Abstract
The effect of storage temperature, pH, and homogenization pressure on the oxidative deterioration of Tween 20 and sodium caseinate sunflower oil-in-water emulsions was studied by monitoring conjugated dienes (CD), lipid hydroperoxides (LH), and thiobarbituric acid reactive substances (TBARs). CD increased linearly with storage time, and the rate constant was temperature dependent according to the Arrhenius equation with an activation energy equal to 37.5 kJ mol−1. The increase in LH and TBARs with temperature (5–60°C) was in good agreement with CD variation. Tween-stabilized emulsions oxidized faster as pH increased from 3 to 7, whereas a different behavior was observed in emulsions stabilized with sodium caseinate or a mixture of both emulsifiers. A change of homogenization pressure (30–900 bars), reflecting variation of emulsion average droplet size, had no effect on the oxidative stability of the emulsions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Factors that influence the oxidative stability of food emulsions are carefully considered recently, given the increased importance of these systems in many industrial applications. Several authors have examined the effect of pH on the oxidative deterioration of oil-in-water food emulsions, a parameter strongly dependent on the type of surfactant and presence of metal ions.1 Latest studies focus on the utilization of proteins as emulsifiers2 as the use of low molecular weight emulsifiers (e.g., Tween) is not extensively applicable in new emulsion products, which are developed by the food industry. Certain proteins at pH values below their pI can form positively charged emulsion droplets that are less susceptible to metal-promoted oxidation.3
Although, it is well known that oxidation is accelerated as temperature increases,4 there are limited published data on temperature dependence of oxidation rate in oil-in-water emulsions, which concern mostly subzero or low temperatures.5,6 A body of recent research7,8 has focused on homogenized, protein-stabilized oil-in-water emulsions, by elucidating their susceptibility at temperature-related microstructural irregularities. Furthermore, a better knowledge of how storage temperature can affect the development of lipid oxidation in these systems would also be of significant technological importance for the development of various products of similar structure and formulation, such as fresh cheese type, dairy spreads, and coffee creams.
High-pressure homogenization is an important process used in the preparation or stabilization of emulsions and suspensions, resulting in a decrease of the average droplet diameter and an increased interfacial area. The net result, from a practical point of view, is a much reduced tendency for creaming, contributing to an enhanced physical stability of the homogenized emulsions. Although several researchers have examined the effect of homogenization pressure on emulsion microstructural stability,7 there is no much literature evidence regarding any association of homogenization pressure with oxidative deterioration of the emulsions. A further elucidation of the effect of varying homogenization pressure (that generated varying droplet sizes) on oxidative deterioration of low molecular weight- and protein-stabilized o/w emulsions could contribute to control processing parameters for the production of high quality emulsified foods.
In a previous work we have examined the influence of several compositional parameters (e.g. type and concentration of emulsifier and lipid phase) on the oxidative stability of food oil-in-water emulsions.9 This paper focuses on the effect of various processing and storage parameters on the oxidative sensitivity of Tween- or sodium caseinate-stabilized emulsions. More specifically, the experimental work is investigating into the effect of storage temperature, pH, and homogenization pressure on emulsion oxidative deterioration, to propose strategies against the nutritional and quality deterioration of relevant products.
Experimental
Materials
The tested refined sunflower oil was donated by Minerva S.A. (Inofita, Greece). The sodium-caseinate (91.5% protein, Campina DMV) protein powder was kindly provided by Unilever R&D (Vlaardingen, the Netherlands). Tween 20 was purchased by Acros Organics. All the used chemicals and solvents were of analytical grade.
Preparation of the emulsions by use of ultrasonic agitation
Sunflower oil was used to prepare 20% w/w oil-in-water emulsions by mixing the appropriate mass of Toil for 1 min in a blender (Waring Commercial, USA), with distilled water or a buffer solution of 5 mÌ sodium acetate and 5 mM imidazole containing the emulsifier. Either Tween 20, sodium caseinate (Na-CAS), or a combination of the two at equal concentrations, was used as emulsifier, at a concentration of 1% w/w in the continuous phase. The pH was adjusted with the addition of 1 N HCl. The pre-emulsion was placed in an ice bath and subjected to sonication (Sonics, Vitracell) for 30 min. A 15-ml sample of each emulsion was transferred in 20-ml cupped vials and placed in a shaking water bath or in incubators for carrying out the oxidation experiment at 5, 15, 25, 40, or 60°C.
Preparation of the emulsions by use of high pressure homogenization
Sunflower oil was used to prepare 30% w/w oil-in-water emulsions by mixing the appropriate mass of oil for 1 min in a blender (Waring Commercial), with distilled water containing 2% of either Tween 20, or Na-CAS or a combination of the two at equal concentrations. The pre-emulsion passed through a high-pressure valve, two-stage APV Lab 1000 homogenizer (Albertslund, Denmark) at 30, 100, 300, and 900 bars. A 15-ml sample of each emulsion was transferred in 20-ml cupped vials and placed in a shaking water bath at 60°C for a week.
Methods of oxidative analysis
Measurement of conjugated diene hydroperoxides (CD)
A modification of the method described by IUPAC 2.50510 has been used for the determination of conjugated diene hydroperoxides. More specifically, the emulsion sample (20 μl) was added to a mixture of 10 ml isooctane/2-propanol (2:1 v/v) and vortexed (1 min). The absorbance was measured at 232 nm using a UV–VIS scanning spectrophotometer (Unicam Helios, Spectronic Unicam EMEA, Cambridge, United Kingdom). In particular, for the protein-based emulsions, a filtration through Macherey-Nagel filters (25 mm, pore 0.2 μm) was applied just before the measurement to remove protein from the sample and thereby diminish its spectrum interference in this region.
The amount of CD in the oxidizing emulsions was calculated by monitoring absorbance at 232 nm and using the relative molecular mass (280 g mol−1) and the molar absorptivity of linoleic acid (e = 26,000).11
Determination of lipid hydroperoxides (ferric thiocyanate method)
The Ferric thiocyanate method12 was alternatively used for the determination of primary oxidation products. Lipid hydroperoxides (LH) were measured by mixing 0.3 ml of emulsion with 1.5 ml of isooctane/2-propanol (3:1 v/v), by vortexing (10 s, three times), and isolation of the organic solvent phase by centrifugation at 1,000×g for 2 min. The organic solvent phase (200 μl) was added to 2.8 ml of methanol/1-butanol (2:1 v/v), followed by the addition of 15 ìl of 3.97 M ammonium thiocyanate and 15 μl of ferrous iron solution (prepared by adding equal amounts of 0.132 M BaCl2 and 0.144 M FeSO4). After 20 min, the absorbance was measured at 510 nm using a UV–VIS scanning spectrophotometer (Unicam Helios). LH concentrations were calculated using a standard curve made from cumene hydroperoxides.
Measurement of thiobarbituric acid-reactive substances (TBARs)
Thiobarbituric acid-reactive substances (TBARs) were determined according to an adapted method of McDonald and Hultin.13 The emulsion (0.3 ml) was combined with 0.7 ml of water and 2.0 ml of TBA solution (prepared by mixing 15 g trichloroacetic acid, 0.375 g thiobarbituric acid, 1.76 ml 12 N HCl and 82.9 ml H2O) in test tubes and placed in a boiling water bath for 15 min. The tubes were cooled to room temperature for 10 min and then centrifuged (2,000×g) for 15 min. The absorbance was measured at 532 nm. Concentrations of TBARs were calculated from a standard curve prepared with 1,1,3,3-tetraethoxypropane.
Microstructural analysis
Restricted diffusion-based droplet size measurements were obtained by means of pfg-NMR using a Minispec MQ20 (Bruker)7 at the Unilever Research and Development Center (Vlaardingen, the Netherlands).
The o/w emulsions were filled to a height of 15 mm in NMR tubes of 10 mm diameter, and thermally equilibrated for 30 min at 20°C. The filled tubes were inserted in a Bruker Minispec MQ20, operating at a proton resonance frequency of 20 MHz, equipped with a 499-10AVGX-diffusion probe especially designed to have high magnetic field homogeneity of 0.4 ms (dead time of the probe: 0.02 ms, magnet temperature 40°C). Obtained values of the volume weighted geometric mean diameter d 3,3 and the width σ of the droplet size distribution are converted to the surface weighted mean diameter d 3,2 using the relation d 3,2 = d 3,3 × exp(−σ 2/2).7
Statistical analysis
Oxidation experiments were carried out in triplicate and during analysis each measurement was repeated three times. Results were averaged (n = 9) and statistically analyzed with two-way ANOVA test (p < 0.05) by use of Statistica 7.0 statistical program. Differences between oxidative indicators for the various treatments were calculated by Post hoc comparison of means according to Duncan’s multiple range test.
Results
Effect of temperature on the oxidative stability of o/w emulsions
The effect of temperature on oxidation rates was studied in sunflower oil-in-water emulsions prepared with distilled water and stabilized with 1% Tween 20. The emulsions were left to oxidize in incubators at 5, 15, 25, 40, or 60°C, reflecting a range from chilling conditions to high temperatures of storage and transport of processed foods. Conjugated dienes (CD) were periodically determined as primary oxidation products, whereas secondary products of hydroperoxide decomposition were estimated by measuring the production of thiobarbituric-acid-related substances (TBARs) at 532 nm. The results of CD (g kg−1) accumulation are presented in Figure 1, showing the increase of oxidative deterioration of sunflower oil-in-water emulsions with increasing temperature. More specifically, a linear increase of CD with oxidation time was observed at all processing temperatures, following the equation:
where
- CD:
-
is the value of conjugated dienes after time t of oxidation (g kg−1)
- CDo :
-
is the initial value in fresh emulsion (g kg−1)
- k :
-
is the rate constant of the reaction (g kg−1 h−1)
- t :
-
is the time of oxidation (h)
The rate constants and the correlation coefficients of Eq. 1 are presented in Table 1. As oxidation proceeds, primary products like CD are further degraded to secondary ones. The determination of TBARs (Table 1) indicated that secondary oxidation products were at very low levels under our experimental conditions. Lipid hydroperoxides (LH) is another index of the primary oxidation products and were determined at the beginning and the end of oxidation experiments. The results, expressed as average increases over the oxidation time at each temperature, are presented in Table 1 and show a generally good agreement with the CD increase.
Plotting the lnk values of CD accumulation versus 1/T (Figure 2) indicated that the Arrhenius equation is followed with a good correlation coefficient (R 2 = 0.949):
where
- k :
-
is the rate constant of CD formation (h−1)
- R :
-
is the molar gas constant (8.32 J K−1 mol−1)
- T :
-
is the absolute temperature (K)
- k o :
-
is the preexponential factor of frequency (h−1)
- E a :
-
is the activation energy (J mol−1)
The activation energy (E a) was calculated through linear regression and found equal to 37.5 kJ mol−1.
Effect of pH on the oxidative stability of o/w emulsions
For the following series of experiments, a buffer solution of 5 mÌ sodium acetate and 5 mM imidazole was used and the pH was adjusted with the addition of 1 N HCl at values ranging from 1.7 to 7.0. The emulsions were stabilized with 1% Tween 20 or Na-CAS as emulsifier. Mixing with oil and subsequent emulsification generally increased the pH slightly (up to 0.14), whereas the accuracy of pH measurement was ±0.07. The presented initial pH values are those measured after emulsification. The emulsions were left to oxidize in a shaking water bath at 40°C and their instability over time was periodically evaluated with the measurement of CD (g kg−1) and LH (mmol kg−1) for the determination of primary oxidation products, whereas secondary products were estimated by measuring TBARs (mmol kg−1).
A pH dependence of oxidation was observed in Tween-stabilized emulsions with higher rate of oxidative deterioration observed at pH 7.0, followed by pH 5.5, and pH 3.0 (Figure 3). This rate of oxidation was in accordance with CD and LH values, as well as, TBARS. The pH values of the emulsions remained almost constant during the oxidation, with the exception of pH 7.0, which slightly decreased.
Contrary to the previous results, no such statistically significant difference was observed for the protein-stabilized emulsions in the pH range of 1.7 to 5.7, whereas the emulsions stabilized at pH 7.0 presented a lower rate of oxidation (data not shown).
To further elucidate the effect of pH on the oxidative deterioration of Tween- or Na-CAS-stabilized emulsions, an additional series of experiments was conducted involving 30% (o/w) emulsions stabilized with a mixture of Tween and Na-CAS (1% each) and adjusted at both acidic and neutral pH values. A mixture of Tween/Na-CAS 1:1 was used and an emulsion with higher oil concentration was prepared, which is closer to the formulation of innovative real products.9 From this point of view, this certain emulsion system was selected as appropriate to further examine any effect of pH. The water bath temperature was adjusted to 60°C to accelerate the oxidation experiment.
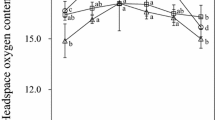
The results, presented in Figure 4, indicated no statistically significant difference in the oxidation rate, although a tendency of a higher accumulation of primary oxidation products was observed at longer oxidation times for neutral as compared to acidic pH.
Effect of neutral and acidic pH values on the production of conjugated diene hydroperoxides (CD—232 nm), lipid hydroperoxides (LH—510 nm), and thiobarbituric acid-reactive substances (TBARs—532 nm) in 30% sunflower o/w emulsion stabilized by 2% Tween 20/Na-CAS (1:1) and fluctuation of pH values during oxidation at 60°C.
Effect of homogenization pressure on the oxidative stability of o/w emulsions
To investigate the effect of homogenization pressure on the oxidation, a series of neutral 30% sunflower oil-in-water emulsions, prepared with varying emulsifiers (the low molecular weight Tween 20, Na-CAS, or an equal mixture of both) were homogenized under varying pressures (30, 100, 300, 900 bars). Emulsions were left to autoxidize for 1 week under accelerated conditions (60°C in a shaking water bath) and their oxidative status was periodically estimated by measurement of CD, whereas total changes of LH and TBARS were recorded as additional oxidative markers.
Droplet sizes of sodium caseinate emulsions (30% sunflower oil, 2% protein), homogenized under varying pressures were calculated with pfg-NMR analysis (results are shown in Table 2). It is clear that an increase of homogenization pressure in the range of 30–900 bars, led to a steady reduction of average oil droplet size (between∼3.2 and 0.7 μm, respectively), with only a very little change observed at >300 bars. A similar tendency has been reported by Kiokias et al.7 in preheated homogenized protein-stabilized systems of similar formulation, where the droplet size (d 3,2) decreased with homogenization pressure (p) according to the equation: d 3,2 = p −0.4. Our previous results9 showed that the emulsifying agent had no effect on droplet size. Therefore, similar sizes to those presented in Table 2 are expected for emulsions stabilized with Tween or mixtures of Tween and Na-CAS.
The results of conjugated dienes change (CDfinal − CDinitial) versus homogenization pressure are presented in Figure 5. According to the results, no significant effect (p < 0.05, ANOVA two-way test) of homogenization pressure on the oxidative destabilization of the emulsions has been observed in the whole tested range, independently of the emulsifier composition. Similar observations were obtained by LH and TBARS values (data not shown).
Discussion
Effect of temperature on the oxidative stability of o/w emulsions
The experimental results of this study indicated that during oxidation of o/w emulsions, CD increased linearly with time (Figure 1) in the studied range of temperature. Conjugated dienes are formed during the propagation stage of oxidation through reactions of the free radicals that have been generated at the initiation stage with di-unsaturated fatty acids. Sunflower oil is rich in di-unsaturated fatty acids, e.g., the oil used in our experiments has a linoleic acid content of 60.6%, whereas free radicals are regenerated through the reaction. Therefore, lack of influence of the reactants concentration on the rate of oxidation is not unexpected. A linear increase of CD values has also been observed by other researchers in accelerated oxidation of emulsions,14 during storage of fried products,15 and, similarly, apparent zero-order rates have been considered for the initial stage of oxidation of bulk oil16 and emulsion,5 based on experimental results of peroxide values.
The rate constants of CD accumulation increased with temperature, following the Arrhenius equation as presented in the “Results” section. The activation energy (E a) was found equal to 37.5 kJ mol−1, which is in agreement with literature data reporting typical E a values for lipid oxidation ranging from 24 to 240 kJ mol−1.16,17 The rather low value is not unexpected as CD are formed through reactions of free radicals, which are very active.
Effect of pH on the oxidative stability of o/w emulsions
The oxidation rate of Tween-stabilized emulsions increased as pH increased from 3.0 to 7.0. This observation is in agreement with those presented by other authors. More specifically, Huang et al.1 reported that oxidation of corn oil-in-phosphate buffer emulsions, emulsified with Tween 20, proceeded faster when pH increased from 3 to 7. Similarly, Ruth et al.18 studied the influence of pH on sunflower oil-in-water emulsions stabilized with 1% Tween 60 and found that the formation of conjugated diene hydroperoxides and hexanal were increased at pH 6, compared to pH 3. In addition, Mancuso et al.19 reported that the initial oxidation of emulsions was pH-dependent, with a varying effect for different emulsifiers. They observed a higher oxidation rate at pH 7 than at pH 3, especially in o/w emulsions stabilized with 1% Tween 20. The faster increase of primary oxidation products at higher pH could be caused by increased formation and decreased degradation of these products, or a combination of both factors. Increased formation of primary oxidation products may be associated with the partitioning of iron and other transition metals (that are naturally present in the oil, surfactant, and/or water), which is affected by pH. The increase of pH results in lowering the solubility of the transition metals in the continuous phase so that they precipitate to the droplets’ surface and promote oxidation.19,20 Alamed et al.21 confirmed the presence of endogenous metals that naturally exist in the oil and/or water, after the addition of different concentrations of EDTA, which inhibited lipid oxidation in salmon o/w emulsions stabilized with Brij 35.
On the other hand, in iron-catalyzed lipid oxidation of corn o/w emulsions stabilized with anionic, nonionic, or cationic surfactants, the oxidation rate was pH-dependent only for the samples emulsified with the anionic surfactant.22 These results were attributed to iron association with negatively charged emulsion droplets.23 The lack of influence of pH on the oxidation rate of emulsions stabilized with nonionic or cationic emulsifiers, when FeCl3 was added as oxidation catalyst, possibly indicates that iron concentration at the droplet interface is high enough to promote oxidation even at the low pH. In addition, the pH dependence of lipid oxidation is influenced by the buffer system,1 an observation that might explain some contradictory effects, which were indicated by the results of Paiva-Martins and Gordon 24 in olive oil emulsions stabilized by Tween 20 and adjusted to pH 3.5–7.4.
The observed decrease in pH of neutral emulsions as oxidation proceeds with time (Figure 3) may be attributed to the formation of several short chain aliphatic acids resulting from lipid oxidation as secondary products. Ruth et al.18 identified acetic, propanoic, 2-methylopropanoic, butanoic, pentanoic, hexanoic, heptanoic, octanoic, and nonanoic acids among the oxidation products of sunflower o/w emulsions. Diminished formation of acidic compounds is expected at lower pH as the oxidation rate is lower and more acid is required to alter the pH. In particular, for the protein-stabilized emulsions, it has been reported that alteration of basic amino acids of the proteins because of their reaction with primary or secondary products of oxidation may also be involved in this pH decrease with aging.2
In emulsions stabilized with the protein Na-CAS, the change of pH to values above and below the pI of the protein, (a value ∼4.6), results to a negative or positive charge of lipid droplets, respectively, which is expected to induce a different rate of emulsion oxidative deterioration. Therefore, at pH value below the pI of the protein, where emulsions droplets are charged positively, a lower oxidation rate would be expected because of the Coulombic repulsive forces between iron and droplet interface.25 However, the experimental results indicated a slightly lower accumulation of CD at pH 7.0. A similar pH effect on primary oxidation products was observed by Osborn-Barnes and Akoh26 in protein-stabilized emulsions and was attributed not to increased formation but to more rapid decomposition of the primary oxidation products at pH 7.0 by transition metals. Na-CAS has pronounced metal-binding properties, and the oxidative stability of Na-CAS-stabilized emulsions was found to depend on metal availability, but physicochemical conditions of the system may also influence its radical scavenging activity.2 Hu et al.3 reported that casein contains phosphoseryl groups that remain anionic at low pH; thus, they can chelate iron near the lipid droplet and consequently promote lipid oxidation. In fact, the charge of emulsion droplets is not the only factor affecting the oxidative stability of lipids in protein-stabilized emulsions.3,25
The different effects of the pH on the oxidation rates of emulsions stabilized with Na-CAS or Tween was further confirmed by the results obtained with the mixture of the emulsifiers. The contradictory behaviors observed with Tween and Na-CAS were balanced and the oxidation rate was unaffected by the pH change.
Effect of homogenization pressure on the oxidative stability of o/w emulsions
Concerning the strong relationship between homogenization pressure and droplet size, it could be hypothesized that a change in homogenization pressure could be a determining factor for monitoring oxidative deterioration. In general, data concerning the influence of oil droplet size and interfacial area on lipid oxidation of emulsions is scarce and sometimes confusing. Several researchers27,28 found no effect of droplets size on lipid oxidation of soybean and structured lipid-based o/w emulsions, respectively. On the other hand, others14,29 concluded that oil-in water emulsions with smaller droplets were faster oxidized, whereas Nakaya et al.30 reported the opposite result.
It is known that the increase of homogenization pressure generally leads to reduction of oil droplet size, as already previously discussed. Moreover, smaller droplet sizes (produced at increasing pressures) signify a larger surface area, which could reasonably associate with a higher potential of contact between diffusing oxygen, water-soluble free radicals, and the oil–water interface. Therefore, a general trend of increased oxidative sensitivity in emulsion containing smaller droplets would be expected. However, the results of the current experiment indicated no statistically significant difference between different homogenization pressures and oxidative destabilization of sunflower o/w emulsions, independent of the type of emulsifier. These results are in agreement with those of Shimada et al.27 and Osborn and Akoh.28 Moreover, the value of activation energy (E a = 37.5 kJ mol−1) calculated in this work, together with values reported by other researchers (24–240 kJ mol−1), indicate that oil oxidation is controlled by chemical reactions rather than diffusion phenomena. Thus, the effect of interfacial area and droplet size is expected not to be significant. Contrary to the present results, Lethuaut et al.14 found higher oxidation rates when the interfacial area was increased, but their experiments were conducted in sealed vials, implying limited oxygen availability in the headspace so that diffusion might have been the rate-limiting factor. In addition, they observed that the increase in oxidation rate was much lower than the increase in interfacial surface, although the result was probably attributed to the antioxidant potential of protein that was used as emulsifier. Coupland et al.31 commented that when oxygen and oxy-radicals concentration in the aqueous phase is not reduced (e.g., by interactions with food components), changes in droplet size would affect oxidation kinetics only if they allow a higher proportion of the oxidizable triglycerides to accumulate at the interface. Considering the lack of a universal correlation between particle size and oxidation, Shen et al.32 suggested that the mobility of particles in an emulsion has a more predominant influence on oxidation rates.
Interestingly, but not surprisingly, emulsions prepared by Tween at all homogenization pressures were much faster oxidized than the rest, whereas the pure protein preparations were the most stable. Such an influence of emulsifier composition on oxidation has been recently reported and discussed by Kiokias et al.9
The results of this study indicated that the formation of primary oxidation products in emulsions is strongly temperature-dependent and the quantification of the reaction rate’s reliance with temperature could help to monitor the oxidative alterations. On the other hand, the effect of pH is associated with the emulsifier used, and is more pronounced in Tween-stabilized emulsions. Homogenization pressure and, consequently, droplet size seemed not to affect the oxidation rate so far as availability and mobility of radicals is not eliminated.
References
S.W. Huang, E.N. Frankel, K. Schwarz and J.B. German, J Agric Food Chem 44, 2496–2502 (1996).
A. Villiere, M. Viau, I. Bronnec, N. Moreau and C. Genot, J Agric Food Chem 53, 1514–1520 (2005).
M. Hu, D.J. McClements and E.A. Decker, J Agric Food Chem 51, 1696–1700 (2003).
M.P. Almajano and M. H. Gordon, J Am Oil Chem Soc 81, 275–279 (2004).
S. Calligaris, L. Manzocco and M.C. Nicoli, Food Chem 101, 1019–1024 (2007).
L.B. Fomuso, M. Corredig and C.C. Akoh, J Agric Food Chem 50, 7114–7119 (2002).
S. Kiokias, C.K. Reiffers-Magnani and A. Bot, J Agric Food Chem 52, 3823–3830 (2004).
S. Kiokias and A. Bot, Food Hydrocolloids 19, 493–501 (2005).
S.N. Kiokias, C.P. Dimakou, I.V. Tsaprouni and V. Oreopoulou, Food Biophysics FOBI 1, 115–123 (2006).
IUPAC. In: Standard Methods of Analysis of Oils Fats and Derivatives 7th ed., edited by C. Paquot and H. Hautfenne (Blackwell Scientific Publications, Oxford, UK 1987), pp. 212–213.
S. Kiokias and M. Gordon, Eur J Clin Nutr 57, 1135–1140 (2003).
C.D. Nuchi, P. Hernadez, D.J. McClements and E.A. Decker, J Agric Food Chem 50, 5445–5449 (2002).
R.E. McDonald and H.O. Hultin, J Food Sci 52, 15–21, 27 (1987).
L. Lethuaut, F. Metro and C. Genot, J Am Oil Chem Soc 79, 425–430 (2002).
D.P. Houhoula and V. Oreopoulou, J Food Eng 65, 427–432 (2004).
S. Calligaris, L. Manzocco, L.S. Conte and M.C. Nicoli, J Food Sci 69, 361–366 (2004).
C.P. Tan, Y.B. Che Man, J. Selemat and M.S.A. Yusoff, J Am Oil Chem Soc 78, 1133–1137 (2001).
S.M Ruth, J.P. Roozen, M.A. Posthumus and F.J.H.M. Jansen, J Agric Food Chem 47, 4365–4369 (1999).
J.R. Mancuso, D.J. McClements and E.A. Decker, J Agric Food Chem 47, 4112–4116 (1999).
Y.J. Cho, D.J. McClements and E.A. Decker, J Agric Food Chem 50, 5704–5710 (2002).
J. Alamed, D.J. McClements and E.A. Decker, Food Chem 95, 585–590 (2006).
L. Mei, D.J. McClements, J. Wu and E.A Decker, Food Chem 61, 307–312 (1998).
L. Mei, D.J. McClements and E.A Decker, J Agric Food Chem 46, 5072–5077 (1998).
F. Paiva-Martins and M.H. Gordon, J Am Oil Chem Soc 79, 571–576 (2002).
M. Hu, D.J. McClements and E.A. Decker, J Agric Food Chem 51, 1435–1439 (2003).
H.T. Osborn-Barnes and C.C. Akoh, J Agric Food Chem 51, 6851–6855 (2003).
K. Shimada, H. Okada, K. Matsuo and S. Yoshioka, Biosci Biotechnol Biochem 60, 125–127 (1996).
H.T Osborn and C.C. Akoh, Food Chem 84, 451–456 (2004).
S. Gohtani, M. Sirendi., N. Yamamoto, K. Kajikawa and Y. Yamamoto, J Dispers Sci Technol 20, 1319–1325 (1999).
K. Nakaya, H. Ushio, S. Matsukawa, M. Shimizu and T. Ohshima, Lipid 40, 501–507 (2005).
J.N. Coupland, Z. Zhu, H. Wan, D.J. McClements, W.W Nawar and P. Chinachoti, J Am Oil Chem Soc 73, 795–801 (1996).
Z. Shen, P. Udabage, I. Burgar and M.A. Augustin, J Am Oil Chem Soc 82, 797–802 (2005).
Acknowledgment
The current project was financed by a European Marie-Cure Reintegration Program (6th Framework, Contract no. 513675).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dimakou, C.P., Kiokias, S.N., Tsaprouni, I.V. et al. Effect of Processing and Storage Parameters on the Oxidative Deterioration of Oil-in-Water Emulsions. Food Biophysics 2, 38–45 (2007). https://doi.org/10.1007/s11483-007-9027-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-007-9027-6