposition method. The charge, thickness, permeability, and environmental responsiveness of interfacial layers surrounding lipid droplets can be controlled using this method.64–67 The LbL approach involves successive deposition of oppositely charged polymers onto a droplet surface, which may then be cross-linked using a suitable method (e.g., physically, enzymatically, or chemically). By building thick, impermeable interfacial layers from biopolymers (proteins or polysaccharides) that are resistant to enzymatic degradation and displacement, it may be possible to reduce lipid bioavailability. Alternatively, it may be possible to encapsulate a lipophilic component and release it at a particular site-of-action (e.g., the stomach, small intestine, or large intestine) by controlling the environmental responsiveness of the interfacial layers (e.g., to pH, ionic strength of specific enzymes). Another approach for modulating the interfacial properties of emulsified lipids involves forming covalently cross-linked interfacial protein–polysaccharide complexes based on the Maillard reaction.6
In principle, careful design of the composition and properties of the interfacial layers surrounding lipid droplets may be a useful tool for food manufacturers to control lipid digestibility and absorption, although more research is needed in this area to understand how interfacial layers behave within animals and humans.
Controlling the Physical State of the Lipid Phase
The lipid phases in most foods are usually liquid at body temperature, but in some foods, they may be either fully or partially crystalline (Figure 3). In addition, delivery systems based on solid lipid particles (where the lipid phase is at least partly crystalline) have recently been developed for encapsulation, protection, and delivery of lipophilic functional components.68–70 It is therefore important to understand the influence of the physical state of the lipid phase on lipid digestion and absorption. Potentially, the crystallinity of the lipid phase may alter the ability of enzymes to digest the emulsified lipids (i.e., to convert the TAGs to FFAs and monoacyglycerols) or to alter the absorption of the digestion products (e.g., solubilization and transport in mixed micelles). Some studies have shown that long-chain fatty acids form insoluble soaps in the presence of multivalent cations (such as Ca2+ and Mg2+), thereby retarding lipid digestion.71,72 Other in vitro and in vivo studies have shown that encapsulated lipophilic molecules are released from solid lipid particles, which would suggest that the solidified lipids are still digested and the encapsulated lipophilic components are still bioavailable.73,74 Nevertheless, the release rate of lipophilic molecules from emulsified lipids during digestion depends on the crystallinity and structural organization of the lipid phase. For example, it has been shown that the release rate of a lipophilic drug decreased with increasing crystallinity of the lipid droplets.74 Recent experiments in our laboratory have also shown that solid tripalmitin particles are digested more slowly by lipase than liquid tripalmitin particles of the same size (Figure 5). These results are reported as the percentage of FFAs released compared to the total amount that would be released if all the TAG molecules present were converted to one MAG and two FFA molecules. These experiments show that it may be possible to use solid lipid particles to control the rate of digestion and the release of lipophilic bioactive components.
Desirable Characteristics of Structured Lipid Delivery Systems
There are a number of characteristics that an edible delivery system must have if it is going to be suitable for controlling the bioavailability of food lipids. Some of the most important of these attributes are listed below:
-
Food grade: The delivery system must be fabricated entirely from permitted food ingredients (generally recognized as safe) using processing operations that have regulatory approval.
-
Economic production: The delivery system should be capable of being economically manufactured from inexpensive ingredients.
-
Protection against chemical degradation: The delivery system may have to protect an encapsulated bioactive lipid against some form of chemical degradation during storage, e.g., oxidation, hydrolysis, etc.
-
Loading capacity and retention: A delivery system should be capable of encapsulating a relatively large amount of bioactive lipid per unit mass of carrier material and should efficiently retain the encapsulated component until it needs to be delivered.
-
Delivery mechanism: The delivery system may have to be designed so that it releases the bioactive lipid at a particular site of action, at a controlled rate or in response to a specific environmental stimulus (e.g., pH, ionic strength, enzyme activity, or temperature).
-
Food matrix compatibility: The delivery system should be compatible with the surrounding food matrix; that is, it should not adversely affect the appearance, texture, flavor, or stability of the final product.
-
Bioavailability/bioactivity: A delivery system should be capable of controlling the bioavailability/bioactivity of the encapsulated component in a known way.
Conclusions
Knowledge of the physicochemical basis of the mastication, ingestion, digestion, and absorption of particular food components is becoming increasingly important as the food industry attempts to rationally design and fabricate foods for specific nutritional performance. Nevertheless, the interaction of foods with the human body is extremely complex, involving many different molecular, physicochemical, and physiological processes, which depend on the composition, properties, and structure of the initial food, the characteristics of the individual consuming the food (e.g., age, sex, genetics, health, etc.), and various other factors (time of consumption, previous food consumed, food temperature). For this reason, there is currently a relatively poor understanding of the major factors that influence the digestion and absorption of specific food components. The authors believe that a highly integrated approach involving scientists from many different disciplines will be required to advance our understanding of this important field, e.g., food technologists, engineers, biochemists, biophysicists, sensory scientists, nutritionists, medical researchers, and psychologists. In particular, researchers will have to use a combination of in vitro and in vivo (animal and human) studies to understand the complex physicochemical processes that occur and to use this knowledge to develop effective strategies to design foods that can either increase or decrease the bioavailability of lipids.
References
P. Sanguansri, M.A. Augustin, Trends Food Sci. Technol. 17(10), 547–556 (2006)
J. Parada, J.M. Aguilera, J. Food Sci. 72(2), R21–R32 (2007)
E. Bauer, S. Jakob, R. Mosenthin, Asian–Australas. J. Anim. Sci. 18(2), 282–295 (2005)
G. Fave, T.C. Coste, M. Armand, Cell. Mol. Biol. 50(7), 815–831 (2004)
Y. Pafumi, D. Lairon, P.L. de la Porte et al., J. Biol. Chem. 277(31), 28070–28079 (2002)
M.A. Augustin, L. Sanguansri, O. Bode, J. Food Sci. 71(2), E25–E32 (2006)
C.J.H. Porter, N.L. Trevaskis, W.N. Charman, Nat. Rev. Drug Discov. 6(3), 231–248 (2007)
H. Verhagen, S. Coolen, G. Duchateau, M. Hamer, J. Kyle, A. Rechner, Mutat. Res., Fundam. Mol. Mech. Mutagen. 551(1–2), 65–78 (2004)
D. Lairon, B. Play, D. Jourdheuil-Rahmani, J. Nutr. Biochem. 18(4), 217–227 (2007)
P. Tso, ed. by K.D. Crissinger, M.H. Stipanuk. Biochemical and Physiological Aspects of Human Nutrition (Saunders, Philadelphia, PA, 2000), pp. 75–90
M.C. Carey, D.M. Small, C.M. Bliss, Annu. Rev. Physiol. 45, 651–677 (1983)
T. Sanz, H. Luyten, Food Hydrocoll. 21(2), 203–211 (2007)
T. Sanz, H. Luyten, Food Hydrocoll. 20(6), 892–900 (2006)
T. Sanz, H. Luyten, Food Hydrocoll. 20(5), 703–711 (2006)
M.H. Vingerhoeds, T.B.J. Blijdenstein, F.D. Zoet, G.A. van Aken, Food Hydrocoll. 19(5), 915–922 (2005)
M.E. Malone, I.A.M. Appelqvist, I.T. Norton, Food Hydrocoll. 17(6), 763–773 (2003)
M.E. Malone, I.A.M. Appelqvist, I.T. Norton, Food Hydrocoll. 17(6), 775–784 (2003)
T.B.J. Blijdenstein, E. van der Linden, T. van Vliet, G.A. van Aken, Langmuir 20(26), 11321–11328 (2004)
E.H.A. de Hoog, J.F. Prinz, L. Huntjens, D.M. Dresselhuis, G.A. van Aken, J. Food Sci. 71(7), E337–E341 (2006)
E. Silletti, M.H. Vingerhoeds, W. Norde, G.A. Van Aken, Food Hydrocoll. 21(4), 596–606 (2007)
E.A. Gwartney, D.K. Larick, E.A. Foegeding, J. Food Sci. 69(9), S333–S339 (2004)
G.A. Van Aken, ed. by D.J. McClements. Understanding and Controlling the Microstructure of Complex Foods (CRC, Boca Raton, FL, 2007), pp. 449–482
J. Bakker, D.J. Mela, ed. by R.J. McGorrin, J.V. Leland. Flavor–Food Interactions (American Chemical Society, Washington, DC, 1996), pp. 36–47
N.W. Weisbrodt, ed. by L.R. Johnson. Gastrointestinal Physiology, 6th edn. (Mosby, St. Louis, MO, 2001), pp. 37–45
N.W. Weisbrodt, ed. by L.R. Johnson. Gastrointestinal Physiology, 6th edn. (Mosby, St. Louis, MO, 2001), pp. 47–56
L. Kalantzi, K. Goumas, V. Kalioras, B. Abrahamsson, J.B. Dressman, C. Reppas, Pharm. Res. 23(1), 165–176 (2006)
M. Armand, Curr. Opin. Clin. Nutr. Metab. Care 10(2), 156–164 (2007)
D.C. Whitcomb, M.E. Lowe, Dig. Dis. Sci. 52(1), 1–17 (2007)
E. Jurado, F. Camacho, G. Luzon, M. Fernandez-Serrano, M. Garcia-Roman, Chem. Eng. Sci. 61(15), 5010–5020 (2006)
M. Mukherjee, J. Mol. Catal., B Enzym. 22(5–6), 369–376 (2003)
M. Armand, B. Pasquier, M. Andre et al., Am. J. Clin. Nutr. 70(6), 1096–1106 (1999)
M. Armand, B. Pasquier, P. Borel et al., OCL–Oleagineux Corps Gras Lipides 4(3), 178–185 (1997)
E. Whitney, S. Rolfes, Understanding Nutrition, 9th edn. (Wadsworth, Belmont, CA, 1999)
M. Armand, P. Borel, P. Ythier et al., J. Nutr. Biochem. 3(7), 333–341 (1992)
D.J. McClements, Food Emulsions: Principles, Practice, and Techniques, 2nd edn. (CRC, Boca Raton, FL, 2005)
M. Armand, P. Borel, C. Dubois et al., Gastroenterology 104(4), A608–A608 (1993)
S. Mun, E.A. Decker, Y. Park, J. Weiss, D.J. McClements, Food Biophys. 1(1), 21–29 (2006)
B. Pasquier, M. Armand, C. Castelain et al., Biochem. J. 314, 269–275 (1996)
D. Lairon, Dietary Fiber in Health and Disease (Plenum, New York, NY, 1997)
D. Lairon, Adv. Exp. Med. Biol. 427, 99–108 (1997)
C.H.M. Versantvoort, E. Van de Kamp, C.J.M. Rompelberg, Report no. RIVM Report 320102002/2004, 2004
L.Y. Chen, G.E. Remondetto, M. Subirade, Trends Food Sci. Technol. 17(5), 272–283 (2006)
S.W. Cui, Food Carbohydrates: Chemistry, Physical Properties and Applications (Taylor and Francis, Boca Raton, FL, 2005)
I.T. Norton, W.J. Frith, Food Hydrocoll. 15(4–6), 543–553 (2001)
I.T. Norton, W.J. Frith, S. Ablett, Food Hydrocoll. 20(2–3), 229–239 (2006)
B. Wolf, W.J. Frith, I.T. Norton, J Rheol 45(5), 1141–1157 (2001)
K.G.H. Desai, H.J. Park, Dry Technol. 23(7), 1361–1394 (2005)
S. Wongsasulak, T. Yoovidhya, S. Bhumiratana, P. Hongsprabhas, D.J. McClements, J. Weiss, Food Res. Int. 39(3), 277–284 (2006)
W. Li, B.J. Dobraszczyk, P.J. Wilde, J. Cereal Sci. 39(3), 403–411 (2004)
K. Eskins, G.F. Fanta, F.C. Felker, F.L. Baker, Carbohydr. Polym. 29(3), 233–239 (1996)
G. Yilmaz, R.O.J. Jongboom, H. Feil, W.E. Hennink, Carbohydr. Polym. 45(4), 403–410 (2001)
P. Borel, M. Armand, P. Ythier et al., J. Nutr. Biochem. 5(3), 124–133 (1994)
M. Armand, M. Hamosh, N.R. Mehta et al., Pediatr. Res. 40(3), 429–437 (1996)
M.C. Michalski, V. Briard, M. Desage, A. Geloen, Eur. J. Nutr. 44(7), 436–444 (2005)
P. Reis, K. Holmberg, T. Debeche, B. Folmer, L. Fauconnot, H. Watzke, Langmuir 22(19), 8169–8177 (2006)
A.W. Sonesson, U.M. Elofsson, H. Brismar, T.H. Callisen, Langmuir 22(13), 5810–5817 (2006)
S. Friberg, K. Larsson, J. Sjoblom, Food Emulsions, 4th edn. (Marcel Dekker, New York, 2004)
A.W. Sonesson, H. Brismar, T.H. Callisen, U.M. Elofsson, Langmuir 23(5), 2706–2713 (2007)
A. Mackie, P. Wilde, Adv. Colloid Interface Sci. 117(1–3), 3–13 (2005)
L.A. Pugnaloni, E. Dickinson, R. Ettelaie, A.R. Mackie, P.J. Wilde, Adv. Colloid Interface Sci. 107(1), 27–49 (2004)
S. Mun, E.A. Decker, D.J. McClements, Food Res. Int. 40(6), 770–781 (2007)
S. Mun, E.A. Decker, Y. Park, J. Weiss, D.J. McClements, Food Biophys 1(1), 21–29 (2006)
G.Y. Park, S. Mun, Y. Park et al., Food Chem. 104(2), 761–767 (2007)
G. Decher, Science 277, 1232–1237 (1997)
D. Guzey, D.J. McClements, Adv. Colloid Interface Sci. 128, 227–248 (2006)
D. Guzey, D.J. McClements, J. Agric. Food Chem. 55(2), 475–485 (2007)
S. Mun, E.A. Decker, D.J. McClements, Langmuir. 21(14), 6228–6234 (2005)
P.M. Bummer, Crit. Rev. Ther. Drug Carr. Syst. 21(1), 1–19 (2004)
R.H. Muller, C.M. Keck, J. Biotechnol. 113(1–3), 151–170 (2004)
R.H. Muller, K. Mader, S. Gohla, Eur. J. Pharm. Biopharm. 50(1), 161–177 (2000)
I. Read, Drugs Aging 21(1), 7–17 (2004)
T. Vaskonen, J. Nutr. Biochem. 14(9), 492–506 (2003)
C. Olbrich, O. Kayser, R.H. Muller, J. Nanopart. Res. 4(1–2), 121–129 (2002)
C. Olbrich, O. Kayser, R.H. Muller, Int. J. Pharm. 237(1–2), 119–128 (2002)
Acknowledgments
This material is based upon the work supported by the Cooperative State Research, Extension, Education Service, US Department of Agriculture, Massachusetts Agricultural Experiment Station (Project no. 831), and an US Department of Agriculture, CREES, NRI Grant (Award number 2005-01357).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McClements, D.J., Decker, E.A., Park, Y. et al. Designing Food Structure to Control Stability, Digestion, Release and Absorption of Lipophilic Food Components. Food Biophysics 3, 219–228 (2008). https://doi.org/10.1007/s11483-008-9070-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-008-9070-y