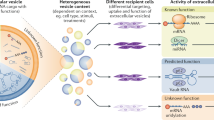
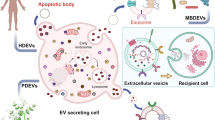
Abstract
Extracellular vesicles (EVs) are nanosized, membrane-bound vesicles released from eukaryotic and prokaryotic cells that can transport cargo containing DNA, RNA, lipids and proteins, between cells as a means of intercellular communication. Although EVs were initially considered to be cellular debris deprived of any essential biological functions, emerging literature highlights the critical roles of EVs in the context of intercellular signaling, maintenance of tissue homeostasis, modulation of immune responses, inflammation, cancer progression, angiogenesis, and coagulation under both physiological and pathological states. Based on the ability of EVs to shuttle proteins, lipids, carbohydrates, mRNAs, long non-coding RNAs (lncRNAs), microRNAs, chromosomal DNA, and mitochondrial DNA into target cells, the presence and content of EVs in biofluids have been exploited for biomarker research in the context of diagnosis, prognosis and treatment strategies. Additionally, owing to the characteristics of EVs such as stability in circulation, biocompatibility as well as low immunogenicity and toxicity, these vesicles have become attractive systems for the delivery of therapeutics. More recently, EVs are increasingly being exploited as conduits for delivery of therapeutics for anticancer strategies, immunomodulation, targeted drug delivery, tissue regeneration, and vaccination. In this review, we highlight and discuss the multiple strategies that are employed for the use of EVs as delivery vehicles for therapeutic agents, including the potential advantages and challenges involved.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracellular vesicles (EVs) are nanosized, membrane-bound vesicles released from eukaryotic and prokaryotic cells that can transport cargo including DNA, RNA, lipids, and proteins, between cells as a form of intercellular communication (Fevrier and Raposo 2004; Mathivanan et al. 2010; Raposo and Stoorvogel 2013; Colombo et al. 2014; Zaborowski et al. 2015). EVs have been found in various body fluids such as amniotic fluid, ascites, bile, blood, breast milk, cerebrospinal fluid, saliva, semen, and urine. In the literature, EV is the general name for various cell-derived vesicles, such as microparticles, microvesicles, nanovesicles, nanoparticles, calcifying matrix vesicles, argosomes, tolerosomes, oncosomes, prostasomes, secretomes, exosomes, exovesicles, exosome-like vesicles, and ectosomes (Colombo et al. 2014; Thery et al. 2018). Though the nomenclature of EVs is still a matter of debate (Gould and Raposo 2013; Thery et al. 2018; Chiang and Chen 2019), the terms ectosomes, microparticles, and microvesicles mainly refer to vesicles ranging in size from 150 to 1000 nm that are released from the cell membrane by the budding process. The term “exosome” was initially used to tag vesicles whose size ranged from 40 to 1000 nm with a 5’-nucleotidase activity (Trams et al. 1981). In the late 1980s, however, the use of this term was restricted to only include vesicles of the endosomal origin ranging in size from 30 to 100 nm (Johnstone et al. 1987; Fevrier and Raposo 2004).
Almost three decades ago EVs were considered to be cellular debris that was deprived of any essential biological function(s). However, emerging literature strongly implicates critical roles of EVs in the context of intercellular signaling, maintenance of tissue homeostasis, modulation of immune responses, inflammation, cancer progression, angiogenesis, and coagulation, under both physiological and pathological states (Hu et al. 2012; Andaloussi et al. 2013; Yanez-Mo et al. 2015; Di Rocco et al. 2016; Iraci et al. 2016; Kalra et al. 2016; Hu et al. 2017a, b). Based on the ability of EVs to shuttle proteins, lipids, carbohydrates, mRNAs, long non-coding RNAs (lncRNAs), microRNAs, chromosomal DNA, and mitochondrial DNA into target cells, the presence and content of EVs in biofluids has been exploited for biomarker research in the context of diagnosis, prognosis and treatment strategies (Walker et al. 1988; Valadi et al. 2007; Lin et al. 2015; Ratajczak and Ratajczak 2016; Wang et al. 2017a; Samanta et al. 2018; Abdel-Haq 2019). EVs are comprised of lipid bilayer membranes coated with various ligands, which, in turn, can interact with receptors on target cells, thereby making these vesicles promising candidates for targeted delivery (Agrahari et al. 2019). Due to their increased stability in circulation and biocompatibility, as well as low immunogenicity and toxicity, EVs are attractive systems for transport and delivery of therapeutics. EVs are increasingly being exploited as conduits for delivery of therapeutics for anticancer strategies, immunomodulation, targeted drug delivery, tissue regeneration, and vaccination (Gyorgy et al. 2015; Ohno et al. 2016). In this review, we highlight and discuss the various strategies employed for the use of EVs as delivery vehicles, including the potential advantages and challenges involved.
Basic and Therapeutic Implications of EVs
EVs can be secreted in vitro by a variety of cells including adipocytes, fibroblasts, glial cells, hematopoietic cells (B cells, T cells, dendritic cells, mast cells, and platelets), intestinal epithelial cells, neuronal cells, Schwann cells and numerous tumor cell lines (Yanez-Mo et al. 2015; Hu et al. 2016a). Additionally, in vivo EVs exist in various biological fluids including blood, urine, saliva, epididymal fluid, amniotic liquid, malignant and pleural effusions or ascites, bronchoalveolar lavage fluid, synovial fluid and breast milk. Within these fluid compartments, EVs serve as mediators for cellular communication and cargo transportation, thereby regulating various physiological processes (Schorey and Bhatnagar 2008; Kooijmans et al. 2012; Vlassov et al. 2012; Hu et al. 2013; Antimisiaris et al. 2018; Bunggulawa et al. 2018; Yang et al. 2018a). EVs bear combinations of ligands that engage different cell surface receptors simultaneuosly and can communicate without the need of direct cell-to-cell contact. For example, EVs can transfer MHC II / peptide complex from antigen presenting cells to T cells and subsequently, antigen presentation to secondary T lymphocytes, thereby facilitating antigen-specific communication between nonadjacent APC and T cells (Arnold and Mannie 1999). Additionaly, EVs stimulated from dendritic cells in response to IL10 treatment, suppressed inflammation and collagen-induced arthritis in mice, thereby underscoring the use of EVs as a better therapeutic approach compared with DCs for the treatment of autoimmune diseases such as rheumatoid arthritis (Kim et al. 2005; Schorey and Bhatnagar 2008). In addition to the role of EVs in antigen-specific communication, EVs released from epithelial cell origin are known to carry antimicrobial peptides such as cathelicidin-37 and beta-defensin 2, which during the infection by a protozoan parasite Cryptosporidium parvum, leads to increased release of EV, thereby resulting in protection of epithilial cells (Hu et al. 2013). Additionally, EV-cargo such as miRNAs and lncRNAs are known to be stabilized in circulation via protection of the vesicular structure, and are subsequently transferred to target cells to inhibit the expression of target genes. EV-miRNAs have also been shown to trigger malignancy by entering the tumor microenvironment. For example, Felicetti et al., demonstrated that vesicles released from miR-222-overexpressing cells were able to transfer miR-222-dependent malignancy to recipient primary melanomas (Felicetti et al. 2016). EVs are also involved in a variety of physiological events such as the cross talk among glial cells. As an example, EV-miR-9 released from HIV Tat protein stimulated astrocytes can be taken up by microglia resulting in increased migration of the latter cells (Yang et al. 2018a). A study by Hu et al. has also demonstrated that lincRNA-Cox2 expression was increased in EVs derived from astrocytes exposed to morphine, in turn, leading to impaired phagocytosis in microglial cells (Hu et al. 2018).
During erythrocyte maturation, EV secretion serves an excretory function by which the unwanted proteins and RNA are cleared from the cells. However, in cells that lack efficient degradation capability or are located in close proximity to a drainage system such as the tubules of the kidney or the gut, it is EV release rather than lysosomal processing that is beneficial for the cells (Johnstone et al. 1987; Johnstone 2006; Vlassov et al. 2012).
The composition of the EV is primarily governed by the physiological state of its environment as well as the type of producer cell. While the membranes of all EVs are enriched with cholesterol (Morelli et al. 2004; Llorente et al. 2013), glycosphingolipids (Llorente et al. 2013), and phosphatidylserine (Laulagnier et al. 2004; Morelli et al. 2004), the exact lipid profile of specific EVs tends to be similar to, yet distinguishable from that of its cell of origin (Vidal et al. 1989). The proteomic content of the EV is multifactorial; some proteins are present in most EVs, including HSP70, Alix, CD6, CD81, CD9, and major histocompatibility complex class II proteins (Simpson et al. 2009; Mathivanan et al. 2012; Pleet et al. 2018). Other proteins are associated with specific EV subsets, including receptors and other membrane proteins, that confer various functions to the EV. As with lipids and nucleic acids, the proteins incorporated into EVs are related to, but distinct from, the overall protein pool in the cell of origin, suggesting the existence of an intracellular sorting mechanism that helps to determine the EV protein content. The nucleic acid content of EVs is also variable, including various types and quantities of DNA, ribosomal RNA, mRNA, and non-coding RNAs such as miRNAs and lncRNAs.
The mechanism(s) by which EVs interact with their recipient cells still remain elusive. EVs are proposed to interact primarily via the docking of the ligand on the vesicle surface to the receptor(s) on the recipient cells. This docking elicits a signaling response, followed by the transfer of membrane proteins from the vesicle to the cell membrane, fusion of the vesicle with the recipient cell membrane, vesicle uptake through endocytotic processes (clathrin-coated pits, pinocytosis, caveolae, macropinocytosis, and phagocytosis), and ultimately extrusion through a vesicle-cell channel (de Curtis and Meldolesi 2012; Mittelbrunn and Sanchez-Madrid 2012). The fate of vesicular components in recipient cells could depend on the mode of uptake, with processing through the endosomal pathway potentially leading to degradation of EV contents (Tian et al. 2013). Although the mechanism(s) of cargo transfer remains to be elucidated, it is well-recognized that endogenous EVs can exert diverse and potent effects on recipient cells. The diversity of mechanisms by which EVs are generated and can confer functional effects provides a platform for both opportunities and challenges for developing EV-based therapeutics.
In recent years, various novel EV functions have been elucidated, with much of the diversity of the functions ascribed to their cell of origin (Vlassov et al. 2012). For instance, EVs have been investigated as immune response mediators with roles specifically in antigen presentation (Thery et al. 2002, 2009). Furthermore, the role of EVs in angiogenesis, apoptosis, coagulation, and inflammation has now been well-established (Janowska-Wieczorek et al. 2005; Becker et al. 2016; Todorova et al. 2017; Fu et al. 2018; Fujita et al. 2018; Deng et al. 2019; Silachev et al. 2019). Emerging literature has also demonstrated that distinctive properties of EVs make them suitable carriers and vehicles for delivery of various drugs and biomolecules, thereby underscoring their use in therapeutic applications (Table 1) (Srivastava et al. 2016b, a). EVs generated from various cell types, including but not restricted to stem cells, stromal cells, progenitor cells, neuronal cells, cancer cells, and circulating cells, have been tested for their therpaeutic efficacy involving delivery via intraperitoneal, intranasal, intrathecal and intravenous routes in various in vivo model systems of disease pathogeneis. Role of administration routes for EV drug delivery in animal models will be further discussed in section 5 of this review.
Isolation and Characterization of Blood-Derived EVs
EVs can be derived from various sources, including blood, and have been shown to exhibit a change in their composition as well as numbers, under various pathological conditions. It has been shown that blood-derived EVs from healthy individuals can be derived from endothelial cells, erythrocytes, leukocytes, megakaryocytes and/or platelets. Under diseased states however, the numbers and composition of these EVs has been shown to be altered (Zara et al. 2019). As an example, there are reports demonstrating increased numbers of EVs derived from endothelial cells of patients with systemic lupus erythematosus and cardiac failure and this was shown to positively correlate with increased risk for cardiovascular problems (Nozaki et al. 2010; Parker et al. 2014). It has also been shown that increased platelet-specific EVs are a biological marker for cerebral dysfunction(s) in patients with malaria and furhter, that platelet-derived EV numbers are directly associated with coma depth and thrombocytopenia (Pankoui Mfonkeu et al. 2010; Sierro and Grau 2019). Several excellent review articles have described the biology and role of EVs in various disease pathogenesis (Brites and Fernandes 2015; Withrow et al. 2016; Zhang et al. 2016c; Gopal et al. 2017; Huang-Doran et al. 2017; Kinoshita et al. 2017; Castro-Marrero et al. 2018; Fujita et al. 2018; Konoshenko et al. 2018; Letsiou and Bauer 2018; Murphy et al. 2018; Yang et al. 2018b; Aghabozorgi et al. 2019; Pegtel and Gould 2019; Watson et al. 2019; Wu et al. 2019; Zara et al. 2019; Zhu et al. 2019), and the isolation and characterization of EVs from various cells including blood (Aatonen et al. 2014; Nguyen et al. 2016; Xu et al. 2016; Menck et al. 2017; Mushahary et al. 2018; Gorgun et al. 2019; Kim et al. 2019; Pulliam et al. 2019; Richter et al. 2019; Rossi et al. 2019; Skalnikova et al. 2019; Weber et al. 2019).
Bioengineering of EVs
In order to boost their therapeutic potential, EVs can be bioengineered through modifications such as the loading of drugs or attachment of molecules to their surface. Another type of bioengineered EVs relies on the development of artificial exosomes, exosome-based semisynthetic vesicles, exosome-like nanovesicles, and exosome-mimetic nanovesicles (De La Pena et al. 2009; Bryniarski et al. 2013; Jang et al. 2013; Forterre et al. 2014; Jeong et al. 2014; Yoon et al. 2015). These two main categories of EV bioengineering will be referred to in the following sections as engineered EVs and EV mimetics.
Engineered EVs
As mentioned previously, engineered EVs are primarily modified through the loading of drugs as well as via alteration or attachment of molecules on their surface, to enhance delivery efficacy of the therapeutic contents. The in vivo clearance of unmodified EVs is very rapid following their administration. Thus, these engineered surface modifications are meant to extend the biodistribution, stability, and pharmacokinetic profiles of the EVs, thereby facilitating the proposed drug delivery. Several examples of successful engineering of EVs exist in the literature, with the most recent examples listed in Table 2.
EV mimetics
A possible substitute for naturally derived or purified EVs in the development of drug delivery systems and therapeutics is synthetically designed EV mimetics. Synthesis of EV mimetics permits scalable production for use in the clinical setting and provides additional advantages over naturally occurring EVs, in that EV mimetics are sterile and uniform in size and content. EV mimetics, however, do not always function in the same way as endogenous EVs due to the lack of several additional components that are essential for the primary functions of the EVs. Furthermore, the process of screening the core component, extraction, and incorporation of the screened core components into the liposomal complex is cumbersome and labor intensive. Despite these limitations, several studies have begun to evaluate the use of EV mimetics for therapeutic applications. To date, three primary sub-types of EV mimetics have emerged: artificial EV mimetics, physical-origin EV mimetics, and hybrid EV mimetics (Antimisiaris et al. 2018). Recent discussions on nanoparticles for drug delivery include an elegant review by DeMarino et al. covering various aspects of nanoparticle formulation and their applications in improving the delivery efficiency of drugs (DeMarino et al. 2017).
Artificial EV mimetics
Artificial EVs are generated through the assembly of lipids into a bilayer model to resemble the membranes of natural EVs, followed by functionalizing the vesicle surface with proteins or other modifications, thereby allowing the surface to have direct contact with the receptors of the target cells. In some cases, artificial EVs are also tagged with hydrophilic molecules to decrease their elimination and extend their time in circulation. One limitation of artificial EV mimetics is that it is based on the premise that EV function does not require all the components of natural EVs for target-specific, efficient drug delivery. Examples of artificial EV mimetics that have been produced for drug discovery and therapeutics are listed in Table 3. It is important to note that most of the artificial EV mimetics investigated to date are primarily liposomes.
Physical-Origin EV Mimetics
In this category of EV-mimetics, the starting material is not artificial, but rather is derived from other non-EV cellular components. These includeEV mimetics derived from whole cells (termed “cellular vesicles” or “cell-derived vesicles”). Nanovesicles can be generated from whole cells using a variety of techniques, including extrusion through nanopores or cutting the cells with blade-lined microchannels (Jo et al. 2014; Yoon et al. 2015). These physical-origin EV mimetics are able to overcome some of the limitations of other types of EVs such as the low-yield of EVs isolation from cell media or other sources and the lack of a true physiological cell membrane in artificial EV mimetics. Cell-derived vesicles make up the majority of the physical-origin EVs that have been investigated to date (Table 4), demonstrating promising features that could augment the efficiencies of drug targeting (Fuhrmann et al. 2015a).
Hybrid EV Mimetics
Other types of EV mimetics have also been described in the literature; the most common other type of EV mimetic is a hybrid model. Hybrid EV mimetics link EVs to another biological messaging system in order to take advantage of the characteristics of both systems. For example, exosomes have been fused with liposomes, thereby altering the cellular uptake of the EV through changes in the lipid composition or the properties of the lipids making up the liposome (Sato et al. 2016). Another example is the fusion of non-enveloped viruses with EVs to create virus-EV particles (Feng et al. 2013). This natural defense of the virus allows it to escape neutralizing antibodies using the EV-like membrane as camouflage.
Methods of Preparation and Engineering of Engineered EVs and EV-Mimetics
EV Loading
On the basis of EVs biogenesis, the methods of EV loading have been primarily categorized as follows: (a) strategies including site of exosomal functional entities, e.g. transmembrane proteins and the use of their natural tropism to co-localize the exogenous components; (b) strategies involving the use of molecular mechanisms for the effective incorporation of exogenous molecules into EVs for their cytosolic delivery; and (c) strategies involving enrichment of the quantity of molecules into the cellular plasma membrane to be encapsulated by passive mechanism during multivesicular body formation. The techniques for the production of bioengineered EVs are generally classified by the presence or timing of EV isolation, including: (a) pre-loading modifications, (b) post-loading modifications, and (c) creation of artificial mimetic structures of the natural exosomes.
Pre-loading Modifications
The mechanisms of pre-isolation engineering can be grouped into the following three primary categories: (a) use of “exosome display” to engineer the expression of transmembrane proteins for co-localization to exogenous entities; (b) use of molecular mechanisms to directly incorporate exogenous molecules into EVs for their cytosolic delivery; and (c) enriching the quantity of molecules into the origin cell’s plasma membrane to be encapsulated by passive mechanism during multivesicular body formation (Garcia-Manrique et al. 2018). In each of these cases, the drug of interest is loaded directly into or onto the surface of the parental source cells and, as a result, the EVs are released or isolated from the source cells pre-loaded with the drug of interest. This approach is often employed when specific oligonucleotides or proteins of interest are to be loaded in the EVs wherein the parental source cells are designed to release the EVs that are pre-loaded with either the specific oligonucleotides or the protein of interest. Pre-loading modifications of EVs can be achieved by the treatment of parental source cells with drugs of interest or engineering of parental source cells (Luan et al. 2017).
In a simple example of pre-loading modification, the parental source cells are exposed to the drug of interest for a stipulated time, resulting in the drug-exposed cells secreting EVs that are pre-loaded with the drug of interest. While this method is simple, it is limited by a lack of control over the loading efficiency of the drug into the secreted EVs. Despite the limitations, several studies have successfully used this approach. One such study exposed the murine mesenchymal stromal cell line, SR4987 treated with paclitaxel for 24 h, and found significant anti-proliferative effects on CFPAC-1 cells (a paclitaxel-sensitive, human pancreatic cell line) when compared with cells that were treated with conditioned medium from untreated mesenchymal stromal cells (Pascucci et al. 2014). In another study, human adipose-derived mesenchymal stem cells that were incubated in p5 (a peptide derived from p35) for 24 h were able to release biologically functional p5 to inhibit p35 cleavage, CDK5 phosphorylation and calpain-mediated p53 upregulation in bovine aortic endothelial cells. The p5-incubated cells protected the aortic endothelial cells from stress like hypoxia/ischemia, oxidative stress, and inflammation (Fang et al. 2016).
The effect of drug exposure is not limited to cellular uptake of the drug, as the exposure can also cause reactive changes within the cell that are reflected in the secreted EVs. For example, exposure of human hepatocellular carcinoma cells to heat shock or anticancer drugs such as paclitaxel, carboplatin, etoposide, or irinotecan hydrochloride result in the release of EVs that are loaded with heat shock proteins on their membrane surface. These heat shock protein-bearing EVs can elicit anti-tumor effects in natural killer cells, in vitro (Lv et al. 2012). In another study, human hepatocellular carcinoma cells were exposed to the histone deacetylase inhibitor MS-275 for 72 h, and following exposure, EVs that were isolated from the culture medium demonstrated increased cytotoxicity of natural killer cells and increased proliferation of peripheral blood mononuclear cells, thereby suggesting a promising therapeutic strategy against hepatocellular carcinoma (Xiao et al. 2013). Recently, Yuan et al. have also demonstrated the anti-cancer potential of EVs that were released from human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-transduced mesenchymal stromal cells in 11 different cancer cell lines, suggesting the efficacy of these EVs in inducing selective apoptosis in various cancer cells (Yuan et al. 2017).
Extrusion
Extrusion is a process by which EVs are derived from cells through filters of reducing pore sizes (Jang et al. 2013). The vesicles are produced artificially by breaking up the cells and reforming the contents in the exosome mimetics. This technique has shown to produce higher quantities of EVs when compared to the EVs released by the cells (Jang et al. 2013; Jang and Gho 2014; Lunavat et al. 2016). Investigators have used this method to develop the exosome mimetic nanovesicles to effectively deliver chemotherapeutics such as doxorubicin, 5-fluorouracil, gemcitabine, and carboplatin and study their effects on tumor growth (Jang et al. 2013). EVs were also harvested from the same cells to compare the efficacy of EVs to that of the exosome mimetic nanovesicles. Both of the vesicles have similar efficacy in reducing tumor growth, however, when compared to free drug exosome mimetics were more efficient compared to natural EVs. Another interesting finding was that when the exosome mimetics were isolated from the two cell lines containing cancer drugs and injected into an immunocompetent mouse tumor model, they both exhibited similar anti-tumor effects with no reported systemic side effects. Other investigators have shown that loading of RNAi in the exosome-mimetic nanovesicles was therapeutically active (Lunavat et al. 2016). From this study, it was reported that both exogenous and endogenous loading methods were efficient to cause a reduction in the expression levels of c-Myc. The positive findings of the study imply that the exosome-mimetic nanovesicles could, in fact, be used to overcome some of the scale-up issues currently associated with the development of EV therapeutics.
Microfluidic Method
This method has been recently used for purification of vesicles from cell media or biological fluids (Wang et al. 2013; Liang et al. 2017; Liu et al. 2017; Wu et al. 2017) and whole cells (Yoon et al. 2015). This method has been used in the preparation of liposomes and other types of nanoparticles for drug delivery. In fact, it has been established that one-step, the fully automated, and the scalable microfluidic system can be used for ligand-targeted liposomes (Ran et al. 2016; Rosenblum et al. 2018). This technique can also be used to prepare exosome mimetics for efficient drug delivery, however, this remains unexplored until now.
Post-Isolation Modifications
Incubation with Drugs
Through a similar process, as is used pre-isolation, drug loading of EVs can also be performed by incubating the EVs post-isolation with the drug. Liposomes have long been used to improve the therapeutic and pharmacokinetic profiles of therapeutic drugs through increased bioavailability and retention in the target tissues, although opsonization and rapid clearance continue to be a significant hurdle for some of these nanoparticles (Zhang et al. 2005).
Recent reports have shown that curcumin, doxorubicin, and paclitaxel can be passively loaded within EVs to improve their therapeutic efficacy (Sun et al. 2010; Zhuang et al. 2011; Yang et al. 2015). Doxorubicin- and paclitaxel-loaded EVs have been demonstrated to cross the blood-brain barrier in zebrafish (Yang et al. 2015), with paclitaxel-loaded EVs demonstrating anti-tumorigenic effects (Pascucci et al. 2014; Rani et al. 2015). Curcumin, on the other hand, interacts with the lipid membrane of the EV to form a complex which, upon administration to macrophages, exhibits better anti-inflammatory efficacy than curcumin delivered alone (Sun et al. 2010). Curcumin complex has also been delivered in vivo in the lipopolysaccharide (LPS)-induced mouse model of shock. This study demonstrated the stability of the curcumin complex over a longer period, and also showed that administration of curcumin-loaded EVs intranasally protected mice from LPS-induced brain inflammation and autoimmune encephalomyelitis, and delayed tumor growth (Zhuang et al. 2011). Based on these findings, a phase I clinical trial (NCT01294072) is currently ongoing to evaluate the efficacy of plant exosomes to deliver curcumin drug to colon cancer patients.
Sonication
Several techniques have now been developed to increase the efficiency of transferring drug into EVs, including sonication. In this process, EVs are mixed with the drugs or proteins of interest followed by sonication with a homogenizer probe. The sonicator induces mechanical shear forces that affect the EV membrane integrity and allow increased drug entry into the EV (Kim et al. 2016). Although the membrane integrity is affected, the sonication process does not appear to alter other contents within the EVs significantly, and the membrane integrity is restored within an hour of incubation. In addition to being encapsulated inside the EV following sonication, drugs could also adhere to the outer surface of the EV membrane, resulting in two phases of drug release. The first burst release phase results from the release of the drug adhered to the outer membrane of the EVs, followed by the slow release of the drug encapsulated inside the EV (Kim et al. 2016).
Electroporation
A popular method to load cargo into EVs is electroporation, a process by which transient pores are made into the membranes of the EVs. In this method, purified EVs and the therapeutic cargo are mixed together in a buffer followed by electroporation and incubation (Shtam et al. 2013; Tian et al. 2014; Lamichhane et al. 2015). After incubation, the EVs are washed with PBS to remove unloaded cargo followed by ultracentrifugation. A study by Alvarez-Erviti et al. successfully engineered bone marrow dendritic cells to express rabies virus glycoprotein peptide that was fused to an EV membrane expressing Lamp2b. Intravenous injection of these EVs to mice resulted in neuron-specific gene silencing (Alvarez-Erviti et al. 2011). Similar studies have been carried out with RAD51, luciferase and MAPK1 siRNAs loaded into EVs through electroporation and delivered to HeLa cells, endothelial cells, monocytes and lymphocytes respectively (Shtam et al. 2013; Banizs et al. 2014). Other investigators have also loaded dsDNAs and chemotherapeutic drugs in EVs using this technique (Tian et al. 2014; Lamichhane et al. 2015). Overall, electroporation is one of the most useful techniques for delivery of siRNA, DNA, chemotherapeutic agents as well as miRNA, mRNA and proteins into EVs. Although this technique results in a minimal effect on the EV components, it may produce aggregation (Hertzberg and Wolff 1990; Weaver 1993; Hood et al. 2014; Johnsen et al. 2016) and lacks significant scalability, that would be necessary for large clinical investigations.
Saponin Assisted Loading
Another method for EV loading is the permeabilization of the EV membrane through the use of saponin. Saponin is a detergent-like molecule that interacts with cholesterol in the EV membrane resulting in pore formation (Jacob et al. 1991; Jamur and Oliver 2010). This technique was used in a study assessing the use of catalase-loaded EVs derived from macrophages for drug delivery in Parkinson’s disease, which resulted in protection against oxidative stress and neurodegeneration (Haney et al. 2015). The authors compared different loading techniques and showed that EVs loaded by saponin permeabilization showed no alterations in EV size or morphology and had similar loading efficiencies and sustained release compared to sonication and extrusion methods. The EVs that underwent sonication appeared to have more non-spherical morphology than those undergoing saponin permeabilization (Haney et al. 2015). Others have shown similar success with saponin in preparing a porphyrin-EV complex, which was shown to be taken up by MDA cells (Fuhrmann et al. 2015b). Although saponin permeabilization is a simple and easy procedure for loading therapeutic proteins, it has not yet been well-studied. Additionally, it is important to ensure removal of the saponins after use, as prolonged exposure may affect the EV morphology, uptake, and stability.
Freezing and Thawing
Another simple method of instilling drug within the EVs is through freeze and thaw cycles. In this method, drugs are incubated with the EVs at 37°C followed by rapid freezing at -80 °C and then thawed to room temperature. This process is repeated for a minimum of 3 cycles for drug encapsulation (Sato et al. 2016). The major drawback of this procedure is that it can induce aggregation, and it tends to result in lower drug loading than many of the other methods. Of note, however, this method can be used for fusion of exosomes with liposomes to develop exosome mimetic particles (Sato et al. 2016).
Surface Modification Method
The proteins located on the surface of EVs are significantly associated with the biodistribution characteristics of the EV. Modification of the surface proteins through gene transfer vectors can, therefore, improve the targeting efficiency of the exosomes (Sato et al. 2016). Some of the transmembrane proteins that are most commonly altered include tetraspanins, Lamp-2b, glycosyl-phosphatidyl-inositol, platelet-derived growth-factor receptors, and lactadherin (Mentkowski et al. 2018). For example, fusing rabies viral glycoprotein with Lamp-2b on EVs results in specific delivery of the EVs to neurons and glia (Alvarez-Erviti et al. 2011; Liu et al. 2015). Similarly, immature dendritic cells have been modified to express Lamp-2b fused with αv integrin-specific iRGD peptide to target tumor cells (Tian et al. 2014). Several methods have been adapted for surface modification of EVs while ensuring that functionality is retained. One such method is copper-catalyzed azide-alkyne cycloaddition (CCAAC) (Smyth et al. 2014; Oude Blenke et al. 2015; Wang et al. 2015) which has been used successfully for delivering chemotherapeutics (Lee et al. 2016). Recently, modified methods have been developed for loading and surface modification of exosomes. Anchor peptides such as CP05 have been demonstrated to aid in targeting, loading and purification of diverse-origin-exosomes through binding to CD63- exosomal surface protein. Furthermore, it was shown that exosomal anchor peptide could be used as a tool for exosomal engineering, probing gene function in vivo, as well as targeted therapeutic drug delivery (Gao et al. 2018).
Methods of EV Delivery
Therapeutic efficacy and toxicity of EVs are critically influenced by their biodistribution (Wiklander et al. 2015). For relevance in a clinical setting, EVs must be stable and capable of delivering their cargo through the commonly used (preferably non-invasive) administration routes. Here we compare various administration routes currently used for effective EV delivery of therapeutics in vivo (Johnsen et al. 2014; Lener et al. 2015).
It has been well-established that systemic administration of EVs results in accumulation in the liver, kidneys and spleen resulting in the rapid removal of the EVs from blood circulation. Multimodal imaging of systemically administered luciferase-loaded EVs in vivo revealed that the half-life of EVs was less than 30 minutes in most tissues and the EVs were completely cleared from the animals by 6 hours (Lai et al. 2014). Likewise, a pharmacokinetic analysis revealed that the half-life of EVs loaded with luciferase-lactadherin fusion protein in the circulation is approximately 2 minutes and only weakly detectable after 4 hours, indicating rapid clearance in vivo (Takahashi et al. 2013). These results are in line with previous studies demonstrating that EVs can be detected in the liver and/or spleen, but not in circulation, at 24 h after systemic administration (Peinado et al. 2012; Ohno et al. 2013).
Despite the advanced development of drugs for the treatment of various brain disorders, delivery of these drugs to the brain, however, remains a significant challenge because of difficulty in penetrating the blood-brain barrier (Gabathuler 2009). Intranasal delivery provides a practical, noninvasive method for delivering therapeutic agents to the brain, the quantities of drug administered nasally and that are transported directly from nose to brain, however, is small (Johnson et al. 2010). Zhuang and colleagues showed that intranasal delivery of EV-encapsulated curcumin or Stat3 inhibitor, JSI-124 (cucurbitacin I), resulted in the compounds reaching microglial cells. Additionally, administration of the curcumin or JSI-124 EVs inhibited LPS-induced microglial cell activation, delayed experimental autoimmune encephalomyelitis (EAE) disease, and inhibited tumor growth in vivo (Zhuang et al. 2011). Recently, intranasal delivery of lincRNA-Cox2 siRNA loaded EVs showed decrease of LPS-Induced microglial proliferation in mice (Liao et al. 2019). Table 5 summarizes the administration routes used for EV drug delivery in the studies published to date.
Therapeutic Applications of EVs
With increased promising preclinical and early clinical evidence, exploring the potential of EVs as therapeutic agents have attracted a lot of attention and have made its way into the clinics. As shown in Table 6, EVs are currently being tested as drug delivery vehicles in several different trials. Given the ability of EVs to modulate various responses in recipient cells and strong candidacy as a biomarker for a variety of diseases, there is a growing interest in using them as therapeutic entities (Fais et al. 2016). In non-small cell lung cancer (NSCLC), tumor-associated antigen (TAA) loaded dendritic cell-derived EVs (Dex) showed immunomodulatory response and underwent phase I clinical trial (Escudier et al. 2005; Morse et al. 2005). In this study, MAGE antigen-loaded Dex therapy in 15 MAGE3+ advanced melanoma patients resulted in no detectable MAGE-specific T cell responses in peripheral blood, although enhanced NK cell effector functions were observed in 8 out of 13 patients (Escudier et al. 2005). In another study, 3 out of 9 patients with advanced MAGE+ NSCLC who received MAGE3 A1-loaded Dex successfully developed MAGE3 A1-specific systemic immune responses as determined by delayed type hypersensitivity (DTH) reactivity, although only minimal increases in peptide-specific T cell activity were detected (Morse et al. 2005). The demonstration of Dex administration safety profile, the feasibility of therapy and success in some patients resulted in the clinical phase II trial for the treatment of non-small-cell lung cancer patients (Besse et al. 2016). In this study, to overcome the minimal peptide-specific activity, TLR4L- or interferon (IFN)-γ-maturated Dex was used to induce greater T cell stimulation compared to Dex from immature DCs (Segura et al. 2005; Viaud et al. 2011).
In another series of cancer trials, the ascites-derived exosomes in combination with the granulocyte-macrophage-colony-stimulating factor (GM-CSF) in the immunotherapy of colorectal cancer (CRC) were proven safe and well tolerated in phase I clinical trial. The ascites-derived exosomes isolated by sucrose/D(2)O density gradient ultracentrifugation is 60-90-nm vesicles that contain the diverse immunomodulatory markers of exosomes and tumor-associated carcinoembryonic antigen (CEA) (Dai et al. 2008). The above studies indicate that this therapeutic concept is safe and feasible, thus reinforcing the use of EVs as a new therapeutic approach against diseases (Figs. 1 and 2).
Examples of organ systems targeted by EV administration. The organ disease-specific studies have utilized various EV-related interventions, including: a umbilical cord mesenchymal stem cell (MSC)-derived, cardiac progenitor cell-derived, hypoxic cardiosphere-derived, or embryonic stem cell-derived exosomes, or curcumin-loaded exosomes; b hypoxia preconditioned MSC-derived exosomes or bone marrow-derived A1 exosomes; c MSC-derived exosomes; d umbilical cord MSC-derived or urine-derived stem cell exosomes; e MSC-derived exosomes; f iron oxide exosomes, tumor associated antigen containing exosomes, paclitaxel containing exosomes, trastuzmab-emtansine containing exosomes, or GM-CSF expressing embryonic stem cell-derived exosomes; g adipose-derived, induced pluripotent stem cell-derived, or umbilical cord MSC-derived exosomes; h MSC-derived or adipose-derived stem cell exosomes; i miR-223 containing exosomes; j LDL-stimulated macrophage-derived exosomes
Conclusions and Future Perspectives
The significant advancements in the knowledge surrounding the biology of EVs over the past several years have opened new avenues in the field of life sciences, especially in medicine. Not the least of these is the work that has been done in investigating the role of EVs in both health and diseases, resulting in novel prospects for the advancement of enriched therapeutic EVs. These interventions could help in the synthesis of new cargos inspired by natural vesicles or conventional synthetic alternatives (liposomes, polymersomes, inorganic nanoparticles, and so on) without serious inconveniences. Furthermore, the development of EVs for drug delivery has generated significant excitement in the field; however, the main limitations/challenges of EVs as genuine therapeutic agents include developing methods for efficient, large-scale clinical grade production, isolation, storage, modification, purification as well as target delivery. For example, storage and retrieval conditions of EVs and EV mimetics can can alter their characteristics (Thery et al. 2018). Although efforts have been made in this regard (Zhou et al. 2006; Yuana et al. 2015; Reiner et al. 2017; Leiferman et al. 2019), currently there are no standard operating procedures for long term storage of various types of EVs. Additionally, organ- or cell- specific delivery of therapeutics with EVs poses yet another challenge. Indeed, targeted delivery of EVs has gained increasing attention in the field (Table 5). Recent investigations are focused on overcoming these limitations by establishing artificial EV mimetics or by generating vesicles from membrane fragments created by the extrusion or slicing of cells. Ultimately, regardless of the methods used, the development of multidisciplinary teams with skills in applied biology, pharmacology, chemical engineering, material sciences, and medicine will be required to translate EV-based therapy to clinical practices successfully.
References
Aatonen MT, Ohman T, Nyman TA, Laitinen S, Gronholm M, Siljander PR (2014) Isolation and characterization of platelet-derived extracellular vesicles. J Extracell Vesicles 3.
Abdel-Haq H (2019) Blood exosomes as a tool for monitoring treatment efficacy and progression of neurodegenerative diseases. Neural Regen Res 14:72–74
Aghabozorgi AS, Ahangari N, Eftekhaari TE, Torbati PN, Bahiraee A, Ebrahimi R, Pasdar A (2019) Circulating exosomal miRNAs in cardiovascular disease? New emerging hopes. J Cell Physiol, Pathogenesis
Agrahari V, Agrahari V, Burnouf PA, Chew CH, Burnouf T (2019) Extracellular Microvesicles as New Industrial Therapeutic Frontiers. Trends Biotechnol
Altanerova U, Babincova M, Babinec P, Benejova K, Jakubechova J, Altanerova V, Zduriencikova M, Repiska V, Altaner C (2017) Human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia. Int J Nanomedicine 12:7923–7936
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–345
Andaloussi SEL, Mager I, Breakefield XO, Wood MJ (2013) Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12:347–357
Antimisiaris SG, Mourtas S, Marazioti A (2018) Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery Pharmaceutics 10.
Arnold PY, Mannie MD (1999) Vesicles bearing MHC class II molecules mediate transfer of antigen from antigen-presenting cells to CD4+ T cells. Eur J Immunol 29:1363–1373
Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP (2013) Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 10:301–312
Banizs AB, Huang T, Dryden K, Berr SS, Stone JR, Nakamoto RK, Shi W, He J (2014) In vitro evaluation of endothelial exosomes as carriers for small interfering ribonucleic acid delivery. Int J Nanomedicine 9:4223–4230
Barok M, Puhka M, Vereb G, Szollosi J, Isola J, Joensuu H (2018) Cancer-derived exosomes from HER2-positive cancer cells carry trastuzumab-emtansine into cancer cells leading to growth inhibition and caspase activation. BMC Cancer 18:504
Beauvillain C, Juste MO, Dion S, Pierre J, Dimier-Poisson I (2009) Exosomes are an effective vaccine against congenital toxoplasmosis in mice. Vaccine 27:1750–1757
Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D (2016) Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 30:836–848
Bellavia D, Raimondo S, Calabrese G, Forte S, Cristaldi M, Patinella A, Memeo L, Manno M, Raccosta S, Diana P, Cirrincione G, Giavaresi G, Monteleone F, Fontana S, De Leo G, Alessandro R (2017) Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo Chronic Myelogenous Leukemia cell growth. Theranostics 7:1333–1345
Besse B et al (2016) Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 5:e1071008
Braun RK, Chetty C, Balasubramaniam V, Centanni R, Haraldsdottir K, Hematti P, Eldridge MW (2018) Intraperitoneal injection of MSC-derived exosomes prevent experimental bronchopulmonary dysplasia. Biochem Biophys Res Commun 503:2653–2658
Brites D, Fernandes A (2015) Neuroinflammation and Depression: Microglia Activation, Extracellular Microvesicles and microRNA Dysregulation. Front Cell Neurosci 9:476
Bryniarski K et al (2013) Antigen-specific, antibody-coated, exosome-like nanovesicles deliver suppressor T-cell microRNA-150 to effector T cells to inhibit contact sensitivity. J Allergy Clin Immunol 132:170–181
Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y, Wang G (2018) Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnology 16:81
Busato A, Bonafede R, Bontempi P, Scambi I, Schiaffino L, Benati D, Malatesta M, Sbarbati A, Marzola P, Mariotti R (2016) Magnetic resonance imaging of ultrasmall superparamagnetic iron oxide-labeled exosomes from stem cells: a new method to obtain labeled exosomes. Int J Nanomedicine 11:2481–2490
Castro-Marrero J, Serrano-Pertierra E, Oliveira-Rodriguez M, Zaragoza MC, Martinez-Martinez A, Blanco-Lopez MDC, Alegre J (2018) Circulating extracellular vesicles as potential biomarkers in chronic fatigue syndrome/myalgic encephalomyelitis: an exploratory pilot study. J Extracell Vesicles 7:1453730
Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y (2013) Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun 431:566–571
Chen L, Lu FB, Chen DZ, Wu JL, Hu ED, Xu LM, Zheng MH, Li H, Huang Y, Jin XY, Gong YW, Lin Z, Wang XD, Chen YP (2018) BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol Immunol 93:38–46
Cheng Y, Schorey JS (2013) Exosomes carrying mycobacterial antigens can protect mice against Mycobacterium tuberculosis infection. Eur J Immunol 43:3279–3290
Chiang CY, Chen C (2019) Toward characterizing extracellular vesicles at a single-particle level. J Biomed Sci 26:9
Ciullo A, Biemmi V, Milano G, Bolis S, Cervio E, Fertig ET, Gherghiceanu M, Moccetti T, Camici GG, Vassalli G, Barile L (2019) Exosomal Expression of CXCR4 Targets Cardioprotective Vesicles to Myocardial Infarction and Improves Outcome after Systemic Administration. Int J Mol Sci 20
Colombo M, Raposo G, Thery C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289
Cosenza S, Toupet K, Maumus M, Luz-Crawford P, Blanc-Brude O, Jorgensen C, Noel D (2018) Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 8:1399–1410
Cui GH, Wu J, Mou FF, Xie WH, Wang FB, Wang QL, Fang J, Xu YW, Dong YR, Liu JR, Guo HD (2018) Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J 32:654–668
Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G (2008) Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther 16:782–790
Danesh A, Inglis HC, Jackman RP, Wu S, Deng X, Muench MO, Heitman JW, Norris PJ (2014) Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood 123:687–696
de Curtis I, Meldolesi J (2012) Cell surface dynamics - how Rho GTPases orchestrate the interplay between the plasma membrane and the cortical cytoskeleton. J Cell Sci 125:4435–4444
De La Pena H, Madrigal JA, Rusakiewicz S, Bencsik M, Cave GW, Selman A, Rees RC, Travers PJ, Dodi IA (2009) Artificial exosomes as tools for basic and clinical immunology. J Immunol Methods 344:121–132
De Miguel D, Basanez G, Sanchez D, Malo PG, Marzo I, Larrad L, Naval J, Pardo J, Anel A, Martinez-Lostao L (2013) Liposomes decorated with Apo2L/TRAIL overcome chemoresistance of human hematologic tumor cells. Mol Pharm 10:893–904
DeMarino C, Schwab A, Pleet M, Mathiesen A, Friedman J, El-Hage N, Kashanchi F (2017) Biodegradable Nanoparticles for Delivery of Therapeutics in CNS Infection. J NeuroImmune Pharmacol 12:31–50
Deng ZB, Zhuang X, Ju S, Xiang X, Mu J, Liu Y, Jiang H, Zhang L, Mobley J, McClain C, Feng W, Grizzle W, Yan J, Miller D, Kronenberg M, Zhang HG (2013) Exosome-like nanoparticles from intestinal mucosal cells carry prostaglandin E2 and suppress activation of liver NKT cells. J Immunol 190:3579–3589
Deng Z, Rong Y, Teng Y, Mu J, Zhuang X, Tseng M, Samykutty A, Zhang L, Yan J, Miller D, Suttles J, Zhang HG (2017) Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol Ther 25:1641–1654
Deng W, Tang T, Hou Y, Zeng Q, Wang Y, Fan W, Qu S (2019) Extracellular vesicles in atherosclerosis. Clin Chim Acta 495:109–117
Di Rocco G, Baldari S, Toietta G (2016) Towards Therapeutic Delivery of Extracellular Vesicles: Strategies for In Vivo Tracking and Biodistribution Analysis. Stem Cells Int 2016:5029619
Didiot MC, Hall LM, Coles AH, Haraszti RA, Godinho BM, Chase K, Sapp E, Ly S, Alterman JF, Hassler MR, Echeverria D, Raj L, Morrissey DV, DiFiglia M, Aronin N, Khvorova A (2016) Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing. Mol Ther 24:1836–1847
Doeppner TR, Herz J, Gorgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM (2015) Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl Med 4:1131–1143
Escudier B et al (2005) Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med 3:10
Fais S et al (2016) Evidence-Based Clinical Use of Nanoscale Extracellular Vesicles in Nanomedicine. ACS Nano 10:3886–3899
Fang WH, Kumar S, McDowell G, Smith D, Krupinski J, Olah P, Al-Baradie RS, Al-Rukban MO, Petcu EB, Slevin M (2016) Mesenchymal Stem Cells Loaded with p5, Derived from CDK5 Activator p35, Inhibit Calcium-Induced CDK5 Activation in Endothelial Cells. Stem Cells Int 2016:2165462
Felicetti F, De Feo A, Coscia C, Puglisi R, Pedini F, Pasquini L, Bellenghi M, Errico MC, Pagani E, Care A (2016) Exosome-mediated transfer of miR-222 is sufficient to increase tumor malignancy in melanoma. J Transl Med 14:56
Feng Z, Hensley L, McKnight KL, Hu F, Madden V, Ping L, Jeong SH, Walker C, Lanford RE, Lemon SM (2013) A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 496:367–371
Fevrier B, Raposo G (2004) Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 16:415–421
Forterre A, Jalabert A, Berger E, Baudet M, Chikh K, Errazuriz E, De Larichaudy J, Chanon S, Weiss-Gayet M, Hesse AM, Record M, Geloen A, Lefai E, Vidal H, Coute Y, Rome S (2014) Proteomic analysis of C2C12 myoblast and myotube exosome-like vesicles: a new paradigm for myoblast-myotube cross talk? PLoS One 9:e84153
Fu H, Hu D, Zhang L, Tang P (2018) Role of extracellular vesicles in rheumatoid arthritis. Mol Immunol 93:125–132
Fuhrmann G, Herrmann IK, Stevens MM (2015a) Cell-derived vesicles for drug therapy and diagnostics: opportunities and challenges. Nano Today 10:397–409
Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM (2015b) Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release 205:35–44
Fujita Y, Kadota T, Araya J, Ochiya T, Kuwano K (2018) Extracellular Vesicles: New Players in Lung Immunity. Am J Respir Cell Mol Biol 58:560–565
Gabathuler R (2009) Blood-brain barrier transport of drugs for the treatment of brain diseases. CNS Neurol Disord Drug Targets 8:195–204
Gallego-Lleyda A, De Miguel D, Anel A, Martinez-Lostao L (2018) Lipid Nanoparticles Decorated with TNF-Related Aptosis-Inducing Ligand (TRAIL) Are More Cytotoxic than Soluble Recombinant TRAIL in Sarcoma. Int J Mol Sci 19.
Gangadaran P, Rajendran RL, Lee HW, Kalimuthu S, Hong CM, Jeong SY, Lee SW, Lee J, Ahn BC (2017) Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J Control Release 264:112–126
Gao W, Liu H, Yuan J, Wu C, Huang D, Ma Y, Zhu J, Ma L, Guo J, Shi H, Zou Y, Ge J (2016) Exosomes derived from mature dendritic cells increase endothelial inflammation and atherosclerosis via membrane TNF-alpha mediated NF-kappaB pathway. J Cell Mol Med 20:2318–2327
Gao X, Ran N, Dong X, Zuo B, Yang R, Zhou Q, Moulton HM, Seow Y, Yin H (2018) Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Sci Transl Med 10
Garcia-Manrique P, Matos M, Gutierrez G, Pazos C, Blanco-Lopez MC (2018) Therapeutic biomaterials based on extracellular vesicles: classification of bio-engineering and mimetic preparation routes. J Extracell Vesicles 7:1422676
Goh WJ, Lee CK, Zou S, Woon EC, Czarny B, Pastorin G (2017a) Doxorubicin-loaded cell-derived nanovesicles: an alternative targeted approach for anti-tumor therapy. Int J Nanomedicine 12:2759–2767
Goh WJ, Zou S, Ong WY, Torta F, Alexandra AF, Schiffelers RM, Storm G, Wang JW, Czarny B, Pastorin G (2017b) Bioinspired Cell-Derived Nanovesicles versus Exosomes as Drug Delivery Systems: a Cost-Effective Alternative. Sci Rep 7:14322
Gopal SK, Greening DW, Rai A, Chen M, Xu R, Shafiq A, Mathias RA, Zhu HJ, Simpson RJ (2017) Extracellular vesicles: their role in cancer biology and epithelial-mesenchymal transition. Biochem J 474:21–45
Gorgun C, Reverberi D, Rotta G, Villa F, Quarto R, Tasso R (2019) Isolation and Flow Cytometry Characterization of Extracellular-Vesicle Subpopulations Derived from Human Mesenchymal Stromal Cells. Curr Protoc Stem Cell Biol 48:e76
Gould SJ, Raposo G (2013) As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles 2
Grange C, Tapparo M, Bruno S, Chatterjee D, Quesenberry PJ, Tetta C, Camussi G (2014) Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med 33:1055–1063
Guo X, Qiu W, Liu Q, Qian M, Wang S, Zhang Z, Gao X, Chen Z, Xue H, Li G (2018) Immunosuppressive effects of hypoxia-induced glioma exosomes through myeloid-derived suppressor cells via the miR-10a/Rora and miR-21/Pten Pathways. Oncogene 37:4239–4259
Gyorgy B, Hung ME, Breakefield XO, Leonard JN (2015) Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol 55:439–464
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV (2015) Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release 207:18–30
Hertzberg H, Wolff K (1990) Which diagnosis do you suggest? Schweiz Arch Tierheilkd 132:331–334
Hood JL, Scott MJ, Wickline SA (2014) Maximizing exosome colloidal stability following electroporation. Anal Biochem 448:41–49
Hoshino A et al (2015) Tumour exosome integrins determine organotropic metastasis. Nature 527:329–335
Hu G, Yao H, Chaudhuri AD, Duan M, Yelamanchili SV, Wen H, Cheney PD, Fox HS, Buch S (2012) Exosome-mediated shuttling of microRNA-29 regulates HIV Tat and morphine-mediated neuronal dysfunction. Cell Death Dis 3:e381
Hu G, Gong AY, Roth AL, Huang BQ, Ward HD, Zhu G, Larusso NF, Hanson ND, Chen XM (2013) Release of luminal exosomes contributes to TLR4-mediated epithelial antimicrobial defense. PLoS Pathog 9:e1003261
Hu L, Wickline SA, Hood JL (2015) Magnetic resonance imaging of melanoma exosomes in lymph nodes. Magn Reson Med 74:266–271
Hu G, Yang L, Cai Y, Niu F, Mezzacappa F, Callen S, Fox HS, Buch S (2016a) Emerging roles of extracellular vesicles in neurodegenerative disorders: focus on HIV-associated neurological complications. Cell Death Dis 7:e2481
Hu L, Wang J, Zhou X, Xiong Z, Zhao J, Yu R, Huang F, Zhang H, Chen L (2016b) Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci Rep 6:32993
Hu G, Witwer KW, Bond VC, Haughey N, Kashanchi F, Pulliam L, Buch S (2017a) Proceedings of the ISEV symposium on "HIV, NeuroAIDS, drug abuse & EVs". J Extracell Vesicles 6:1294360
Hu G, Yelamanchili S, Kashanchi F, Haughey N, Bond VC, Witwer KW, Pulliam L, Buch S (2017b) Proceedings of the 2017 ISEV symposium on "HIV, NeuroHIV, drug abuse, & EVs". J Neuro-Oncol 23:935–940
Hu G, Liao K, Niu F, Yang L, Dallon BW, Callen S, Tian C, Shu J, Cui J, Sun Z, Lyubchenko YL, Ka M, Chen XM, Buch S (2018) Astrocyte EV-Induced lincRNA-Cox2 Regulates Microglial Phagocytosis: Implications for Morphine-Mediated Neurodegeneration. Mol Ther Nucleic Acids 13:450–463
Huang C, Huang Y, Zhou Y, Nie W, Pu X, Xu X, Zhu J (2018) Exosomes derived from oxidized LDL-stimulated macrophages attenuate the growth and tube formation of endothelial cells. Mol Med Rep 17:4605–4610
Huang-Doran I, Zhang CY, Vidal-Puig A (2017) Extracellular Vesicles: Novel Mediators of Cell Communication In Metabolic Disease. Trends Endocrinol Metab 28:3–18
Ibrahim AG, Cheng K, Marban E (2014) Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports 2:606–619
Iraci N, Leonardi T, Gessler F, Vega B, Pluchino S (2016) Focus on Extracellular Vesicles: Physiological Role and Signalling Properties of Extracellular Membrane Vesicles. Int J Mol Sci 17:171
Jacob MC, Favre M, Bensa JC (1991) Membrane cell permeabilization with saponin and multiparametric analysis by flow cytometry. Cytometry 12:550–558
Jamur MC, Oliver C (2010) Permeabilization of cell membranes. Methods Mol Biol 588:63–66
Jang SC, Gho YS (2014) Could bioengineered exosome-mimetic nanovesicles be an efficient strategy for the delivery of chemotherapeutics? Nanomedicine (London) 9:177–180
Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, Nilsson J, Lotvall J, Kim YK, Gho YS (2013) Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 7:7698–7710
Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ (2005) Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer 113:752–760
Jeong D, Jo W, Yoon J, Kim J, Gianchandani S, Gho YS, Park J (2014) Nanovesicles engineered from ES cells for enhanced cell proliferation. Biomaterials 35:9302–9310
Jiang ZZ, Liu YM, Niu X, Yin JY, Hu B, Guo SC, Fan Y, Wang Y, Wang NS (2016) Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther 7:24
Jo W, Kim J, Yoon J, Jeong D, Cho S, Jeong H, Yoon YJ, Kim SC, Gho YS, Park J (2014) Large-scale generation of cell-derived nanovesicles. Nanoscale 6:12056–12064
Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M (2014) A comprehensive overview of exosomes as drug delivery vehicles - endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta 1846:75–87
Johnsen KB, Gudbergsson JM, Skov MN, Christiansen G, Gurevich L, Moos T, Duroux M (2016) Evaluation of electroporation-induced adverse effects on adipose-derived stem cell exosomes. Cytotechnology 68:2125–2138
Johnson NJ, Hanson LR, Frey WH (2010) Trigeminal pathways deliver a low molecular weight drug from the nose to the brain and orofacial structures. Mol Pharm 7:884–893
Johnstone RM (2006) Exosomes biological significance: A concise review. Blood Cells Mol Dis 36:315–321
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C (1987) Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262:9412–9420
Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H, Xiang X, Deng ZB, Wang B, Zhang L, Roth M, Welti R, Mobley J, Jun Y, Miller D, Zhang HG (2013) Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol Ther 21:1345–1357
Ju Z, Ma J, Wang C, Yu J, Qiao Y, Hei F (2017) Exosomes from iPSCs Delivering siRNA Attenuate Intracellular Adhesion Molecule-1 Expression and Neutrophils Adhesion in Pulmonary Microvascular Endothelial Cells. Inflammation 40:486–496
Jung KO, Youn H, Lee CH, Kang KW, Chung JK (2017) Visualization of exosome-mediated miR-210 transfer from hypoxic tumor cells. Oncotarget 8:9899–9910
Kalani A, Kamat PK, Chaturvedi P, Tyagi SC, Tyagi N (2014) Curcumin-primed exosomes mitigate endothelial cell dysfunction during hyperhomocysteinemia. Life Sci 107:1–7
Kalani A, Chaturvedi P, Kamat PK, Maldonado C, Bauer P, Joshua IG, Tyagi SC, Tyagi N (2016) Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Cell Biol 79:360–369
Kalra H, Drummen GP, Mathivanan S (2016) Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int J Mol Sci 17:170
Khan M et al (2015) Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res 117:52–64
Kim SH, Lechman ER, Bianco N, Menon R, Keravala A, Nash J, Mi Z, Watkins SC, Gambotto A, Robbins PD (2005) Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. Journal of immunology (Baltimore, Md : 1950) 174:6440-6448.
Kim SH, Bianco N, Menon R, Lechman ER, Shufesky WJ, Morelli AE, Robbins PD (2006) Exosomes derived from genetically modified DC expressing FasL are anti-inflammatory and immunosuppressive. Mol Ther 13:289–300
Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, Hingtgen SD, Kabanov AV, Batrakova EV (2016) Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 12:655–664
Kim YJ, Yoo SM, Park HH, Lim HJ, Kim YL, Lee S, Seo KW, Kang KS (2017a) Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem Biophys Res Commun 493:1102–1108
Kim YS, Kim JY, Cho R, Shin DM, Lee SW, Oh YM (2017b) Adipose stem cell-derived nanovesicles inhibit emphysema primarily via an FGF2-dependent pathway. Exp Mol Med 49:e284
Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL, Kabanov AV, Batrakova EV (2018) Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine 14:195–204
Kim SY, Phan TH, Limantoro C, Kalionis B, Chrzanowski W (2019) Isolation and Characterization of Extracellular Vesicles from Mesenchymal Stromal Cells. Methods Mol Biol 2029:15–23
Kinoshita T, Yip KW, Spence T, Liu FF (2017) MicroRNAs in extracellular vesicles: potential cancer biomarkers. J Hum Genet 62:67–74
Kobayashi H, Ebisawa K, Kambe M, Kasai T, Suga H, Nakamura K, Narita Y, Ogata A, Kamei Y (2018) Editors' Choice Effects of exosomes derived from the induced pluripotent stem cells on skin wound healing. Nagoya J Med Sci 80:141–153
Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP (2018) Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Res Int 2018:8545347
Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM (2012) Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine 7:1525–1541
Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire C, Chen JW, Tannous BA, Breakefield XO (2014) Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 8:483–494
Lamichhane TN, Raiker RS, Jay SM (2015) Exogenous DNA Loading into Extracellular Vesicles via Electroporation is Size-Dependent and Enables Limited Gene Delivery. Mol Pharm 12:3650–3657
Lamichhane TN, Jeyaram A, Patel DB, Parajuli B, Livingston NK, Arumugasaamy N, Schardt JS, Jay SM (2016) Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell Mol Bioeng 9:315–324
Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux JF, Kobayashi T, Salles JP, Perret B, Bonnerot C, Record M (2004) Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem J 380:161–171
Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S (2012) Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 126:2601–2611
Lee J, Lee H, Goh U, Kim J, Jeong M, Lee J, Park JH (2016) Cellular Engineering with Membrane Fusogenic Liposomes to Produce Functionalized Extracellular Vesicles. ACS Appl Mater Interfaces 8:6790–6795
Leiferman A, Shu J, Upadhyaya B, Cui J, Zempleni J (2019) Storage of Extracellular Vesicles in Human Milk, and MicroRNA Profiles in Human Milk Exosomes and Infant Formulas. J Pediatr Gastroenterol Nutr
Lener T et al (2015) Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles 4:30087
Letsiou E, Bauer N (2018) Endothelial Extracellular Vesicles in Pulmonary Function and Disease. Curr Top Membr 82:197–256
Li Q, Huang Q, Huyan T, Wang Y, Huang Q, Shi J (2018a) Bifacial effects of engineering tumour cell-derived exosomes on human natural killer cells. Exp Cell Res 363:141–150
Li W, Liu Y, Zhang P, Tang Y, Zhou M, Jiang W, Zhang X, Wu G, Zhou Y (2018b) Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl Mater Interfaces 10:5240–5254
Liang LG, Kong MQ, Zhou S, Sheng YF, Wang P, Yu T, Inci F, Kuo WP, Li LJ, Demirci U, Wang S (2017) An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Sci Rep 7:46224
Liao K, Niu F, Dagur RS, He M, Tian C, Hu G (2019) Intranasal Delivery of lincRNA-Cox2 siRNA Loaded Extracellular Vesicles Decreases Lipopolysaccharide-Induced Microglial Proliferation in Mice. J NeuroImmune Pharmacol
Lin J, Li J, Huang B, Liu J, Chen X, Chen XM, Xu YM, Huang LF, Wang XZ (2015) Exosomes: novel biomarkers for clinical diagnosis. ScientificWorldJournal 2015:657086
Liu Y, Li D, Liu Z, Zhou Y, Chu D, Li X, Jiang X, Hou D, Chen X, Chen Y, Yang Z, Jin L, Jiang W, Tian C, Zhou G, Zen K, Zhang J, Zhang Y, Li J, Zhang CY (2015) Targeted exosome-mediated delivery of opioid receptor Mu siRNA for the treatment of morphine relapse. Sci Rep 5:17543
Liu C, Guo J, Tian F, Yang N, Yan F, Ding Y, Wei J, Hu G, Nie G, Sun J (2017) Field-Free Isolation of Exosomes from Extracellular Vesicles by Microfluidic Viscoelastic Flows. ACS Nano 11:6968–6976
Llorente A, Skotland T, Sylvanne T, Kauhanen D, Rog T, Orlowski A, Vattulainen I, Ekroos K, Sandvig K (2013) Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta 1831:1302–1309
Long Q, Upadhya D, Hattiangady B, Kim DK, An SY, Shuai B, Prockop DJ, Shetty AK (2017) Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci U S A 114:E3536–E3545
Lu M, Zhao X, Xing H, Xun Z, Zhu S, Lang L, Yang T, Cai C, Wang D, Ding P (2018) Comparison of exosome-mimicking liposomes with conventional liposomes for intracellular delivery of siRNA. Int J Pharm 550:100–113
Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D (2017) Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin 38:754–763
Lunavat TR, Jang SC, Nilsson L, Park HT, Repiska G, Lasser C, Nilsson JA, Gho YS, Lotvall J (2016) RNAi delivery by exosome-mimetic nanovesicles - Implications for targeting c-Myc in cancer. Biomaterials 102:231–238
Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, Lin HM, Shang CZ, Chen YJ, Min J (2012) Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem 287:15874–15885
Martinez-Lostao L, Garcia-Alvarez F, Basanez G, Alegre-Aguaron E, Desportes P, Larrad L, Naval J, Martinez-Lorenzo MJ, Anel A (2010) Liposome-bound APO2L/TRAIL is an effective treatment in a rabbit model of rheumatoid arthritis. Arthritis Rheum 62:2272–2282
Mathivanan S, Ji H, Simpson RJ (2010) Exosomes: extracellular organelles important in intercellular communication. J Proteome 73:1907–1920
Mathivanan S, Fahner CJ, Reid GE, Simpson RJ (2012) ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res 40:D1241–D1244
Menck K, Bleckmann A, Schulz M, Ries L, Binder C (2017) Isolation and Characterization of Microvesicles from Peripheral Blood. J Vis Exp.
Mentkowski KI, Snitzer JD, Rusnak S, Lang JK (2018) Therapeutic Potential of Engineered Extracellular Vesicles. AAPS J 20:50
Miranda AM, Lasiecka ZM, Xu Y, Neufeld J, Shahriar S, Simoes S, Chan RB, Oliveira TG, Small SA, Di Paolo G (2018) Neuronal lysosomal dysfunction releases exosomes harboring APP C-terminal fragments and unique lipid signatures. Nat Commun 9:291
Mittelbrunn M, Sanchez-Madrid F (2012) Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol 13:328–335
Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD Jr, Thomson AW (2004) Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 104:3257–3266
Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK (2005) A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 3:9
Munagala R, Aqil F, Jeyabalan J, Agrawal AK, Mudd AM, Kyakulaga AH, Singh IP, Vadhanam MV, Gupta RC (2017) Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett 393:94–102
Murphy C, Withrow J, Hunter M, Liu Y, Tang YL, Fulzele S, Hamrick MW (2018) Emerging role of extracellular vesicles in musculoskeletal diseases. Mol Asp Med 60:123–128
Mushahary D, Spittler A, Kasper C, Weber V, Charwat V (2018) Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A 93:19–31
Namazi H, Mohit E, Namazi I, Rajabi S, Samadian A, Hajizadeh-Saffar E, Aghdami N, Baharvand H (2018) Exosomes secreted by hypoxic cardiosphere-derived cells enhance tube formation and increase pro-angiogenic miRNA. J Cell Biochem 119:4150–4160
Nguyen DB, Ly TB, Wesseling MC, Hittinger M, Torge A, Devitt A, Perrie Y, Bernhardt I (2016) Characterization of Microvesicles Released from Human Red Blood Cells. Cell Physiol Biochem 38:1085–1099
Nozaki T, Sugiyama S, Sugamura K, Ohba K, Matsuzawa Y, Konishi M, Matsubara J, Akiyama E, Sumida H, Matsui K, Jinnouchi H, Ogawa H (2010) Prognostic value of endothelial microparticles in patients with heart failure. Eur J Heart Fail 12:1223–1228
Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M (2013) Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 21:185–191
Ohno S, Drummen GP, Kuroda M (2016) Focus on Extracellular Vesicles: Development of Extracellular Vesicle-Based Therapeutic Systems. Int J Mol Sci 17:172
Oude Blenke E, Klaasse G, Merten H, Pluckthun A, Mastrobattista E, Martin NI (2015) Liposome functionalization with copper-free "click chemistry". J Control Release 202:14–20
Pankoui Mfonkeu JB, Gouado I, Fotso Kuate H, Zambou O, Amvam Zollo PH, Grau GE, Combes V (2010) Elevated cell-specific microparticles are a biological marker for cerebral dysfunctions in human severe malaria. PLoS One 5:e13415
Parker B, Al-Husain A, Pemberton P, Yates AP, Ho P, Gorodkin R, Teh LS, Alexander MY, Bruce IN (2014) Suppression of inflammation reduces endothelial microparticles in active systemic lupus erythematosus. Ann Rheum Dis 73:1144–1150
Pascucci L, Cocce V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, Vigano L, Locatelli A, Sisto F, Doglia SM, Parati E, Bernardo ME, Muraca M, Alessandri G, Bondiolotti G, Pessina A (2014) Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release 192:262–270
Pegtel DM, Gould SJ (2019) Exosomes. Annu Rev Biochem 88:487–514
Peinado H et al (2012) Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 18:883–891
Perets N, Hertz S, London M, Offen D (2018) Intranasal administration of exosomes derived from mesenchymal stem cells ameliorates autistic-like behaviors of BTBR mice. Mol Autism 9:57
Pleet ML, Branscome H, DeMarino C, Pinto DO, Zadeh MA, Rodriguez M, Sariyer IK, El-Hage N, Kashanchi F (2018) Autophagy, EVs, and Infections: A Perfect Question for a Perfect Time. Front Cell Infect Microbiol 8:362
Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D (2019) Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease. J Neuro-Oncol
Rajendran RL, Gangadaran P, Bak SS, Oh JM, Kalimuthu S, Lee HW, Baek SH, Zhu L, Sung YK, Jeong SY, Lee SW, Lee J, Ahn BC (2017) Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci Rep 7:15560
Ran R, Middelberg APJ, Zhao CX (2016) Microfluidic synthesis of multifunctional liposomes for tumour targeting. Colloids Surf B: Biointerfaces 148:402–410
Rani S, Ryan AE, Griffin MD, Ritter T (2015) Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther 23:812–823
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383
Ratajczak MZ, Ratajczak J (2016) Horizontal transfer of RNA and proteins between cells by extracellular microvesicles: 14 years later. Clin Transl Med 5:7
Reiner AT et al (2017) Concise Review: Developing Best-Practice Models for the Therapeutic Use of Extracellular Vesicles. Stem Cells Transl Med 6:1730–1739
Richter M, Fuhrmann K, Fuhrmann G (2019) Evaluation of the Storage Stability of Extracellular Vesicles. J Vis Exp.
Rosenblum D, Joshi N, Tao W, Karp JM, Peer D (2018) Progress and challenges towards targeted delivery of cancer therapeutics. Nat Commun 9:1410
Rossi IV, Gavinho B, Ramirez MI (2019) Isolation and Characterization of Extracellular Vesicles Derived from Trypanosoma cruzi. Methods Mol Biol 1955:89–104
Samanta S, Rajasingh S, Drosos N, Zhou Z, Dawn B, Rajasingh J (2018) Exosomes: new molecular targets of diseases. Acta Pharmacol Sin 39:501–513
Sato YT, Umezaki K, Sawada S, Mukai SA, Sasaki Y, Harada N, Shiku H, Akiyoshi K (2016) Engineering hybrid exosomes by membrane fusion with liposomes. Sci Rep 6:21933
Schorey JS, Bhatnagar S (2008) Exosome function: from tumor immunology to pathogen biology. Traffic 9:871–881
Segura E, Amigorena S, Thery C (2005) Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol Dis 35:89–93
Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV (2013) Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal 11:88
Sierro F, Grau GER (2019) The Ins and Outs of Cerebral Malaria Pathogenesis: Immunopathology, Extracellular Vesicles, Immunometabolism, and Trained Immunity. Front Immunol 10:830
Silachev DN, Goryunov KV, Shpilyuk MA, Beznoschenko OS, Morozova NY, Kraevaya EE, Popkov VA, Pevzner IB, Zorova LD, Evtushenko EA, Starodubtseva NL, Kononikhin AS, Bugrova AE, Evtushenko EG, Plotnikov EY, Zorov DB, Sukhikh GT (2019) Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation. Cells 8.
Simpson RJ, Lim JW, Moritz RL, Mathivanan S (2009) Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics 6:267–283
Skalnikova HK, Bohuslavova B, Turnovcova K, Juhasova J, Juhas S, Rodinova M, Vodicka P (2019) Isolation and Characterization of Small Extracellular Vesicles from Porcine Blood Plasma, Cerebrospinal Fluid, and Seminal Plasma. Proteomes 7
Smyth T, Petrova K, Payton NM, Persaud I, Redzic JS, Graner MW, Smith-Jones P, Anchordoquy TJ (2014) Surface functionalization of exosomes using click chemistry. Bioconjug Chem 25:1777–1784
Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ (2015) Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release 199:145–155
Srivastava A, Babu A, Filant J, Moxley KM, Ruskin R, Dhanasekaran D, Sood AK, McMeekin S, Ramesh R (2016a) Exploitation of Exosomes as Nanocarriers for Gene-, Chemo-, and Immune-Therapy of Cancer. J Biomed Nanotechnol 12:1159–1173
Srivastava A, Amreddy N, Babu A, Panneerselvam J, Mehta M, Muralidharan R, Chen A, Zhao YD, Razaq M, Riedinger N, Kim H, Liu S, Wu S, Abdel-Mageed AB, Munshi A, Ramesh R (2016b) Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells. Sci Rep 6:38541
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG (2010) A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 18:1606–1614
Sun X, Shan A, Wei Z, Xu B (2018) Intravenous mesenchymal stem cell-derived exosomes ameliorate myocardial inflammation in the dilated cardiomyopathy. Biochem Biophys Res Commun 503:2611–2618
Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, Takakura Y (2013) Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol 165:77–84
Tao SC, Rui BY, Wang QY, Zhou D, Zhang Y, Guo SC (2018) Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv 25:241–255
Thery C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2:569–579
Thery C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9:581–593
Thery C et al (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7:1535750
Tian T, Zhu YL, Hu FH, Wang YY, Huang NP, Xiao ZD (2013) Dynamics of exosome internalization and trafficking. J Cell Physiol 228:1487–1495
Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G (2014) A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35:2383–2390
Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F (2017) Extracellular Vesicles in Angiogenesis. Circ Res 120:1658–1673
Trams EG, Lauter CJ, Salem N Jr, Heine U (1981) Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 645:63–70
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659
Viaud S, Ploix S, Lapierre V, Thery C, Commere PH, Tramalloni D, Gorrichon K, Virault-Rocroy P, Tursz T, Lantz O, Zitvogel L, Chaput N (2011) Updated technology to produce highly immunogenic dendritic cell-derived exosomes of clinical grade: a critical role of interferon-gamma. J Immunother 34:65–75
Vidal M, Sainte-Marie J, Philippot JR, Bienvenue A (1989) Asymmetric distribution of phospholipids in the membrane of vesicles released during in vitro maturation of guinea pig reticulocytes: evidence precluding a role for "aminophospholipid translocase". J Cell Physiol 140:455–462
Vlassov AV, Magdaleno S, Setterquist R, Conrad R (2012) Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 1820:940–948
Wahlgren J, De LKT, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, Valadi H (2012) Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res 40:e130
Walker CM, Steimer KS, Rosenthal KL, Levy JA (1988) Identification of human immunodeficiency virus (HIV) envelope type-specific T helper cells in an HIV-infected individual. J Clin Invest 82:2172–2175
Wang Z, Wu HJ, Fine D, Schmulen J, Hu Y, Godin B, Zhang JX, Liu X (2013) Ciliated micropillars for the microfluidic-based isolation of nanoscale lipid vesicles. Lab Chip 13:2879–2882
Wang M, Altinoglu S, Takeda YS, Xu Q (2015) Integrating Protein Engineering and Bioorthogonal Click Conjugation for Extracellular Vesicle Modulation and Intracellular Delivery. PLoS One 10:e0141860
Wang J, Sun X, Zhao J, Yang Y, Cai X, Xu J, Cao P (2017a) Exosomes: A Novel Strategy for Treatment and Prevention of Diseases. Front Pharmacol 8:300
Wang Y, Chen X, Tian B, Liu J, Yang L, Zeng L, Chen T, Hong A, Wang X (2017b) Nucleolin-targeted Extracellular Vesicles as a Versatile Platform for Biologics Delivery to Breast Cancer. Theranostics 7:1360–1372
Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, Zhou J, Heng BC, Zou XH, Ouyang H, Liu H (2017c) Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther 8:189
Wang L, Pei S, Han L, Guo B, Li Y, Duan R, Yao Y, Xue B, Chen X, Jia Y (2018a) Mesenchymal Stem Cell-Derived Exosomes Reduce A1 Astrocytes via Downregulation of Phosphorylated NFkappaB P65 Subunit in Spinal Cord Injury. Cell Physiol Biochem 50:1535–1559
Wang XL, Zhao YY, Sun L, Shi Y, Li ZQ, Zhao XD, Xu CG, Ji HG, Wang M, Xu WR, Zhu W (2018b) Exosomes derived from human umbilical cord mesenchymal stem cells improve myocardial repair via upregulation of Smad7. Int J Mol Med 41:3063–3072
Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G, Bear J, Monninger M, Sun M, Morales-Kastresana A, Jones JC, Felber BK, Chen X, Gursel I, Pavlakis GN (2016) Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials 105:195–205
Watson LS, Hamlett ED, Stone TD, Sims-Robinson C (2019) Neuronally derived extracellular vesicles: an emerging tool for understanding Alzheimer's disease. Mol Neurodegener 14:22
Weaver JC (1993) Electroporation: a general phenomenon for manipulating cells and tissues. J Cell Biochem 51:426–435
Weber A, Wehmeyer JC, Schmidt V, Lichtenberg A, Akhyari P (2019) Rapid Fluorescence-based Characterization of Single Extracellular Vesicles in Human Blood with Nanoparticle-tracking Analysis. J Vis Exp.
Wiklander OP, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mager I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CI, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE (2015) Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 4:26316
Withrow J, Murphy C, Liu Y, Hunter M, Fulzele S, Hamrick MW (2016) Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther 18:286
Wu M, Ouyang Y, Wang Z, Zhang R, Huang PH, Chen C, Li H, Li P, Quinn D, Dao M, Suresh S, Sadovsky Y, Huang TJ (2017) Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci U S A 114:10584–10589
Wu JY, Ji AL, Wang ZX, Qiang GH, Qu Z, Wu JH, Jiang CP (2018) Exosome-Mimetic Nanovesicles from Hepatocytes promote hepatocyte proliferation in vitro and liver regeneration in vivo. Sci Rep 8:2471
Wu X, Liu Y, Wei W, Liu ML (2019) Extracellular vesicles in autoimmune vasculitis - Little dirts light the fire in blood vessels. Autoimmun Rev 18:593–606
Xiao W, Dong W, Zhang C, Saren G, Geng P, Zhao H, Li Q, Zhu J, Li G, Zhang S, Ye M (2013) Effects of the epigenetic drug MS-275 on the release and function of exosome-related immune molecules in hepatocellular carcinoma cells. Eur J Med Res 18:61
Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ (2016) Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest 126:1152–1162
Yaddanapudi K, Meng S, Whitt AG, Al Rayyan N, Richie J, Tu A, Eaton JW, Li C (2019) Exosomes from GM-CSF expressing embryonic stem cells are an effective prophylactic vaccine for cancer prevention. Oncoimmunology 8:1561119
Yanez-Mo M et al (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4:27066
Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S (2015) Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res 32:2003–2014
Yang L, Niu F, Yao H, Liao K, Chen X, Kook Y, Ma R, Hu G, Buch S (2018a) Exosomal miR-9 Released from HIV Tat Stimulated Astrocytes Mediates Microglial Migration. J NeuroImmune Pharmacol 13:330–344
Yang Y, Boza-Serrano A, Dunning CJR, Clausen BH, Lambertsen KL, Deierborg T (2018b) Inflammation leads to distinct populations of extracellular vesicles from microglia. J Neuroinflammation 15:168
Yoon J, Jo W, Jeong D, Kim J, Jeong H, Park J (2015) Generation of nanovesicles with sliced cellular membrane fragments for exogenous material delivery. Biomaterials 59:12–20
Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y, Ashraf M, Xu M (2015) Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol 182:349–360
Yuan Z, Kolluri KK, Gowers KH, Janes SM (2017) TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J Extracell Vesicles 6:1265291
Yuana Y, Boing AN, Grootemaat AE, van der Pol E, Hau CM, Cizmar P, Buhr E, Sturk A, Nieuwland R (2015) Handling and storage of human body fluids for analysis of extracellular vesicles. J Extracell Vesicles 4:29260
Yuyama K, Sun H, Sakai S, Mitsutake S, Okada M, Tahara H, Furukawa J, Fujitani N, Shinohara Y, Igarashi Y (2014) Decreased amyloid-beta pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J Biol Chem 289:24488–24498
Zaborowski MP, Balaj L, Breakefield XO, Lai CP (2015) Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 65:783–797
Zara M, Guidetti GF, Camera M, Canobbio I, Amadio P, Torti M, Tremoli E, Barbieri SS (2019) Biology and Role of Extracellular Vesicles (EVs) in the Pathogenesis of Thrombosis. Int J Mol Sci 20
Zhang JS, Liu F, Huang L (2005) Implications of pharmacokinetic behavior of lipoplex for its inflammatory toxicity. Adv Drug Deliv Rev 57:689–698
Zhang M, Xiao B, Wang H, Han MK, Zhang Z, Viennois E, Xu C, Merlin D (2016a) Edible Ginger-derived Nano-lipids Loaded with Doxorubicin as a Novel Drug-delivery Approach for Colon Cancer Therapy. Mol Ther 24:1783–1796
Zhang M, Viennois E, Prasad M, Zhang Y, Wang L, Zhang Z, Han MK, Xiao B, Xu C, Srinivasan S, Merlin D (2016b) Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 101:321–340
Zhang W, Zhou X, Zhang H, Yao Q, Liu Y, Dong Z (2016c) Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Ren Physiol 311:F844–F851
Zhang M, Xu C, Liu D, Han MK, Wang L, Merlin D (2018a) Oral Delivery of Nanoparticles Loaded With Ginger Active Compound, 6-Shogaol, Attenuates Ulcerative Colitis and Promotes Wound Healing in a Murine Model of Ulcerative Colitis. J Crohns Colitis 12:217–229
Zhang W, Zhang J, Cheng L, Ni H, You B, Shan Y, Bao L, Wu D, Zhang T, Yue H, Chen J (2018b) A disintegrin and metalloprotease 10-containing exosomes derived from nasal polyps promote angiogenesis and vascular permeability. Mol Med Rep 17:5921–5927
Zhou H, Yuen PS, Pisitkun T, Gonzales PA, Yasuda H, Dear JW, Gross P, Knepper MA, Star RA (2006) Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int 69:1471–1476
Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, Qian H (2013) Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther 4:34
Zhu T, Wang Y, Jin H, Li L (2019) The role of exosome in autoimmune connective tissue disease. Ann Med 51:101–108
Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, Miller D, Zhang HG (2011) Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther 19:1769–1779
Zhuang X, Deng ZB, Mu J, Zhang L, Yan J, Miller D, Feng W, McClain CJ, Zhang HG (2015) Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles 4:28713
Acknowledgments
This work was supported by grants DA041751, DA043164, DA040397 (to S.B.), MH112848, DA043138 (to S.B. and G.H.), DA042704, DA046831 (to G.H.), and DA044087 (to P.P.) from the NIH. The support of the Nebraska Center for Substance Abuse Research is acknowledged. The project described was also supported by the NIH, National Institute of Mental Health (grant 2P30MH062261). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sil, S., Dagur, R.S., Liao, K. et al. Strategies for the use of Extracellular Vesicles for the Delivery of Therapeutics. J Neuroimmune Pharmacol 15, 422–442 (2020). https://doi.org/10.1007/s11481-019-09873-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-019-09873-y