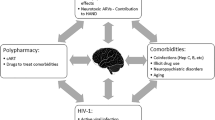
Abstract
The development of novel antiretroviral treatments has led to a significant turning point in the fight against HIV. Although therapy leads to virologic suppression and prolonged life expectancies, HIV-associated neurocognitive disorder (HAND) remains prevalent. While various hypotheses have been proposed to explain this phenomenon, a growing body of literature explores the neurotoxic effects of antiretroviral therapy. Research to date brings into question the potential role of such medications in neurocognitive and neuropsychiatric impairment seen in HIV-positive patients. This review highlights recent findings and controversies in cellular, molecular, and clinical neurotoxicity of antiretrovirals. It explores the pathogenesis of such toxicity and relates it to clinical manifestations in each medication class. The concept of accelerated aging in persons living with HIV (PLWH) as well as potential treatments for HAND are also discussed. Ultimately, this article hopes to educate clinicians and basic scientists about the neurotoxic effects of antiretrovirals and spur future scientific investigation into this important topic.

Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The HIV epidemic led to the development of a myriad of antiretroviral therapies. First discovered was azidothymidine (AZT), and after patterns of AZT-resistance emerged, other nucleoside reverse transcriptase inhibitors (NRTIs) were developed. Next came non-nucleoside reverse transcriptase inhibitors (NNRTIs) and protease inhibitors (PIs). Later came integrase inhibitors, fusion inhibitors, and entry inhibitors. A pharmacokinetic enhancer class (cobicistat) was recently introduced designed to improve the pharmacokinetics and increase effectiveness of HIV medications. Today, a regimen combining two NRTIs and one integrase inhibitor is typically recommended, though a multitude of other options exist based on individual circumstances such as genotypic resistance, prior exposure and demonstrated medication intolerance (Saag et al. 2018). With the advent of combination antiretroviral therapy (cART; sometimes referred to as highly active antiretroviral therapy or HAART), a once fatal disease has become indefinitely controllable, leading to drastically increased life expectancies in affected patients (Marcus et al. 2016). Since a definitive cure is not yet available, patients require life-long therapy, and with such a prolonged exposure to medications (in addition to long-term toxicity from the first-generation medications), a careful consideration of neurological adverse effects is warranted.
In particular, antiretroviral use has been associated with a range of neurological toxicity, from peripheral neuropathy to neuropsychiatric and neurocognitive deficits in the central nervous system (CNS) (Meeker et al. 2014). However, it is often difficult to distinguish certain adverse effects caused by HIV medications from direct and indirect deleterious effects from the virus itself (Treisman and Soudry 2016). One such instance is HIV-associated neurocognitive disorder (HAND), a term which describes several disorders based on severity of neurocognitive impairment. They are asymptomatic neurocognitive impairment (ANI), mild neurocognitive disorder (MND), and HIV-associated dementia (HAD), a progressive and life-threatening form of dementia (Antinori et al. 2007; Letendre 2011). To date, no specific treatment exists for HAND nor is a diagnostic biomarker available (Saylor et al. 2016). Although other non-neurological conditions have declined in prevalence due to the efficacy of cART, HAND remains common in the cART era. It is estimated that about one third of HIV+ patients have a HAND diagnosis and over half have neuropsychological impairment (Heaton et al. 2010; Sacktor et al. 2016). Interestingly, the compositional prevalence of its subgroups has changed in the cART era. HAD has become increasingly uncommon (2%) while rates of ANI and MND actually increased (Heaton et al. 2011; Singer and Nemanim 2017). This suggests that either cART is unable to adequately suppress HIV in the nervous system or that cART use is contributing to the development of HAND (Etherton et al. 2015).
In this article, we evaluate each of the classes of HIV therapy, reviewing the latest concepts and controversies regarding the clinical manifestations and cellular mechanisms of ART-induced CNS neurotoxicity. Where applicable, we include antiretroviral routes of administration in in vivo studies (intraperitoneal, CSF, etc.), and mention when medications used in studies are clinically relevant. However, note that estimating clinically-relevant concentrations is difficult, given lack of data on antiretroviral CSF:plasma area under the curve, predictions that parenchymal concentrations can reach greater levels than in the CSF, and the fact that HIV disrupts the blood brain barrier (BBB), allowing for increased antiretroviral CSF accessibility (Decloedt et al. 2015; Jensen et al. 2015). We discuss how CNS penetrance by ART may affect neurotoxicity, explore the concept of accelerated aging in PLWH (persons living with HIV), and highlight recent advancements in the possible treatment of HAND. Peripheral nervous system toxicity is beyond the scope of this review and only briefly covered.
Nucleoside Reverse Transcriptase Inhibitors (NRTIs)
NRTIs, the first class of HIV medications discovered, work by blocking reverse transcriptase, thereby preventing the virus from generating functional cDNA via premature DNA strand termination (Shah et al. 2016). In ascending order of approval date, the NRTIs are azidothymidine/zidovudine (AZT), didanosine (ddI), stavudine (d4T), lamivudine (3TC), abacavir (ABC), tenofovir disoproxil fumarate (TDF), emtricitabine (FTC), and tenofovir alafenamide fumarate (TAF). Older NRTIs such as AZT were found to have more off-target effects, limiting their clinical use relative to newer agents (Schweinsburg et al. 2005).
Although potent inhibitors of reverse transcriptase, NRTIs also cause off-target inhibition of mitochondrial polymerase γ, the enzyme responsible for normal mitochondrial DNA replication (Kakuda 2000). Through this inhibition, the primary mechanism of NRTI toxicity appears to be mitochondrial toxicity, energy depletion, and oxidative stress, which have been demonstrated both in vitro and in vivo (Lewis et al. 2003; Kohler and Lewis 2007; Nooka and Ghorpade 2018). The extent of mitochondrial polymerase γ inhibition among NRTIs is ddI > d4T > > 3TC > TDF ≥ FTC ≥ AZT ≥ ABC (Bienstock and Copeland 2004). This type of mitochondrial toxicity is considerably cell/tissue-dependent. Stavudine impairs mitochondria in axons and Schwann cells causing peripheral neuropathy, AZT impairs mitochondria in skeletal muscles and causes myopathy, and others can cause lipoatrophy and lactic acidosis (White 2001; Abers et al. 2014; Margolis et al. 2014). Mitochondrial DNA (mtDNA) depletion from NRTI exposure is also persistent, dependent on cumulative exposure, and can cause long-term effects even after discontinuation (Poirier et al. 2003; Underwood et al. 2015). TAF, a prodrug of tenofovir and a component of the vast majority of modern regimens, produces greater intracellular concentrations than TDF, which might lead to worse neurotoxicity.
It was previously thought that NRTI neurotoxicity was limited to the periphery, but emerging evidence has called this into question. From a clinical standpoint, AZT is known to cause insomnia, nausea, and severe headaches, and in high doses can cause seizures (Richman et al. 1987; Saracchini et al. 1989). Other NRTIs have been linked to retinal atrophy, and dose-dependent psychiatric disturbances (Turjanski and Lloyd 2005; Gabrielian et al. 2013). One study used magnetic resonance spectroscopy in patients as a proxy for brain mitochondrial integrity and their results suggested that didanosine and/or stavudine may cause depleted brain mitochondria (Schweinsburg et al. 2005). On a cellular level too, NRTIs have been implicated in CNS toxicity. Abacavir induced endoplasmic reticulum (ER) stress in human astrocytes at therapeutic doses, activating all three unfolded protein response (UPR) pathways in vitro (Nooka and Ghorpade 2017; Nooka and Ghorpade 2018). Oligodendrocyte dysfunction (both in vitro and in vivo with intravenous administration) seen with other ART drugs (such as ritonavir and lopinavir) was not observed in NRTIs (Jensen et al. 2015). In mice, long-term intraperitoneal NRTI administration at clinically relevant concentrations led to mtDNA deletion and mitochondrial toxicity in cortical neurons (Zhang et al. 2014; Hung et al. 2017). Additionally, TDF has been associated with increased risk of developing chronic kidney disease (Scherzer and Shlipak 2015) (presumably through mitochondrial nephrotoxicity (Rodriguez-Nóvoa et al. 2010)) which, in itself, is known to cause cognitive decline (Etgen et al. 2012). Overall, given the link between mitochondrial dysfunction and cognitive impairment (Finsterer 2012), researchers have suggested that although no direct clinical association has been found, NRTI-related mitochondrial toxicity may directly or indirectly contribute to the development of HAND (Hung et al. 2017).
Non-nucleoside Reverse Transcriptase Inhibitors (NNRTI)
NNRTIs include, in order of approval, nevirapine, delavirdine, efavirenz, etravirine, rilpivirine and doravirine. Unlike NRTIs, these drugs do not resemble nucleotides/nucleosides and act on reverse transcriptase noncompetitively to impair cDNA synthesis. Although this class is generally better tolerated than NRTIs, resistant HIV strains became problematic, necessitating that NNRTIs be used in combination with other antiretrovirals (hence cART) (Margolis et al. 2014). As a class, the most common adverse event is rash, though individual drugs in this class have their own specific side effect profiles (Drake 2000).
Of the NNRTIs, the most infamous for CNS toxicity is efavirenz, which in the past was also one of the most commonly prescribed cART components due to its efficacy and favorable pharmacokinetics (Shah et al. 2016). Efavirenz has been associated with both neurological (dizziness, insomnia, vivid dreams, headache, and impaired concentration) and psychiatric (paranoia, hallucinations, anxiety, mania, and depression) adverse effects (Apostolova et al. 2015). These adverse effects occur in upwards of half of patients taking efavirenz and although they typically resolve after several weeks, some can be more persistent (Arendt et al. 2007). The adverse effect most classically associated with efavirenz is vivid dreams. An ambulatory electroencephalogram (EEG) study found that patients taking efavirenz (in a dose-dependent manner) had longer sleep latencies and shorter duration of rapid eye movement (REM) sleep, which was theorized to result in more intense REM periods (i.e. vivid dreams) (Gallego et al. 2004). This lack of sleep efficacy (which typically persists for over 3 months of therapy) also would explain the daytime fatigue and somnolence experienced by patients on the medication (Moyle et al. 2006). Psychiatric symptoms caused by efavirenz exposure can be even more disabling for certain patients. The population of PLWH already have higher rates of psychiatric disorders than the general population (with nearly half of PLWH screening positive) (Bing et al. 2001). Clinicians therefore need to carefully screen and monitor their patients when prescribing efavirenz, especially since it may cause increased rates of suicidality (Mollan et al. 2014), although this remains controversial (Kenedi and Goforth 2011). However, when mental illness contraindicates this drug, using alternative regimens which have less convenient dosing schedules could lead to decreased ART adherence (Kenedi and Goforth 2011).
The mechanisms responsible for efavirenz neurotoxicity (or more relevantly, its main metabolite, 8-hydroxy-efavirenz, a more potent neurotoxin than the parent drug) are currently not well elucidated (Apostolova et al. 2015; Grilo et al. 2017). Recently, there has been considerable scientific interest in understanding how pharmacogenetics impacts its CNS side effects. Research suggests that, similar to NRTIs, the toxicity of efavirenz is mediated by oxidative stress and consequent mitochondrial dysfunction (in addition to elevating intracellular pro-inflammatory factors) (Shah et al. 2016; Ciavatta et al. 2017). Furthermore, efavirenz is consistently found to be more neurotoxic than other ART drugs tested, consistent with its clinical side effect profile. In one experiment of four antiretrovirals in primary rat neurons, efavirenz was the only one to cause ER stress and mitochondrial toxicity at clinically-relevant concentrations (Blas-García et al. 2014). In an in vitro study, efavirenz elicited a dose-dependent (encompassing the range of clinical concentrations) impairment in striatal nerve terminal mitochondrial respiration, leading to depleted ATP levels at the synapse (Stauch et al. 2017). In a recent in vitro and ex vivo study, efavirenz was the only NNRTI (and more potently than ART drugs in other classes) that demonstrated detrimental effects on neuronal viability, morphology, respiration, and excitability when exposed to rat cortical neurons at target plasma concentrations (Ciavatta et al. 2017).
Given the well-characterized CNS side effect profile of efavirenz and the persistence of HAND in the cART era, researchers were interested in its effect on cognitive function. As expected, efavirenz is associated with long-term cognitive impairment. In a recent large cohort study, patients taking long-term efavirenz had significant neurocognitive impairment in many domains compared to those taking lopinavir-ritonavir. This effect was less among HCV seropositive individuals (Ma et al. 2016). Another large study observed efavirenz use was associated with HAND, with higher education acting as a protective factor (Ciccarelli et al. 2011). Switching patients from efavirenz to an alternative regimen did not lead to improvement in neurocognitive measures after 10 weeks, suggesting that efavirenz likely leads to persistent neurocognitive dysfunction (Payne, Chadwick et al. 2017).
Other drugs in the NNRTI class in addition to efavirenz are known to have CNS toxicity, with nevirapine being more toxic than the remaining NNRTIs (Shah et al. 2016). However, compared to efavirenz, these drugs’ CNS side effects are less studied, less frequent, and less significant in clinical practice (Abers et al. 2014).
Protease Inhibitors (PI)
In the HIV life cycle, once mRNA is translated into protein precursors, a virally-encoded protease is required to cleave these into mature proteins (Flexner 1998; Brik and Wong 2003). The protease enzyme as a therapeutic target led to the development of protease inhibitors, including saquinavir mesylate, ritonavir, indinavir, nelfinavir mesylate, lopinavir, atazanavir sulfate, fosamprenavir calcium, tipranavir, and darunavir. Of note, after discovering the cytochrome P450-inhibiting effects of ritonavir, it is now used mostly as a pharmacokinetic booster, allowing for less-frequent dosing of PI-containing regimens (Lv et al. 2015). In comparison to NNRTI-containing regimens, PI-based regimens were found to have lower rates of resistance (Riddler et al. 2008), though the use of PIs has been limited by their drug-drug interactions and off-target toxicities. In particular, they can cause lipodystrophy syndrome (due to homology between protease enzyme and two lipid metabolism enzymes) and insulin resistance (which in some cases, can lead to the development of diabetes), in addition to cardiovascular disease (Carr 2000; Brown et al. 2005; Lv et al. 2015). Newer PIs, such as darunavir, have been designed specifically to minimize these off-target effects (Pokorná et al. 2009).
Results from cell and animal studies of PI neurotoxicity have been mixed. In one in vitro study, darunavir did not cause mitochondrial toxicity in rat neurons at clinically relevant concentrations, unlike efavirenz (Blas-García et al. 2014). Lopinavir and to a lesser extent, amprenavir, caused disruption of astrocytic glutamate homeostasis in vitro and were associated with gliosis and neurobehavioral deficits in mice exposed to oral doses (Vivithanaporn et al. 2016). Lopinavir, but not darunavir, was neurotoxic to primary rat neuroglial cultures. This was thought to be mediated by oxidative stress (Stern et al. 2018). In another in vitro study, darunavir caused reactive oxygen species (ROS) production in astrocytes although not at clinically relevant concentrations (Latronico et al. 2018). Intravenous ritonavir and lopinavir (at doses based on human plasma and CSF levels) had detrimental effects on mice oligodendrocyte maturation in vivo which was reversed with drug cessation (Jensen et al. 2015). Investigators studying the effects of ART on neurotransmitter release found that indinavir reduced in vitro synaptic acetylcholine transmission at plasmalevel concentrations, although at supraphysiological concentrations.
PIs also appear to cause certain CNS effects on a clinical level. Ritonavir was shown to be more neurotoxic than other PIs and can cause nausea, dizziness, and circumoral paresthesia (Markowitz et al. 1995). However, using ritonavir as an pharmacokinetic enhancer allows for lower doses, which reduces the frequency of adverse events (Hill et al. 2009). Several studies (Bacellar et al. 1994; Pettersen et al. 2006) have found increased risk of peripheral neuropathy with PI use (although a recent analysis found the independent risk from PIs is small (Ellis et al. 2008)). Based on results from aforementioned cell and animal studies, it is feasible that PI use could contribute to neurocognitive dysfunction. HAND has been associated with myelin disruption (with reduced levels of myelin basic protein) and structural white matter deterioration on imaging (ritonavir and lopinavir have oligodendrocyte toxicity (Jensen et al. 2015)). Furthermore, since neurotransmitter system dysfunction could help explain ART CNS toxicity, the authors who found impaired synaptic acetylcholine transmission with indinavir suggested that this may contribute to cognitive dysfunction (Ekins et al. 2017). An autopsy study found that PI exposure increased the risk of cerebral small vessel disease, which was, in turn, associated with neurocognitive impairment (Soontornniyomkij et al. 2014). A large study did not find differences in neurocognitive performance with PI use, in comparison to triple therapy, after several years (Arenas-Pinto et al. 2016). Another study found that CSF viral escape (when HIV is detectable in CSF but not in the serum) is associated with PI use, but did not lead to worse neurocognitive performance (Pérez-Valero et al. 2019). PI use is associated with hyperbilirubinemia, but this was not shown to affect neurocognitive function (Barber et al. 2016). Despite the link between PIs and certain neurologic adverse effects, there is little, if any, clinical or preclinical evidence of a link between their use and HAND.
Integrase Inhibitors
Integrase is an HIV-encoded protein necessary for integration of viral cDNA into host DNA and after 12 years of development, the first agent in the integrase inhibitor class, raltegravir, was introduced in 2007 (Pommier et al. 2005), followed by dolutegravir, elvitegravir, and most recently approved, bictegravir in 2018. In general, these drugs are some of the most efficacious among antiretrovirals, have low rates of resistance, and are relatively tolerable in the clinical setting (Patel 2018). The most common side effects of this class include diarrhea, nausea, and headache (del Mar Gutierrez et al. 2014). In clinical trials, raltegravir had lower rates of CNS adverse events than efavirenz and similar rates of severe adverse effects relative to placebo (Lennox et al. 2010; Steigbigel et al. 2010; Nguyen et al. 2011). Subsequent studies found higher rates of myalgia in patients taking raltegravir although this was rarely a cause for discontinuation (Lee et al. 2013). A large study in Botswana found evidence for neural tube defects associated with dolutegravir use during pregnancy (Zash et al. 2017; Zash et al. 2018). The most common neuropsychiatric effect reported with raltegravir and dolutegravir is insomnia which was reversible after drug cessation and can be improved by switching to morning dosing schedules (Gray and Young 2009; Capetti et al. 2017). Other neuropsychiatric effects linked to integrase inhibitors include depression and anxiety and have been found to have higher rates than initially suggested by clinical trials (Harris et al. 2008; Curtis et al. 2014; Fettiplace et al. 2017; Harris 2018). A large clinical study found that the discontinuation rates due to adverse events for raltegravir, dolutegravir, and elvitegravir were 3.6, 3.8, and 5.0% (Penafiel et al. 2017). Dolutegravir had higher rates of discontinuation due to neuropsychiatric effects compared to raltegravir and elvitegravir. These results were consistent with findings from a previous cohort study which additionally showed an almost three-fold increase in discontinuation rate in female patients and older patients (Hoffmann et al. 2017). When bictegravir was introduced, trials found rates of neuropsychiatric effects comparable to dolutegravir, suggesting a class effect of integrase inhibitors (Gallant et al. 2017; Sax et al. 2017).
Although reports of neuropsychiatric effects from integrase inhibitors suggest neurotoxicity, underlying mechanisms for such toxicity are not fully understood. In one in vitro study, raltegravir did not cause mitochondrial toxicity in rat neurons at clinically-relevant concentrations, unlike efavirenz (Blas-García et al. 2014). In another, raltegravir caused ROS production in astrocytes, although not at clinically relevant concentrations (Latronico et al. 2018). However, an in vitro study found that elvitegravir but not raltegravir nor dolutegravir was neurotoxic to primary rat neuroglial cultures at clinically relevant plasma level concentrations. This effect was thought to be mediated by the integrated stress response (ISR) rather than strictly oxidative stress (Stern et al. 2018). The ISR is normally an adaptive response to cellular stressors which restores homeostasis but with prolonged exposure to certain insults, this response activates pathways that lead to cell death (Pakos-Zebrucka et al. 2016).
A clinical study of dolutegravir-containing ART found high dolutegravir concentrations in the CSF, suggesting a possible mechanism by which concentration-dependent neurotoxicity causes CNS adverse effects (Letendre et al. 2014). Other than neuropsychiatric effects, integrase inhibitors do not appear to cause significant neurocognitive impairment. On the contrary, dolutegravir is being studied as a possible treatment for HAND, as discussed later.
Entry Inhibitors
To infect a host cell, the HIV envelope proteins gp41 and gp120 bind to host CD4 and then to a co-receptor, typically CCR5 or CXCR4. In 2003, enfuvirtide, a gp41 inhibitor was approved and later maraviroc, a CCR5 antagonist, gained FDA approval. Very recently, ibalizumab, a monoclonal antibody against CD4, gained approval in 2018. These drugs prevent viral entry into host cells. Of note, HIV-2 uses different chemokine receptors and therefore this class is only effective with HIV-1 (Saraiya et al. 2018).
Enfuvirtide use in ART is limited by its requirement of twice-daily parenteral administration due to poor solubility and rapid removal from circulation (although research shows that conjugating it with polyethylene glycol may help with this problem) (Cheng et al. 2016). However, it remains an effective therapy for drug-resistant HIV when other regimens have been exhausted (Lalezari et al. 2003). Enfuvirtide was initially thought to have increased rates of peripheral neuropathy (Fung and Guo 2004), yet subsequent studies found no clear evidence of this link (Cherry et al. 2008). To date, there have been no significant reports of CNS toxicity in enfuvirtide, and in general, it has a favorable safety profile with adverse events mostly limited to injection-site reactions (LaBonte et al. 2003; Oldfield et al. 2005; Manfredi and Sabbatani 2006; Treisman and Soudry 2016).
Maraviroc is a slowly reversible, noncompetitive CCR5 antagonist. Similar to enfuvirtide, maraviroc has favorable tolerability, a limited resistance pattern, and is a potent agent in virologic failure cases (Emmelkamp and Rockstroh 2007; Emmelkamp and Rockstroh 2008). In clinical trials, maraviroc monotherapy achieved rapid viral load reduction in a matter of days (Fatkenheuer et al. 2005), and the most common side effects were similar between maraviroc and placebo (Yost et al. 2009). However, maraviroc is only effective in patients with CCR5-tropic HIV-1, a feature that limits its use and requires tropism testing prior to use (Emmelkamp and Rockstroh 2008). Unfortunately, all trials on CXCR4 inhibitors have failed due to peripheral toxicity (Shah et al. 2016). In in vitro toxicology studies, maraviroc was the least toxic to astrocytes compared to a number of ART drugs from other classes, with a TC50 10,000-fold higher than CSF concentrations (Latronico et al. 2018). One in vitro study showed that it may cause pro-inflammatory activation of microglia cells in rats (Lisi et al. 2012). However, a subsequent study provided evidence against this claim, showing that by blocking CCR5 in the CNS, maraviroc could ameliorate neuropathic pain (when administered intrathecally in rats) by restoring the balance of pro- and antinociceptive factors in astrocytes and microglia (Piotrowska et al. 2016). There have been no substantial clinical reports of neurocognitive impairment with maraviroc. Rather, maraviroc and a similar investigational drug, cenicriviroc, are being studied as potential treatment options for HAND, as discussed below.
Ibalizumab, the most recent entry inhibitor, has advantages over others in the class. Its weekly dosing could improve adherence and its unique mechanism of action could prevent cross-resistance of HIV. Although data on neurotoxicity screening in this medication is sparse, it has also been fairly well-tolerated with no significant neurological effects reported (Jacobson et al. 2009; Bruno and Jacobson 2010).
Pharmacokinetic Enhancers
When ritonavir was initially approved at a 600 mg twice daily dose, toxicity (nausea, vomiting, diarrhea, etc.) led to discontinuation in up to a third of patients (Rublein et al. 1999; d'Arminio et al. 2000). Additionally, it led to many drug-drug interactions due to its cytochrome P450 inhibiting effects (predominantly CYP3A4 but also CYP2D6) (Kumar et al. 1996; Rathbun and Rossi 2002). In humans, ritonavir increased the area under the curve (AUC) of CYP3A-metabolized drugs by up to 20-fold in humans and increased AUC of CYP2D6-metabolized drugs by 145% (Hsu et al. 1998). Given that most PIs undergo metabolism through the CYP3A pathway, researchers quickly realized the potential of using ritonavir to “boost” levels of these drugs. Trials comparing ritonavir to dual protease inhibition with ritonavir and another drug led to substantial improvements in viral suppression and allowed ritonavir to be used at less toxic doses (Yu and Daar 2000; Michelet et al. 2001). With this discovery, the pharmacokinetic enhancer class was incidentally created. Adding an enhancer to an ART regimen allows for reduced pill burden, simpler regimens, and improved adherence, which all lead to increased antiviral efficacy (Xu and Desai 2009). Ritonavir itself does not appear to have serious CNS effects although by boosting levels of other drugs, it theoretically has the potential to indirectly propagate such neurotoxic effects of antiretrovirals (Danner et al. 1995; Carr and Cooper 2000).
Cobicistat is a CYP3A inhibitor designed to enhance the activity of antiretrovirals similar to ritonavir, but holds several unique advantages such as an easier dosing schedule and a more favorable side effect profile (Xu et al. 2010; Larson et al. 2014; Marzolini et al. 2016; Tseng et al. 2017). Similar to ritonavir, it is possible that it could promote potential neurotoxic effects of the medications it enhances. Although no evidence of neurotoxicity has been reported, it has not been extensively tested relative to other HIV medications.
Blood Brain Barrier (BBB)
HIV invasion of the CNS occurs early in disease progression, with the virus being detected in CSF as early as 8 days after initial exposure, leading to activation of pro-inflammatory responses in the CSF and brain parenchyma (Valcour et al. 2012). In around 5–20% of HIV+ patients on ART, HIV is detected in the CSF despite elimination in the plasma below detectable limits, a term called CSF viral escape (Canestri et al. 2010; Joseph et al. 2016). This entity can be divided into three categories- asymptomatic, neuro-symptomatic (clinical and progressive CNS disease), and secondary (increased CSF virus resulting from a secondary infection) (Ferretti et al. 2015). The CSF reservoir created by this escape is associated with elevated CSF levels of neopterin (a marker of macrophage activation), and is thought to increase the risk of HAND (Chen et al. 2014; Gisslén and Hunt 2019). It was theorized that if antiretroviral drugs could penetrate the BBB, this HIV reservoir could be effectively reduced, leading to improvement in CNS insult. To estimate exposure to the CNS by antiretrovirals, researchers developed the CNS penetration effectiveness (CPE) scale. Each drug is ranked from one (lowest penetrance) to four (highest penetrance) based on factors such as CSF concentration and drug pharmacology (Letendre et al. 2010). The CPE scale’s negative correlation with viral RNA in the CNS (the higher the score, the lower the viral load) was validated in several studies (Letendre et al. 2008; Marra et al. 2009). CPE correlation with neurocognitive performance is less clear (Table 1).
Several studies found that regimens with higher CPE were associated with better neurocognitive function in addition to lower CNS levels of TNF-α, a prominent inflammatory marker (Cysique et al. 2011; Smurzynski et al. 2011; Tiraboschi et al. 2015; Carvalhal et al. 2016). In contrast, other studies found either no effect or the opposite effect with higher CPE scores correlating with lower neurocognitive performance or higher risk of dementia (Marra et al. 2009; Cross et al. 2013; Caniglia et al. 2014). Some found that ART intensification with high-CPE medications did not a translate to reduced intrathecal immunoactivation (Yilmaz et al. 2010; Dahl et al. 2011). Furthermore, one study found that interrupting ART is associated with improved neurocognitive performance (Robertson et al. 2010). Participants in this study took older, more toxic regimens, so the relevance of this finding for newer ART is unclear. Another study found that placing patients on higher CPE regimens only improved neurocognition in patients who were impaired at baseline (Tozzi et al. 2009). Authors of these studies suggest that although highly-penetrating regimens are effective at reducing the CNS viral reservoir, they also have higher potential to exert neurotoxicity. Future investigation is required to determine which regimens can optimally suppress HIV in the CNS while simultaneously minimizing neurotoxicity, in the hopes of stabilizing or improving neurocognition.
Aging and Antiretrovirals
With the advent of ART, HIV+ patients have been living longer, and while this is a step in the right direction, the graying of this population brings with it certain clinical ramifications (Kirk and Goetz 2009). For instance, age-related multimorbidity in PLWH (including metabolic syndrome and vascular disease) may also contribute to neurotoxicity, with the resulting polypharmacy increasing the risk of drug-drug interactions that could cause CNS injury (Alonso-Villaverde et al. 2010; Tarr and Telenti 2010). Although the underlying mechanisms remain largely unclear, HIV and aging appear to independently contribute to neurocognitive decline and HAND development (Cañizares et al. 2014; Seider et al. 2014; Coban et al. 2017). This suggests that HIV patients experience premature and accelerated aging, although some researchers question whether the root cause is HIV itself or rather the deleterious effects from therapy (Smith et al. 2012).
A working hypothesis to explain the accelerated aging phenomenon is that age-related CNS injury resulting from toxicity of ART and concomitant drugs enhance vulnerability to CNS complications, even in those with virologic control. Aging-related changes in drug distribution, binding proteins, metabolism and elimination can lead to greater ART drug exposure in the elderly (Mangoni and Jackson 2004; Klotz 2009; Winston et al. 2013). Aging causes structural and functional changes in the BBB, such as decreased endothelial cell counts, choroid plexus epithelium flattening and calcification, as well as thickening of basement and arachnoid membranes. These changes result in increased BBB permeability which may likely affect ART CNS pharmacokinetics (Erdő et al. 2017). PI distribution in the CNS seems to be particularly affected by age, with studies showing that elderly HIV+ patients have decreased clearance of lopinavir and darunavir, longer half-life of indinavir, and higher total exposure of atazanavir (Zhou, Havlir et al. 2000; Crawford et al. 2010; Avihingsanon et al. 2013; Winston et al. 2013; Calza et al. 2017).
Current research is investigating ways to mitigate accelerated cognitive aging in PLWH. One trial (NCT02936401) is currently assessing the use of Mindfulness Based Stress Reduction as a method to improve function in patients older than 60 with HAND. Another (NCT03483740) is testing cognitive remediation group therapy in a similar cohort of older individuals with HAND. A comprehensive review of potential HAND treatment is discussed below.
Experimental HAND Treatment
Given the persistence of HAND in the cART era and the possible contribution from antiretroviral neurotoxicity, a number of previous and current trials have investigated possible therapeutic options to combat HAND (Cross and Kolson 2017). These include drugs already approved for treating other neurodegenerative diseases (selegiline and memantine) (Schifitto et al. 2007a, 2007b), drugs predominantly used for nonneurologic conditions (minocycline, fluconazole, intranasal insulin [NCT03277222], and statins [NCT01600170]) (Rezaie-Majd et al. 2002; Sacktor et al. 2011; Gerena et al. 2012; Nakasujja et al. 2013; Meulendyke et al. 2014; Sacktor et al. 2018), and antioxidants (Coenzyme Q10, heme oxygenase-1, and dimethyl fumarate) (Cross et al. 2011; Louboutin and Strayer 2018; Velichkovska et al. 2018).
Although some ART drugs are associated with neurotoxicity, several ongoing trials are testing treatment intensification approach for cognitive improvement. One trial (NCT01448486) investigated the effects of raltegravir intensification on neurocognitive performance but was unfortunately stopped prematurely due to insufficient patient recruitment. Maraviroc intensification in humans caused an improvement in neuropsychiatric performance, hypothesized to result from reducing the HIV burden in monocytes, leading to two current clinical trials (NCT02159027 and NCT02519777) (Burdo et al. 2013; Ndhlovu et al. 2014). Cenicriviroc, when given to HAND patients, led to decreased inflammatory monocyte activation and subtle improvement in cognitive performance (D'Antoni et al. 2018).
Apart from a few mild successes in trials listed above, we still have not discovered a consistent and efficacious treatment or prevention of HAND. The explanation for this lack of effectiveness is multifactorial. Inherently, clinical trials frequently fail despite promising preclinical results, due to inadequate patient recruitment/retention, fundamental differences between animal models and human subjects, unforeseen adverse effects, etc. More specifically, the underlying epidemiology, natural progression, and pathogenesis behind HAND still eludes us. Does persistent HAND despite virologic suppression result from incomplete antiretroviral CSF penetration, direct or indirect neurotoxicity from antiretrovirals, or something else entirely? Without a clear pathological target, developing specific treatment modalities becomes exceptionally challenging. This is why the impetus for the aforementioned clinical trials came either from medications that showed neuroprotection in other diseases or simply came from incidental findings in the clinic. As such, it is unlikely that these therapies could actually reverse ART-induced specific neurotoxicities rather than simply imparting general neuroprotection. In order to properly confront this disease entity, more research to provide answers to preclinical questions about HAND is essential.
Conclusions
Antiretroviral neurotoxicity is a growing body of research, with novel molecular, cellular, and animal studies uncovering the pathogenesis of such toxicity and relating it to clinical manifestations seen in patients. Each medication has a unique side effect profile, but understanding their long-term effects is becoming increasingly relevant, as the development of new therapy extends the average lifespan of PLWH. New challenges are being uncovered with this aging population, given that they experience longer cumulative ART exposure, have more comorbidities, and develop changes in their pharmacokinetic responses to such drugs (Erdő et al. 2017). Although HIV exerts neurotoxic effects on the brain and can use the CNS as a reservoir for replication, the fact that regimens with higher CPE do not necessarily lead to cognitive improvement has led researchers to hypothesize that ART itself may, in part, contribute to neurotoxicity (Caniglia et al. 2014). This theory is supported by the persistence of HAND in the cART era (Heaton et al. 2010).
Despite the potential for ART-induced neurotoxicity, viral load reduction in the plasma and CNS should remain the principal objective of antiretroviral treatment. Moving forward, we advocate for the following: 1) clinicians maintain a high level of suspicion of HAND (even when sufficiently treated), 2) scientists continue to unravel the epidemiology and pathogenesis of ART-induced neurotoxicity with rigorous studies, and 3) researchers develop and assess novel treatment options for such neurotoxicity, including HAND.
References
Abers MS, Shandera WX, Kass JS (2014) Neurological and psychiatric adverse effects of antiretroviral drugs. CNS Drugs 28(2):131–145
Alonso-Villaverde C, Aragonès G, Beltrán-Debón R, Fernández-Sender L, Rull A, Rodríguez-Sanabria F, Marsillach J, Pardo-Reche P, Camps J, Joven J (2010) Host–pathogen interactions in the development of metabolic disturbances and atherosclerosis in HIV infection: the role of CCL2 genetic variants. Cytokine 51(3):251–258
Antinori A, Arendt G, Becker J, Brew B, Byrd D, Cherner M, Clifford D, Cinque P, Epstein L, Goodkin K (2007) Updated research nosology for HIV-associated neurocognitive disorders. Neurology 69(18):1789–1799
Apostolova N, Funes HA, Blas-Garcia A, Galindo MJ, Alvarez A, Esplugues JV (2015) Efavirenz and the CNS: what we already know and questions that need to be answered. J Antimicrob Chemother 70(10):2693–2708
Arenas-Pinto A, Stöhr W, Jäger HR, Haddow L, Clarke A, Johnson M, Chen F, Winston A, Godi C, Thust S (2016) Neurocognitive function and neuroimaging markers in virologically suppressed HIV-positive patients randomized to ritonavir-boosted protease inhibitor monotherapy or standard combination ART: a cross-sectional substudy from the PIVOT trial. Clin Infect Dis 63(2):257–264
Arendt G, de Nocker D, von Giesen H-J, Nolting T (2007) Neuropsychiatric side effects of efavirenz therapy. Expert Opin Drug Saf 6(2):147–154
Avihingsanon A, Kerr SJ, Punyawudho B, van der Lugt J, Gorowara M, Ananworanich J, Lange JM, Cooper DA, Phanuphak P, Burger DM (2013) Aging not gender is associated with high Atazanavir plasma concentrations in Asian HIV-infected patients. AIDS Res Hum Retrovir 29(12):1541–1546
Bacellar H, Muñoz A, Miller E, Cohen BA, Besley D, Seines O, Becker J, McArthur JC (1994) Temporal trends in the incidence of HTV-1-related neurologic diseases: multicenter AIDS cohort study, 1985-1992. Neurology 44(10):1892–1900
Barber TJ, Moyle G, Hill A, Jagjit Singh G, Scourfield A, Yapa HM, Waters L, Asboe D, Boffito M, Nelson M (2016) A cross-sectional study to evaluate the association of hyperbilirubinaemia on markers of cardiovascular disease, neurocognitive function, bone mineral density and renal markers in HIV-1 infected subjects on protease inhibitors. HIV Clin Trials 17(3):123–130
Bienstock RJ, Copeland WC (2004) Molecular insights into NRTI inhibition and mitochondrial toxicity revealed from a structural model of the human mitochondrial DNA polymerase. Mitochondrion 4(2–3):203–213
Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitiello B (2001) Psychiatric disorders and drug use among human immunodeficiency virus–infected adults in the United States. Arch Gen Psychiatry 58(8):721–728
Blas-García A, Polo M, Alegre F, Funes HA, Martínez E, Apostolova N, Esplugues JV (2014) Lack of mitochondrial toxicity of darunavir, raltegravir and rilpivirine in neurons and hepatocytes: a comparison with efavirenz. J Antimicrob Chemother 69(11):2995–3000
Brik A, Wong C-H (2003) HIV-1 protease: mechanism and drug discovery. Org Biomol Chem 1(1):5–14
Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, Visscher BR, Margolick JB, Dobs AS (2005) Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 165(10):1179–1184
Bruno CJ, Jacobson JM (2010) Ibalizumab: an anti-CD4 monoclonal antibody for the treatment of HIV-1 infection. J Antimicrob Chemother 65(9):1839–1841
Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC (2013) Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS (London, England) 27(9)
Calza L, Colangeli V, Magistrelli E, Bussini L, Conti M, Ramazzotti E, Mancini R, Viale P (2017) Plasma trough concentrations of darunavir/ritonavir and raltegravir in older patients with HIV-1 infection. HIV Med 18(7):474–481
Canestri A, Lescure F-X, Jaureguiberry S, Moulignier A, Amiel C, Marcelin A, Peytavin G, Tubiana R, Pialoux G, Katlama C (2010) Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 50(5):773–778
Caniglia EC, Cain LE, Justice A, Tate J, Logan R, Sabin C, Winston A, van Sighem A, Miro JM, Podzamczer D (2014) Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology 83(2):134–141
Cañizares S, Cherner M, Ellis RJ (2014) HIV and aging: effects on the central nervous system. Seminars in neurology, Thieme Medical Publishers
Capetti A, Di Giambenedetto S, Latini A, Sterrantino G, De Benedetto I, Cossu M, Gori A (2017) Morning dosing for dolutegravir-related insomnia and sleep disorders. HIV Med 838:1–2
Carr A (2000) HIV protease inhibitor-related lipodystrophy syndrome. Clin Infect Dis 30(Supplement_2):S135–S142
Carr A, Cooper DA (2000) Adverse effects of antiretroviral therapy. Lancet 356(9239):1423–1430
Carvalhal A, Gill MJ, Letendre SL, Rachlis A, Bekele T, Raboud J, Burchell A, Rourke SB (2016) Central nervous system penetration effectiveness of antiretroviral drugs and neuropsychological impairment in the Ontario HIV Treatment Network cohort study. J Neuro-Oncol 22(3):349–357
Chen MF, Gill AJ, Kolson DL (2014) Neuropathogenesis of HIV-associated neurocognitive disorders: roles for immune activation, HIV blipping and viral tropism. Curr Opin HIV AIDS 9(6):559–564
Cheng S, Wang Y, Zhang Z, Lv X, Gao GF, Shao Y, Ma L, Li X (2016) Enfuvirtide− PEG conjugate: a potent HIV fusion inhibitor with improved pharmacokinetic properties. Eur J Med Chem 121:232–237
Cherry CL, Duncan AJ, Mackie KF, Wesselingh SL, Brew BJ (2008) A report on the effect of commencing enfuvirtide on peripheral neuropathy. AIDS Res Hum Retrovir 24(8):1027–1030
Ciavatta VT, Bichler EK, Speigel IA, Elder CC, Teng SL, Tyor WR, García PS (2017) In vitro and ex vivo neurotoxic effects of Efavirenz are greater than those of other common Antiretrovirals. Neurochem Res 42(11):3220–3232
Ciccarelli N, Fabbiani M, Di Giambenedetto S, Fanti I, Baldonero E, Bracciale L, Tamburrini E, Cauda R, De Luca A, Silveri MC (2011) Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology 76(16):1403–1409
Coban H, Robertson K, Smurzynski M, Krishnan S, Wu K, Bosch RJ, Collier AC, Ellis RJ (2017) Impact of aging on neurocognitive performance in previously antiretroviral-naive HIV-infected individuals on their first suppressive regimen. AIDS (London, England) 31(11):1565–1571
Crawford KW, Spritzler J, Kalayjian RC, Parsons T, Landay A, Pollard R, Stocker V, Lederman MM, Flexner C (2010) Age-related changes in plasma concentrations of the HIV protease inhibitor lopinavir. AIDS Res Hum Retrovir 26(6):635–643
Cross SA, Kolson DL (2017) Therapeutic considerations in HIV-associated neurocognitive disorders. J Neuroimmune Pharmacol, Springer:737–751
Cross SA, Cook DR, Chi AW, Vance PJ, Kolson LL, Wong BJ, Jordan-Sciutto KL, Kolson DL (2011) Dimethyl fumarate, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and macrophage-mediated neurotoxicity: a novel candidate for HIV neuroprotection. J Immunol 187(10):5015–5025
Cross HM, Combrinck MI, Joska JA (2013) HIV-associated neurocognitive disorders: antiretroviral regimen, central nervous system penetration effectiveness, and cognitive outcomes. S Afr Med J 103(10):758–762
Curtis L, Nichols G, Stainsby C, Lim J, Aylott A, Wynne B, Clark A, Bloch M, Maechler G, Martin-Carpenter L (2014) Dolutegravir: clinical and laboratory safety in integrase inhibitor–naive patients. HIV Clin Trials 15(5):199–208
Cysique LA, Waters EK, Brew BJ (2011) Central nervous system antiretroviral efficacy in HIV infection: a qualitative and quantitative review and implications for future research. BMC Neurol 11(1):148
Dahl V, Lee E, Peterson J, Spudich SS, Leppla I, Sinclair E, Fuchs D, Palmer S, Price RW (2011) Raltegravir treatment intensification does not alter cerebrospinal fluid HIV-1 infection or immunoactivation in subjects on suppressive therapy. J Infect Dis 204(12):1936–1945
Danner SA, Carr A, Leonard JM, Lehman LM, Gudiol F, Gonzales J, Raventos A, Rubio R, Bouza E, Pintado V (1995) A short-term study of the safety, pharmacokinetics, and efficacy of ritonavir, an inhibitor of HIV-1 protease. N Engl J Med 333(23):1528–1534
D'Antoni ML, Paul RH, Mitchell BI, Kohorn L, Fischer L, Lefebvre E, Seyedkazemi S, Nakamoto BK, Walker M, Kallianpur KJ, Ogata-Arakaki D, Ndhlovu LC, Shikuma C (2018) Improved cognitive performance and reduced monocyte activation in virally suppressed chronic HIV after dual CCR2 and CCR5 antagonism. J Acquir Immune Defic Syndr 79(1):108–116
Decloedt EH, Rosenkranz B, Maartens G, Joska J (2015) Central nervous system penetration of antiretroviral drugs: pharmacokinetic, pharmacodynamic and pharmacogenomic considerations. Clin Pharmacokinet 54(6):581–598
del Mar Gutierrez M, Mateo MG, Vidal F, Domingo P (2014) Drug safety profile of integrase strand transfer inhibitors. Expert Opin Drug Saf 13(4):431–445
Drake SM (2000) NNRTIs—a new class of drugs for HIV. J Antimicrob Chemother 45(4):417–420
Ekins S, Mathews P, Saito EK, Diaz N, Naylor D, Chung J, McMurtray AM (2017) α7-nicotinic acetylcholine receptor inhibition by indinavir: implications for cognitive dysfunction in treated HIV disease. AIDS 31(8):1083–1089
Ellis RJ, Marquie-Beck J, Delaney P, Alexander T, Clifford DB, McArthur JC, Simpson DM, Ake C, Collier AC, Gelman BB (2008) Human immunodeficiency virus protease inhibitors and risk for peripheral neuropathy. Ann Neurol 64(5):566–572
Emmelkamp J, Rockstroh J (2007) CCR5 antagonists: comparison of efficacy, side effects, pharmacokinetics and interactions—review of the literature. Eur J Med Res 12(9):409–417
Emmelkamp JM, Rockstroh JK (2008) Maraviroc, risks and benefits: a review of the clinical literature. Expert Opin Drug Saf 7(5):559–569
Erdő F, Denes L, de Lange E (2017) Age-associated physiological and pathological changes at the blood–brain barrier: a review. J Cereb Blood Flow Metab 37(1):4–24
Etgen T, Chonchol M, Förstl H, Sander D (2012) Chronic kidney disease and cognitive impairment: a systematic review and meta-analysis. Am J Nephrol 35(5):474–482
Etherton MR, Lyons JL, Ard KL (2015) HIV-associated neurocognitive disorders and antiretroviral therapy: current concepts and controversies. Curr Infect Dis Rep 17(6):28
Fatkenheuer G, Pozniak AL, Johnson MA, Plettenberg A, Staszewski S, Hoepelman AI, Saag MS, Goebel FD, Rockstroh JK, Dezube BJ, Jenkins TM, Medhurst C, Sullivan JF, Ridgway C, Abel S, James IT, Youle M, van der Ryst E (2005) Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med 11(11):1170–1172
Ferretti F, Gisslen M, Cinque P, Price RW (2015) Cerebrospinal fluid HIV escape from antiretroviral therapy. Curr HIV/AIDS Rep 12(2):280–288
Fettiplace A, Stainsby C, Winston A, Givens N, Puccini S, Vannappagari V, Hsu R, Fusco J, Quercia R, Aboud M, Curtis L (2017) Psychiatric symptoms in patients receiving Dolutegravir. J Acquir Immune Defic Syndr 74(4):423–431
Finsterer J (2012) Cognitive dysfunction in mitochondrial disorders. Acta Neurol Scand 126(1):1–11
Flexner C (1998) HIV-protease inhibitors. N Engl J Med 338(18):1281–1293
Fung HB, Guo Y (2004) Enfuvirtide: a fusion inhibitor for the treatment of HIV infection. Clin Ther 26(3):352–378
Gabrielian A, MacCumber MM, Kukuyev A, Mitsuyasu R, Holland GN, Sarraf D (2013) Didanosine-associated retinal toxicity in adults infected with human immunodeficiency virus. JAMA Ophthalmol 131(2):255–259
Gallant J, Lazzarin A, Mills A, Orkin C, Podzamczer D, Tebas P, Girard P-M, Brar I, Daar ES, Wohl D (2017) Bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection (GS-US-380-1489): a double-blind, multicentre, phase 3, randomised controlled non-inferiority trial. Lancet 390(10107):2063–2072
Gallego L, Barreiro P, del Rio R, Gonzalez de Requena D, Rodriguez-Albarino A, Gonzalez-Lahoz J, Soriano V (2004) Analyzing sleep abnormalities in HIV-infected patients treated with Efavirenz. Clin Infect Dis 38(3):430–432
Gerena Y, Skolasky RL, Velez JM, Toro-Nieves D, Mayo R, Nath A, Wojna V (2012) Soluble and cell-associated insulin receptor dysfunction correlates with severity of HAND in HIV-infected women. PLoS One 7(5):e37358
Gisslén M, Hunt PW (2019) Antiretroviral treatment of acute HIV infection normalizes levels of cerebrospinal fluid markers of central nervous system (CNS) inflammation: a consequence of a reduced CNS reservoir? The Journal of Infectious Diseases
Gray J, Young B (2009) Acute onset insomnia associated with the initiation of raltegravir: a report of two cases and literature review. AIDS Patient Care STDs 23(9):689–690
Grilo NM, Joao Correia M, Miranda JP, Cipriano M, Serpa J, Matilde Marques M, Monteiro EC, Antunes AMM, Diogo LN, Pereira SA (2017) Unmasking efavirenz neurotoxicity: time matters to the underlying mechanisms. Eur J Pharm Sci 105:47–54
Harris M (2018) What did we learn from the bictegravir switch studies? Lancet HIV 5(7):e336–e337
Harris M, Larsen G, Montaner JS (2008) Exacerbation of depression associated with starting raltegravir: a report of four cases. Aids 22(14):1890–1892
Heaton R, Clifford D, Franklin D, Woods S, Ake C, Vaida F, Ellis R, Letendre S, Marcotte T, Atkinson J (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER study. Neurology 75(23):2087–2096
Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neuro-Oncol 17(1):3–16
Hill A, van der Lugt J, Sawyer W, Boffito M (2009) How much ritonavir is needed to boost protease inhibitors? Systematic review of 17 dose-ranging pharmacokinetic trials. Aids 23(17):2237–2245
Hoffmann C, Welz T, Sabranski M, Kolb M, Wolf E, Stellbrink HJ, Wyen C (2017) Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med 18(1):56–63
Hsu A, Granneman GR, Bertz RJ (1998) Ritonavir. Clin Pharmacokinet 35(4):275–291
Hung K-M, Chen P-C, Hsieh H-C, Calkins MJ (2017) Mitochondrial defects arise from nucleoside/nucleotide reverse transcriptase inhibitors in neurons: potential contribution to HIV-associated neurocognitive disorders. Biochim Biophys Acta (BBA) - Mol Basis Dis 1863(2):406–413
Jacobson JM, Kuritzkes DR, Godofsky E, DeJesus E, Larson JA, Weinheimer SP, Lewis ST (2009) Safety, pharmacokinetics, and antiretroviral activity of multiple doses of ibalizumab (formerly TNX-355), an anti-CD4 monoclonal antibody, in human immunodeficiency virus type 1-infected adults. Antimicrob Agents Chemother 53(2):450–457
Jensen BK, Monnerie H, Mannell MV, Gannon PJ, Espinoza CA, Erickson MA, Bruce-Keller AJ, Gelman BB, Briand LA, Pierce RC (2015) Altered oligodendrocyte maturation and myelin maintenance: the role of antiretrovirals in HIV-associated neurocognitive disorders. J Neuropathol Exp Neurol 74(11):1093–1118
Joseph J, Cinque P, Colosi D, Dravid A, Ene L, Fox H, Gabuzda D, Gisslen M, Joseph SB, Letendre S (2016) Highlights of the global HIV-1 CSF escape consortium meeting, 9 June 2016, Bethesda, MD, USA. J Virus Erad 2(4):243–250
Kakuda TN (2000) Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin Ther 22(6):685–708
Kenedi CA, Goforth HW (2011) A systematic review of the psychiatric side-effects of efavirenz. AIDS Behav 15(8):1803–1818
Kirk JB, Goetz MB (2009) Human immunodeficiency virus in an aging population, a complication of success. J Am Geriatr Soc 57(11):2129–2138
Klotz U (2009) Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev 41(2):67–76
Kohler JJ, Lewis W (2007) A brief overview of mechanisms of mitochondrial toxicity from NRTIs. Environ Mol Mutagen 48(3–4):166–172
Kumar GN, Rodrigues AD, Buko AM, Denissen JF (1996) Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther 277(1):423–431
LaBonte J, Lebbos J, Kirkpatrick P (2003) Enfuvirtide. Nature Publishing Group
Lalezari JP, Henry K, O'Hearn M, Montaner JS, Piliero PJ, Trottier B, Walmsley S, Cohen C, Kuritzkes DR, Eron JJ Jr (2003) Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N Engl J Med 348(22):2175–2185
Larson KB, Wang K, Delille C, Otofokun I, Acosta EP (2014) Pharmacokinetic enhancers in HIV therapeutics. Clin Pharmacokinet 53(10):865–872
Latronico T, Pati I, Ciavarella R, Fasano A, Mengoni F, Lichtner M, Vullo V, Mastroianni CM, Liuzzi GM (2018) In vitro effect of antiretroviral drugs on cultured primary astrocytes: analysis of neurotoxicity and matrix metalloproteinase inhibition. J Neurochem 144(3):271–284
Lee FJ, Amin J, Bloch M, Pett SL, Marriott D, Carr A (2013) Skeletal muscle toxicity associated with raltegravir-based combination antiretroviral therapy in HIV-infected adults. J Acquir Immune Defic Syndr 62(5):525–533
Lennox JL, DeJesus E, Berger DS, Lazzarin A, Pollard RB, Madruga JVR, Zhao J, Wan H, Gilbert CL, Teppler H (2010) Raltegravir versus efavirenz regimens in treatment-naive HIV-1–infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr 55(1):39
Letendre S (2011) Central nervous system complications in HIV disease: HIV-associated neurocognitive disorder. Topics in Antiviral Medicine 19(4):137–142
Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S (2008) Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 65(1):65–70
Letendre SL, Ellis RJ, Ances BM, McCutchan JA (2010) Neurologic complications of HIV disease and their treatment. Topics in HIV medicine: a publication of the International AIDS Society, USA 18(2): 45
Letendre SL, Mills AM, Tashima KT, Thomas DA, Min SS, Chen S, Song IH, Piscitelli SC (2014) ING116070: a study of the pharmacokinetics and antiviral activity of Dolutegravir in cerebrospinal fluid in HIV-1–infected, antiretroviral therapy–naive subjects. Clin Infect Dis 59(7):1032–1037
Lewis W, Day BJ, Copeland WC (2003) Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nat Rev Drug Discov 2(10):812–822
Lisi L, Tramutola A, De Luca A, Navarra P, Dello Russo C (2012) Modulatory effects of the CCR5 antagonist maraviroc on microglial pro-inflammatory activation elicited by gp120. J Neurochem 120(1):106–114
Louboutin J-P, Strayer DS (2018) Gene Delivery of antioxidant enzymes in HIV-1-associated neurocognitive disorder. Elsevier, HIV/AIDS, pp 107–123
Lv Z, Chu Y, Wang Y (2015) HIV protease inhibitors: a review of molecular selectivity and toxicity. HIV/AIDS (Auckland, NZ) 7:–95
Ma Q, Vaida F, Wong J, Sanders CA, Kao Y-t, Croteau D, Clifford DB, Collier AC, Gelman BB, Marra CM (2016) Long-term efavirenz use is associated with worse neurocognitive functioning in HIV-infected patients. J Neuro-Oncol 22(2):170–178
Manfredi R, Sabbatani S (2006) A novel antiretroviral class (fusion inhibitors) in the management of HIV infection. Present features and future perspectives of enfuvirtide (T-20). Curr Med Chem 13(20):2369–2384
Mangoni AA, Jackson SH (2004) Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol 57(1):6–14
Marcus JL, Chao CR, Leyden WA, Xu L, Quesenberry CP Jr, Klein DB, Towner WJ, Horberg MA, Silverberg MJ (2016) Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 73(1):39
Margolis AM, Heverling H, Pham PA, Stolbach A (2014) A review of the toxicity of HIV medications. J Med Toxicol 10(1):26–39
Markowitz M, Saag M, Powderly WG, Hurley AM, Hsu A, Valdes JM, Henry D, Sattler F, Marca AL, Leonard JM (1995) A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N Engl J Med 333(23):1534–1540
Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, Ellis RJ, Rodriguez B, Coombs RW, Schifitto G (2009) Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS (London, England) 23(11):1359
Marzolini C, Gibbons S, Khoo S, Back D (2016) Cobicistat versus ritonavir boosting and differences in the drug–drug interaction profiles with co-medications. J Antimicrob Chemother 71(7):1755–1758
Meeker RB, Robertson K, Power C (2014) Neurotoxic consequences of antiretroviral therapies. Encyclopedia of AIDS:1–7
Meulendyke KA, Queen SE, Engle EL, Shirk EN, Liu J, Steiner JP, Nath A, Tarwater PM, Graham DR, Mankowski JL (2014) Combination fluconazole/paroxetine treatment is neuroprotective despite ongoing neuroinflammation and viral replication in an SIV model of HIV neurological disease. J Neuro-Oncol 20(6):591–602
Michelet C, Ruffault A, Sébille V, Arvieux C, Jaccard P, Raffi F, Bazin C, Chapplain J-M, Chauvin J-P, Dohin E (2001) Ritonavir-saquinavir dual protease inhibitor compared to ritonavir alone in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 45(12):3393–3402
Mollan KR, Smurzynski M, Eron JJ, Daar ES, Campbell TB, Sax PE, Gulick RM, Na L, O'keefe L, Robertson KR (2014) Association between efavirenz as initial therapy for HIV-1 infection and increased risk for suicidal ideation or attempted or completed suicide: an analysis of trial data. Ann Intern Med 161(1):1–10
d'Arminio Monforte A, Lepri AC, Rezza G, Pezzotti P, Antinori A, Phillips AN, Angarano G, Colangeli V, De Luca A, Ippolito G (2000) Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. Aids 14(5):499–507
Moyle G, Fletcher C, Brown H, Mandalia S, Gazzard B (2006) Changes in sleep quality and brain wave patterns following initiation of an efavirenz-containing triple antiretroviral regimen. HIV Med 7(4):243–247
Nakasujja N, Miyahara S, Evans S, Lee A, Musisi S, Katabira E, Robertson K, Ronald A, Clifford DB, Sacktor N (2013) Randomized trial of minocycline in the treatment of HIV-associated cognitive impairment. Neurology 80(2):196–202
Ndhlovu LC, Umaki T, Chew GM, Chow DC, Agsalda M, Kallianpur KJ, Paul R, Zhang G, Ho E, Hanks N (2014) Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIV-associated neurocognitive disease (HAND). J Neuro-Oncol 20(6):571–582
Nguyen BYT, Isaacs RD, Teppler H, Leavitt RY, Sklar P, Iwamoto M, Wenning LA, Miller MD, Chen J, Kemp R (2011) Raltegravir: the first HIV-1 integrase strand transfer inhibitor in the HIV armamentarium. Ann N Y Acad Sci 1222(1):83–89
Nooka S, Ghorpade A (2017) HIV-1-associated inflammation and antiretroviral therapy regulate astrocyte endoplasmic reticulum stress responses. Cell Death Dis 3:17061
Nooka S, Ghorpade A (2018) Organellar stress intersects the astrocyte endoplasmic reticulum, mitochondria and nucleolus in HIV associated neurodegeneration. Cell Death Dis 9(3):317
Oldfield V, Keating GM, Plosker G (2005) Enfuvirtide: a review of its use in the management of HIV infection. Drugs 65(8):1139–1160
Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM (2016) The integrated stress response. EMBO Rep 17(10):1374–1395
Patel P, Louie S (2018) Drug interactions in HIV: protease and Integrase inhibitors. In: Pai MKJ, Gubbins P, Rodvold K (eds) Drug interactions in infectious diseases: antimicrobial drug interactions. Humana Press, Cham
Payne B, Chadwick T, Blamire A, Anderson K, Parikh J, Qian J, Hynes A, Wilkinson J, Price D, E. o. S. t. L. R. i. I. C. F. i. E. t. P. s. team (2017) Does efavirenz replacement improve neurological function in treated HIV infection? HIV Med 18(9):690–695
Penafiel J, de Lazzari E, Padilla M, Rojas J, Gonzalez-Cordon A, Blanco JL, Blanch J, Marcos MA, Lonca M, Martinez-Rebollar M, Laguno M, Tricas A, Rodriguez A, Mallolas J, Gatell JM, Martinez E (2017) Tolerability of integrase inhibitors in a real-life setting. J Antimicrob Chemother 72(6):1752–1759
Pérez-Valero I, Ellis R, Heaton R, Deutsch R, Franklin D, Clifford DB, Collier A, Gelman B, Marra C, McCutchan JA (2019) Cerebrospinal fluid viral escape in aviremic HIV-infected patients receiving antiretroviral therapy: prevalence, risk factors and neurocognitive effects. AIDS 33(3):475–481
Pettersen JA, Jones G, Worthington C, Krentz HB, Keppler OT, Hoke A, Gill MJ, Power C (2006) Sensory neuropathy in human immunodeficiency virus/acquired immunodeficiency syndrome patients: protease inhibitor–mediated neurotoxicity. Ann Neurol 59(5):816–824
Piotrowska A, Kwiatkowski K, Rojewska E, Makuch W, Mika J (2016) Maraviroc reduces neuropathic pain through polarization of microglia and astroglia–evidence from in vivo and in vitro studies. Neuropharmacology 108:207–219
Poirier MC, Divi RL, Al-Harthi L, Olivero OA, Nguyen V, Walker B, Landay AL, Walker VE, Charurat M, Blattner WA, Women and G. Infants Transmission Study (2003) Long-term mitochondrial toxicity in HIV-uninfected infants born to HIV-infected mothers. J Acquir Immune Defic Syndr 33(2):175–183
Pokorná J, Machala L, Řezáčová P, Konvalinka J (2009) Current and novel inhibitors of HIV protease. Viruses 1(3):1209–1239
Pommier Y, Johnson AA, Marchand C (2005) Integrase inhibitors to treat HIV/AIDS. Nat Rev Drug Discov 4(3):236
Rathbun RC, Rossi DR (2002) Low-dose ritonavir for protease inhibitor pharmacokinetic enhancement. Ann Pharmacother 36(4):702–706
Rezaie-Majd A, Maca T, Bucek RA, Valent P, Müller MR, Husslein P, Kashanipour A, Minar E, Baghestanian M (2002) Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler Thromb Vasc Biol 22(7):1194–1199
Richman DD, Fischl MA, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, Leedom JM, Groopman JE, Mildvan D, Hirsch MS (1987) The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. N Engl J Med 317(4):192–197
Riddler SA, Haubrich R, DiRienzo AG, Peeples L, Powderly WG, Klingman KL, Garren KW, George T, Rooney JF, Brizz B, Lalloo UG, Murphy RL, Swindells S, Havlir D, Mellors JW, Team ACTGSA (2008) Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med 358(20):2095–2106
Robertson K, Su Z, Margolis D, Krambrink A, Havlir D, Evans S, Skiest D, Team AS (2010) Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology 74(16):1260–1266
Rodriguez-Nóvoa S, Alvarez E, Labarga P, Soriano V (2010) Renal toxicity associated with tenofovir use. Expert Opin Drug Saf 9(4):545–559
Rublein JC, Eron JJ Jr, Butts JD, Raasch RH (1999) Discontinuation rates for protease inhibitor regimens containing ritonavir 600 mg versus ritonavir 400 mg plus saquinavir 400 mg. Ann Pharmacother 33(9):899–905
Saag MS, Benson CA, Gandhi RT, Hoy JF, Landovitz RJ, Mugavero MJ, Sax PE, Smith DM, Thompson MA, Buchbinder SP, Del Rio C, Eron JJ Jr, Fatkenheuer G, Gunthard HF, Molina JM, Jacobsen DM, Volberding PA (2018) Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the international antiviral society-USA panel. JAMA 320(4):379–396
Sacktor N, Miyahara S, Deng L, Evans S, Schifitto G, Cohen BA, Paul R, Robertson K, Jarocki B, Scarsi K (2011) Minocycline treatment for HIV-associated cognitive impairment: results from a randomized trial. Neurology 77(12):1135–1142
Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, Ragin A, Levine A, Miller E (2016) Prevalence of HIV-associated neurocognitive disorders in the multicenter AIDS cohort study. Neurology 86(4):334–340
Sacktor N, Skolasky RL, Moxley R, Wang S, Mielke MM, Munro C, Steiner J, Nath A, Haughey N, McArthur J (2018) Paroxetine and fluconazole therapy for HIV-associated neurocognitive impairment: results from a double-blind, placebo-controlled trial. J Neuro-Oncol 24(1):16–27
Saracchini S, Vaccher E, Covezzi E, Tortorici G, Carbone A, Tirelli U (1989) Lethal neurotoxicity associated to azidothymidine therapy. J Neurol Neurosurg Psychiatry 52(4):544
Saraiya N, Kanagala V, Corpuz M (2018) HIV-2 in the United States: rare but not forgotten. Aids 32(11):1547–1549
Sax PE, Pozniak A, Montes ML, Koenig E, DeJesus E, Stellbrink H-J, Antinori A, Workowski K, Slim J, Reynes J (2017) Coformulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide, for initial treatment of HIV-1 infection (GS-US-380–1490): a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet 390(10107):2073–2082
Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC (2016) HIV-associated neurocognitive disorder—pathogenesis and prospects for treatment. Nat Rev Neurol 12(4):234–248
Scherzer R, Shlipak MG (2015) Risk factors: individual assessment of CKD risk in HIV-positive patients. Nat Rev Nephrol 11(7):392–393
Schifitto G, Navia BA, Yiannoutsos CT, Marra CM, Chang L, Ernst T, Jarvik JG, Miller EN, Singer EJ, Ellis RJ (2007a) Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. Aids 21(14):1877–1886
Schifitto G, Zhang J, Evans S, Sacktor N, Simpson D, Millar L, Hung V, Miller E, Smith E, Ellis R (2007b) A multicenter trial of selegiline transdermal system for HIV-associated cognitive impairment. Neurology 69(13):1314–1321
Schweinsburg BC, Taylor MJ, Alhassoon OM, Gonzalez R, Brown GG, Ellis RJ, Letendre S, Videen JS, McCutchan JA, Patterson TL (2005) Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. J Neuro-Oncol 11(4):356–364
Seider TR, Luo X, Gongvatana A, Devlin KN, de la Monte SM, Chasman JD, Yan P, Tashima KT, Navia B, Cohen RA (2014) Verbal memory declines more rapidly with age in HIV infected versus uninfected adults. J Clin Exp Neuropsychol 36(4):356–367
Shah A, Gangwani MR, Chaudhari NS, Glazyrin A, Bhat HK, Kumar A (2016) Neurotoxicity in the post-HAART era: caution for the antiretroviral therapeutics. Neurotox Res 30(4):677–697
Singer EJ, Nemanim NM (2017) The persistence of HIV-associated neurocognitive disorder (HAND) in the era of combined antiretroviral therapy (cART). Springer, Global Virology II-HIV and NeuroAIDS, pp 375–403
Smith RL, de Boer R, Brul S, Budovskaya Y, van Spek H (2012) Premature and accelerated aging: HIV or HAART? Front Genet 3:328
Smurzynski M, Wu K, Letendre S, Robertson K, Bosch RJ, Clifford DB, Evans S, Collier AC, Taylor M, Ellis R (2011) Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS (London, England) 25(3):357
Soontornniyomkij V, Umlauf A, Chung SA, Cochran ML, Soontornniyomkij B, Gouaux B, Toperoff W, Moore DJ, Masliah E, Ellis RJ (2014) HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS (London, England) 28(9):1297
Stauch KL, Emanuel K, Lamberty BG, Morsey B, Fox HS (2017) Central nervous system-penetrating antiretrovirals impair energetic reserve in striatal nerve terminals. J Neuro-Oncol 23(6):795–807
Steigbigel RT, Cooper DA, Teppler H, Eron JJ, Gatell JM, Kumar PN, Rockstroh JK, Schechter M, Katlama C, Markowitz M (2010) Long-term efficacy and safety of Raltegravir combined with optimized background therapy in treatment-experienced patients with drug-resistant HIV infection: week 96 results of the BENCHMRK 1 and 2 phase III trials. Clin Infect Dis 50(4):605–612
Stern AL, Lee RN, Panvelker N, Li J, Harowitz J, Jordan-Sciutto KL, Akay-Espinoza C (2018) Differential effects of antiretroviral drugs on neurons in vitro: roles for oxidative stress and integrated stress response. J NeuroImmune Pharmacol 13(1):64–76
Tarr PE, Telenti A (2010) Genetic screening for metabolic and age-related complications in HIV-infected persons. F1000 medicine reports 2
Tiraboschi JM, Muñoz-Moreno JA, Puertas M, Alonso-Villaverde C, Prats A, Ferrer E, Rozas N, Masó M, Ouchi D, Martinez-Picado J (2015) Viral and inflammatory markers in cerebrospinal fluid of patients with HIV-1-associated neurocognitive impairment during antiretroviral treatment switch. HIV Med 16(6):388–392
Tozzi V, Balestra P, Salvatori MF, Vlassi C, Liuzzi G, Giancola ML, Giulianelli M, Narciso P, Antinori A (2009) Changes in cognition during antiretroviral therapy: comparison of 2 different ranking systems to measure antiretroviral drug efficacy on HIV-associated neurocognitive disorders. J Acquir Immune Defic Syndr 52(1):56–63
Treisman GJ, Soudry O (2016) Neuropsychiatric effects of HIV antiviral medications. Drug Saf 39(10):945–957
Tseng A, Hughes CA, Wu J, Seet J, Phillips EJ (2017) Cobicistat versus ritonavir: similar pharmacokinetic enhancers but some important differences. Ann Pharmacother 51(11):1008–1022
Turjanski N, Lloyd GG (2005) Psychiatric side-effects of medications: recent developments. Adv Psychiatr Treat 11(1):58–70
Underwood J, Robertson KR, Winston A (2015) Could antiretroviral neurotoxicity play a role in the pathogenesis of cognitive impairment in treated HIV disease? Aids 29(3):253–261
Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, Suwanwela NC, Jagodzinski L, Michael N, Spudich S (2012) Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 206(2):275–282
Velichkovska M, Surnar B, Nair M, Dhar S, Toborek M (2018) Targeted mitochondrial coq10 delivery attenuates antiretroviral drug-induced senescence of neural progenitor cells. Mol Pharm
Vivithanaporn P, Asahchop EL, Acharjee S, Baker GB, Power C (2016) HIV protease inhibitors disrupt astrocytic glutamate transporter function and neurobehavioral performance. AIDS (London, England) 30(4):543
White AJ (2001) Mitochondrial toxicity and HIV therapy. Sex Transm Infect 77(3):158–173
Winston A, Jose S, Gibbons S, Back D, Stohr W, Post F, Fisher M, Gazzard B, Nelson M, Gilson R (2013) Effects of age on antiretroviral plasma drug concentration in HIV-infected subjects undergoing routine therapeutic drug monitoring. J Antimicrob Chemother 68(6):1354–1359
Xu L, Desai MC (2009) Pharmacokinetic enhancers for HIV drugs. Curr Opin Investig Drugs 10(8):775–786
Xu L, Liu H, Murray BP, Callebaut C, Lee MS, Hong A, Strickley RG, Tsai LK, Stray KM, Wang Y (2010) Cobicistat (GS-9350): a potent and selective inhibitor of human CYP3A as a novel pharmacoenhancer. ACS Med Chem Lett 1(5):209–213
Yilmaz A, Verhofstede C, D'Avolio A, Watson V, Hagberg L, Fuchs D, Svennerholm B, Gisslén M (2010) Treatment intensification has no effect on the HIV-1 central nervous system infection in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr 55(5):590–596
Yost R, Pasquale TR, Sahloff EG (2009) Maraviroc: a coreceptor CCR5 antagonist for management of HIV infection. Am J Health Syst Pharm 66(8):715–726
Yu K, Daar ES (2000) Dual protease inhibitor therapy in the management of the HIV-1. Expert Opin Pharmacother 1(7):1331–1342
Zash R, Jacobson DL, Diseko M, Mayondi G, Mmalane M, Essex M, Petlo C, Lockman S, Makhema J, Shapiro RL (2017) Comparative safety of antiretroviral treatment regimens in pregnancy. JAMA Pediatr 171(10):e172222–e172222
Zash R, Makhema J, Shapiro RL (2018) Neural-tube defects with dolutegravir treatment from the time of conception. N Engl J Med 379(10):979–981
Zhang Y, Song F, Gao Z, Ding W, Qiao L, Yang S, Chen X, Jin R, Chen D (2014) Long-term exposure of mice to nucleoside analogues disrupts mitochondrial DNA maintenance in cortical neurons. PLoS One 9(1):e85637
Zhou X-J, Havlir DV, Richman DD, Acosta EP, Hirsch M, Collier AC, Tebas P, Sommadossi J-P, A. C. T. G. S. Investigators (2000) Plasma population pharmacokinetics and penetration into cerebrospinal fluid of indinavir in combination with zidovudine and lamivudine in HIV-1-infected patients. Aids 14(18):2869–2876
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lanman, T., Letendre, S., Ma, Q. et al. CNS Neurotoxicity of Antiretrovirals. J Neuroimmune Pharmacol 16, 130–143 (2021). https://doi.org/10.1007/s11481-019-09886-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-019-09886-7