Abstract
In human immunodeficiency virus-1 (HIV) infected individuals, substance abuse may accelerate the development and/or increase the severity of HIV associated neurocognitive disorders (HAND). It is proposed that CD14+CD16+ monocytes mediate HIV entry into the central nervous system (CNS) and that uninfected and infected CD14+CD16+ monocyte transmigration across the blood brain barrier (BBB) contributes to the establishment and propagation of CNS HIV viral reservoirs and chronic neuroinflammation, important factors in the development of HAND. The effects of substance abuse on the frequency of CD14+CD16+ monocytes in the peripheral circulation and on the entry of these cells into the CNS during HIV neuropathogenesis are not known. PBMC from HIV infected individuals were analyzed by flow cytometry and we demonstrate that the frequency of peripheral blood CD14+CD16+ monocytes in HIV infected substance abusers is increased when compared to those without active substance use. Since drug use elevates extracellular dopamine concentrations in the CNS, we examined the effects of dopamine on CD14+CD16+ monocyte transmigration across our in vitro model of the human BBB. The transmigration of this monocyte subpopulation is increased by dopamine and the dopamine receptor agonist, SKF 38393, implicating D1-like dopamine receptors in the increase in transmigration elicited by this neurotransmitter. Thus, elevated extracellular CNS dopamine may be a novel common mechanism by which active substance use increases uninfected and HIV infected CD14+CD16+ monocyte transmigration across the BBB. The influx of these cells into the CNS may increase viral seeding and neuroinflammation, contributing to the development of HIV associated neurocognitive impairments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies suggest that in some human immunodeficiency virus-1 (HIV) infected individuals, the development of HIV associated neurocognitive disorders (HAND) is accelerated and/or its severity increased with substance abuse, in the absence or presence of prescribed combination antiretroviral therapy (cART) (Rippeth et al. 2004; Hauser et al. 2007; Buch et al. 2012; Meyer et al. 2013; Weber et al. 2013; Meade et al. 2015). The mechanisms by which substance abuse contributes to HIV neuropathogenesis are not completely understood. HIV entry into the central nervous system (CNS) occurs early after peripheral infection (Davis et al. 1992; Valcour et al. 2012) and is thought to be mediated by infected monocyte transmigration across the blood brain barrier (BBB) (Burdo et al. 2013; Saylor et al. 2016). Once within the CNS parenchyma, HIV infected monocytes differentiate into macrophages, establishing a significant long-lived viral reservoir (Crowe et al. 2003; Kramer-Hammerle et al. 2005). This leads to infection and/or activation of CNS resident cells and chronic neuroinflammation, characterized by the production of virus and viral proteins, cytokines, including TNF-α, and IL-1β, and chemokines, including CCL2 (MCP-1) and CXCL12 (SDF-1) (Gonzalez-Scarano and Martin-Garcia 2005; Peng et al. 2006; Wang et al. 2006; Hong and Banks 2015). Chemokine production increases the transmigration of additional uninfected and infected monocytes across the BBB (Gras and Kaul 2010; Spudich and Gonzalez-Scarano 2012). T cells have also been detected within the CNS during HIV/simian immunodeficiency virus (SIV) infection (Marcondes et al. 2001; Sadagopal et al. 2008; Schrier et al. 2015), especially in HIV infected substance abusers before the use of cART (Bell et al. 1993; Tomlinson et al. 1999; Anthony et al. 2003). Whether they play a large role in the cART era is not clear. Although neurons cannot be infected by HIV, chronic viral replication and production of inflammatory and toxic host and viral factors in the CNS can result in neuronal dendritic pruning and cell death (Gonzalez-Scarano and Martin-Garcia 2005). Even complete viral suppression in the periphery with cART does not eliminate the production of HIV Tat and inflammatory mediators in the CNS (Li et al. 2009). Thus, low level chronic neuroinflammation and neuronal damage induced by HIV infection of the CNS, that occurs despite cART, leads to the development of cognitive, behavioral and motor deficits in 40 to 70% of infected individuals (Heaton et al. 2010; Chan et al. 2016).
Peripheral blood monocytes are classified by their surface expression of the LPS receptor, CD14, and the FcγRIII receptor, CD16. Approximately 90% of monocytes in healthy individuals are CD14 positive and CD16 negative (CD14+CD16− classical monocytes). The remaining monocytes are CD14 and CD16 positive and represent a mature monocyte subpopulation with phenotypic and functional characteristics more similar to macrophages than to circulating monocytes (Ziegler-Heitbrock et al. 1993). The CD16+ monocyte population is expanded in the periphery with HIV and SIV infection (Nockher et al. 1994; Thieblemont et al. 1995; Pulliam et al. 1997; Otani et al. 1998). HIV/SIV RNA or DNA sequences are detected in peripheral blood CD16+ monocytes from infected individuals and macaques, in the absence or presence of cART (Shiramizu et al. 2005; Williams et al. 2005; Ellery et al. 2007; Jaworowski et al. 2007) and the levels of HIV DNA in circulating CD16+ monocytes is associated with HAND (Valcour et al. 2010; Kusao et al. 2012). During HIV/SIV infection of the CNS, perivascular accumulation of uninfected and infected CD16+ monocytes/macrophages correlates with neuropathology (Fischer-Smith et al. 2001; Williams et al. 2001; Fischer-Smith et al. 2004; Clay et al. 2007). These studies suggest that peripheral blood CD16+ monocytes are infected by HIV and transport virus into the CNS. Upon their differentiation into macrophages, these HIV infected cells would constitute a viral reservoir within the CNS and contribute to low level viral protein production and viral replication in the brain. In addition, CNS macrophages derived from both infected and uninfected CD16+ monocytes would contribute to chronic neuroinflammation. Thus, the influx of both uninfected and infected CD16+ monocytes into the CNS during the neuropathogenesis of HIV is thought to be a major contributor to the development of HIV associated cognitive impairments (Valcour et al. 2010; Williams et al. 2012; Williams et al. 2014b). Recently, CD16+ monocytes were subdivided into two groups with one group having higher levels of CD14 (CD14+CD16+ intermediate monocytes) as compared to the other group (CD14lowCD16+ non-classical monocytes) (Ziegler-Heitbrock et al. 2010). The specific contributions of each of these CD16+ monocyte subpopulations to the neuropathogenesis of HIV have not been fully characterized.
Use of intravenous and other substances of abuse result in risky behaviors that are major contributors to infection with HIV (El-Bassel et al. 2014; Spiller et al. 2015). Substance abuse is also associated with poor adherence to prescribed cART (Arnsten et al. 2002; Hinkin et al. 2007) and with increased HIV neuropathogenesis (Rippeth et al. 2004; Hauser et al. 2007; Buch et al. 2012; Meyer et al. 2013; Weber et al. 2013; Meade et al. 2015). Drugs of abuse, including cocaine and methamphetamine, increase extracellular concentrations of dopamine in the CNS (Volkow et al. 2009). In this study, we demonstrate that the frequency of peripheral blood CD14+CD16+ and CD14lowCD16+ monocytes, relative to the total monocyte population, is increased in HIV infected substance abusers when compared to those without active substance use. We previously showed that dopamine increases CD14+CD16+ monocyte movement and adhesion (Coley et al. 2015), suggesting that upon transmigration across the BBB, these cells may accumulate within dopaminergic regions of the CNS of HIV infected substance abusers. However, it is not known whether elevated levels of extracellular CNS dopamine increase the entry of peripheral blood CD16+ monocytes or other leukocytes into the brain. To address this, we performed studies using our in vitro model of the human BBB. Our results indicate that dopamine increases CD14+CD16+ monocyte, but not CD14lowCD16+ monocyte or T cell, transmigration once these monocytes have penetrated the BBB in response to low level baseline or constitutive chemokine expression by a mechanism involving D1-like dopamine receptor activation. In addition, dopamine increases the formation of membrane protrusions, indicative of cellular motility, and active a disintegrin and metalloproteinase 17 (ADAM17) expression in CD14+CD16+ monocytes, which may contribute to the dopamine-induced transmigration of these cells across the BBB. Thus, active substance use may amplify CNS viral reservoirs and neuroinflammation in HIV infected individuals by a mechanism involving increased extracellular CNS dopamine, contributing to accelerated and/or augmented HIV neuropathogenesis due to increased uninfected and infected CD14+CD16+ monocyte influx into the CNS.
Materials and Methods
Study Subjects
Blood was obtained from HIV infected individuals from two independent cohorts: the Manhattan HIV Brain Bank (MHBB; U01MH083501, U24MH100931), a member of the National NeuroAIDS Tissue Consortium and a research resource at the Icahn School of Medicine at Mount Sinai, New York, NY (Morgello et al. 2004), and the Women’s Interagency HIV Study (WIHS; U01A1035004, U01A142590), Bronx, NY, a multicenter, prospective cohort study of HIV infected and at-risk HIV uninfected women in the United States (Barkan et al. 1998). Individuals gave written, informed consent for the provision of blood for the purposes of HIV research. Protocols for the acquisition of these samples were approved by the Mount Sinai Program for the Protection of Human Subjects Institutional Review Board and the Institutional Review Board at the Montefiore Medical Center, Albert Einstein College of Medicine. All 48 of the participants from the MHBB cohort were prescribed cART. In the WIHS cohort, most of the women were prescribed cART (n = 6) but some were cART naïve (n = 3). Urine toxicology was used to identify active drug use at each blood draw, testing specifically for amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, opiates, phencyclidine (PCP), methadone and propoxyphene (illicit and prescribed). In many individuals, there was concurrent use of more than one drug. Alcohol use and smoking status were not determined. Table 1 describes demographic and virologic characteristics of individuals from the two cohorts, including CD4 T cell counts, plasma viral loads and cART therapy at the time of blood draw.
Leukocyte Isolation
HIV seronegative blood was obtained from de-identified donors and New York Blood Center leukopaks according to established protocols at the Albert Einstein College of Medicine, Montefiore Medical Center. Blood from HIV seropositive individuals was obtained from the MHBB and WIHS cohorts described above. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Paque PLUS (GE Healthcare, Uppsala, Sweden) density gradient centrifugation of blood samples. Monocytes and T cells were purified from PBMCs obtained from leukopaks of HIV seronegative New York Blood Center donors using antibody and magnetic bead selection with EasySep Human CD14 or CD3 Positive Selection Kits (Stem Cell Technologies, Vancouver, Canada) according to the manufacturer’s instructions. Freshly isolated, purified CD14+ monocytes were then cultured non-adherently in Teflon flasks at 2 × 106 cells/ml in RPMI 1640 + 10% FBS media with 10 ng/ml M-CSF (Peprotech, Rocky Hill, NJ) as previously described (Buckner et al. 2011; Williams et al. 2013; Coley et al. 2015). After 3 days, cultures were highly enriched for CD14+CD16+ monocytes and are termed “mature monocytes” in this study.
Flow Cytometry
The purity of CD14+ monocytes and CD3+ T cells isolated from PBMCs after antibody and magnetic bead selection was determined by flow cytometry using antibodies for CD14 (clone M5E2), CD3 (clone HIT3a), CD19 (clone HIB19), CD56 (clone B159) and appropriate isotype matched negative control antibodies (BD Biosciences, San Jose, CA). Antibodies were titered to determine their optimal concentrations. Antibodies were added to cells (1-3 × 105 in 100 μl) and after 30 min on ice, cells were washed and fixed with 2% paraformaldehyde. For analyses of immunopositive cells, 10,000–40,000 events were acquired using a BD Canto II flow cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar v.9.5.3, Ashland, OR). Positive reactivity with antibodies for CD14, CD3, CD19 and CD56 was indicated by fluorescence intensity that was above that obtained with the appropriate isotype-matched negative control antibodies. Monocyte and T cell purity was >90% and both cell types contained <2% CD19+ B cells and <1% CD56+ NK cells, with monocytes containing <3% CD3+ T cells and T cells containing <3% CD14+ monocytes (Williams et al. 2013).
PBMC and mature monocyte cultures were analyzed by flow cytometry using fluorochrome coupled monoclonal antibodies to CD14, CD16 (clone 3G8) and CD3 (BD Biosciences) to quantify CD14+CD16+ and CD14lowCD16+ monocytes, and CD3+ T cells. For the analysis of CD16+ monocytes in PBMC, the monocyte gate was first determined based on forward scatter and side scatter parameters, and the cells within this gate that were CD16+ with high or low levels of CD14 were then quantified as described previously (Williams et al. 2015).
For dopamine receptor staining, mature monocytes and purified, unactivated T cells (1 × 106 per tube) were stained with antibodies to dopamine D1 receptor (D1R) (324390), dopamine D3 receptor (D3R) (324402), dopamine D5 receptor (D5R) (324408) (1:5 dilution) (Calbiochem/EMD Millipore, Billerica, MA), dopamine D2 receptor (D2R) (B-10 and H-50) (1:10 dilution), dopamine D4 receptor (D4R) (N-20) (1:10 dilution) (Santa Cruz Biotechnology, Santa Cruz, CA) or negative control rabbit serum, rabbit IgG, mouse IgG2a (Sigma-Aldrich, St. Louis, MO) or goat IgG (Santa Cruz Biotechnology) in 50 ul on ice for 30 min. These dopamine receptor antibodies are specific for extracellular portions of each receptor. After two washes, cells were incubated with secondary PE-conjugated anti-rabbit, mouse or goat IgG (1:5 or 1:10 dilution) (Sigma-Aldrich) on ice for 30 min, then washed and fixed in 2% paraformaldehyde. Staining with negative control serum or IgG, rather than the use of unstained cells or cells stained only with secondary antibody, enables the quantification of staining that is specific for each dopamine receptor and is greater than the non-specific staining obtained with negative control antibodies or serum. Reactivity with the appropriate negative control antibody or serum, as determined by flow cytometry, was subtracted from dopamine receptor staining to determine the mean fluorescence intensity (MFI) for each receptor.
Human BBB Model
Human brain microvascular endothelial cells (BMVEC) (Applied Cell Biology Research Institute, Kirkland WA) and human astrocytes isolated from cortical tissue as part of an ongoing approved research protocol at the Albert Einstein College of Medicine, Montefiore Medical Center were cocultured on opposite sides of a gelatin coated tissue culture insert with 3 μm pores (BD Falcon, Franklin Lakes, NJ) as previously described (Eugenin et al. 2006; Buckner et al. 2011; Williams et al. 2013; Williams et al. 2014a; Williams et al. 2015). Briefly, astrocytes were first seeded on the underside of inserts followed by the addition of BMVEC to the upper side. Cocultured BMVEC and astrocytes were incubated for 3 days in 24 well plates at 37 °C, 5% CO2 in M199 media with 5% human serum, 20% newborn calf serum and endothelial cell growth factors to obtain confluent endothelial cell monolayers. After 3 days in culture, astrocytic processes penetrate through the insert pores and contact the BMVEC to induce BBB properties, including impermeability. Cocultures were incubated in low serum media (M199 + 10% newborn calf serum + endothelial cell growth factors) for 24 h before transmigration assays were performed to minimize chemokine production by BMVEC and astrocytes.
BBB Transmigration
Transmigration experiments were performed using PBMC (4 × 105 per coculture) from HIV seronegative individuals or HIV seropositive individuals from the WIHS cohort, and also mature monocytes (2 × 105 per coculture) from HIV seronegative individuals. For each independent donor, cells in low serum media were added to the top of four replicate cocultures per treatment group. Low serum media with dH2O (dopamine and SKF 38393 diluent) or 0.1% BSA in PBS (CXCL12 diluent), was added to the bottom well of cocultures to assay baseline transmigration. Dopamine (Sigma-Aldrich) and/or CXCL12 (R&D Systems, Minneapolis, MN), as well as SKF 38393 alone (Tocris/R&D Systems), were diluted in low serum media and added to the bottom well of cocultures to assay for CD16+ monocyte or T cell transmigration in response to these factors. Transmigrated cells were harvested from the bottom well of cocultures after 24 h and stained for CD14, CD16, and CD3 to quantify by flow cytometry the mean number of transmigrated CD16+ monocytes and T cells from the four replicate cocultures for each treatment group. Evans blue dye coupled albumin was used to assay for the impermeability of BBB cocultures before and after monocyte and T cell transmigration as described previously (Eugenin et al. 2006). To determine the effects of dopamine on chemokine production by cocultured BMVEC and astrocytes, and by transmigrated monocytes, media was collected from the bottom well of cocultures after mature monocytes transmigrated in response to 10 μM dopamine for 24 h. After centrifugation to remove transmigrated monocytes, culture supernatants were then assayed for CCL2 and CXCL12 proteins using human Quantikine ELISA kits (R&D Systems) according to the manufacturer’s instructions.
Immunofluorescence for Membrane Protrusions
Mature monocytes and purified, unactivated T cells from HIV seronegative individuals were treated with dopamine, dH2O (dopamine diluent), CXCL12, or 0.1% BSA in PBS (CXCL12 diluent) for 15, 30 or 45 min. Cells were then fixed with 2% paraformaldehyde in PBS for 1 h, permeabilized with 0.01% Triton X-100, and incubated in blocking solution containing 0.5 M EDTA, 1% fish gelatin, 1% immunoglobulin-free BSA, 1% horse serum, and 1% human serum for 30 min. After blocking, cells were stained with anti-tubulin antibody (1:10.000 dilution, T9026, Sigma-Aldrich) for 1 h at room temperature. The cells were washed and secondary antibody, anti-mouse IgG F(ab’)2-FITC (1:100, F2883, Sigma-Aldrich), mixed with Texas Red®-X phalloidin to stain actin (15 μl/ml, T7471, Life Technologies-Molecular Probes, Eugene, OR) was added, followed by a 2 h incubation at room temperature. After washing, cells were mounted in Prolong Gold antifade reagent with DAPI (P36931, ThermoFisher) and examined by confocal microscopy using a Leica SP2 confocal microscope (Wetzlar, Germany). Multiple fields per treatment group, containing a total of 50 to 100 cells, were examined and the percentage of cells with membrane protrusions in each treatment group was quantified. These data were reported as fold change compared to untreated (dH2O or 0.1% BSA in PBS), which was set to one for each independent donor.
Active ADAM17 Western Blot Analysis
Mature monocytes and purified, unactivated T cells (3 × 106 in 1 ml) from three independent HIV seronegative individuals were treated with media plus dH2O, 10 μM dopamine or 100 ng/ml PMA for times ranging from 5 to 240 min. Protein lysates were prepared with Triton X-100 lysis buffer (Cell Signaling, Beverly, MA) and analyzed by Western blot under reducing conditions using an antibody specific for the activation site of ADAM17 (ab39163, Abcam, Cambridge, MA). Blots were then stripped and probed with an antibody for the housekeeping protein, β-actin (#4967, Cell Signaling).
Statistical Analyses
Statistical analyses were performed using Prism 6.02 software (GraphPad Software, Inc., San Diego, CA). For transmigration and membrane protrusion formation experiments where the results were expressed as fold change compared to baseline, which was set to one, two-tailed non-parametric Wilcoxon signed-rank test comparing medians for each treatment group to a hypothetical value of one was performed to determine statistical significance (p < 0.05). For all other experiments, two-tailed, unpaired, non-parametric Mann-Whitney test was performed to determine statistical significance (p < 0.05).
Results
The Frequency of Peripheral Blood CD16+ Monocytes Is Increased in HIV Infected Active Substance Users
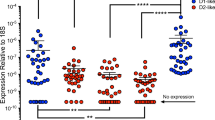
We determined whether the frequency of peripheral blood CD16+ monocytes, relative to the total monocyte population, is increased in HIV infected substance abusers when compared to those without active substance use. Phenotypic analysis of peripheral blood monocytes in PBMC from HIV seronegative individuals and from HIV infected individuals from the MHBB cohort was performed by flow cytometry. The demographic and clinical characteristics of the individuals from this cohort are shown in Table 1. In uninfected individuals (n = 12), 13 ± 4% of peripheral blood monocytes were CD14+CD16+ and CD14lowCD16+ (Fig. 1a). In HIV infected individuals without active substance use (n = 22), as confirmed by urine toxicology, the percentage of CD14+CD16+ and CD14lowCD16+ monocytes increased to 27 ± 13% (p < 0.0001). The percentage of CD14+CD16+ and CD14lowCD16+ monocytes increased to 43 ± 20% in HIV infected individuals who were positive for active substance use by urine toxicology that specifically detected amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, opiates, phencyclidine (PCP), methadone and propoxyphene (n = 26, p < 0.0001). The percentages of individuals in this group that were positive for active use of one drug only were: 39.3% cannabis, 7.1% opiates, 7.1% methadone, 3.6% cocaine, and 3.6% amphetamine. The remaining 39.6% of the active substance users were positive for poly drug use. Thus, the frequency of CD14+CD16+ and CD14lowCD16+ monocytes is increased further in the peripheral circulation of HIV infected individuals who are current substance users as compared to those without active drug use (p < 0.001). These data suggest that the HIV-induced increase in the frequency of the mature CD16+ monocyte subpopulation that is vulnerable to HIV infection (Shiramizu et al. 2005; Williams et al. 2005; Ellery et al. 2007; Jaworowski et al. 2007; Buckner et al. 2011) and transmigrates across the BBB in response to chemokines (Buckner et al. 2011; Williams et al. 2013; Williams et al. 2014b; Williams et al. 2015), is augmented when the infected individuals are actively using psychoactive substances.
The frequency of CD16+ monocytes in the peripheral blood of HIV infected individuals is increased with active substance use and dopamine increases the transmigration of CD14+CD16+ monocytes across the BBB. a PBMC from HIV seronegative donors (n = 12) and from HIV seropositive donors from the MHBB cohort (n = 48) were stained with fluorochrome-coupled CD14, CD16 or isotype matched negative control antibodies and analyzed by flow cytometry. The total number of CD14+ monocytes was determined and the percentage of these monocytes that were CD14+CD16+ and CD14lowCD16+ was calculated. HIV infected donors were divided into non-active drug users (n = 22) and active drug users (n = 26). Data points for each donor are shown with mean ± SD. Significance was determined using two-tailed unpaired Mann-Whitney test. **p < 0.001; ***p < 0.0001. b PBMC from HIV seropositive donors from the WIHS cohort were added to BBB cocultures and CD14+CD16+ monocyte and CD3+ T cell transmigration in response to 10 μM dopamine (DA) was expressed as fold increase over baseline (media plus dH2O), which was set to one (dotted line) for each independent donor. Data are expressed as mean ± SEM. Significance was determined by two-tailed Wilcoxon signed-rank test comparing medians for each treatment group to a hypothetical value of one. *p < 0.05 (DA 10 μM compared to baseline); n = 9 each
CD14+CD16+ Monocytes, but Not T Cells, from HIV Infected Individuals Transmigrate across the BBB in Response to Dopamine
We next examined CD16+ monocyte and CD3+ T cell transmigration across the BBB in response to dopamine. To characterize dopamine mediated transmigration, PBMC from HIV infected women from the WIHS cohort were added to the top of our in vitro model of the human BBB (Eugenin et al. 2006; Buckner et al. 2011; Williams et al. 2013; Williams et al. 2014a; Williams et al. 2015), composed of cocultured human BMVEC and astrocytes. Only PBMC from individuals in the WIHS cohort were used because we did not have sufficient numbers of PBMC from individuals in the MHBB cohort to perform these specific experiments. Two of the nine women from the WIHS cohort from which PBMC were obtained tested positive for active drug use, specifically cannabinoids, at the time of blood draw. Media with or without 10 μM dopamine was added to the bottom of cocultures and after 24 h, transmigrated cells were harvested from the bottom and stained with antibodies for CD14, CD16, and CD3. The number of transmigrated CD16+ monocytes and CD3+ T cells was quantified by flow cytometry. Baseline transmigration of these cells across the BBB in response to media plus dH2O (dopamine diluent) was different in each experiment due to the inherent variability of primary cells. To normalize for these differences, transmigration in response to dopamine in each experiment was expressed as fold change compared to baseline that was set to one for each independent donor. The data from experiments using cells from the indicated number of independent donors were then compiled and presented as mean fold change in transmigration in response to dopamine as compared to cocultures to which there was no dopamine added (baseline) that was set to one as indicated by the dotted line (Fig. 1b). Dopamine (DA) significantly increased CD14+CD16+ monocyte transmigration (n = 9, p < 0.05). To our knowledge, this is the first demonstration that dopamine increases CD14+CD16+ monocyte transmigration across an in vitro model of the human BBB. In contrast, CD14lowCD16+ monocyte (data not shown) and CD3+ T cell (Fig. 1b, n=9) transmigration was not increased by dopamine. In previous studies using PBMC from HIV seronegative and positive individuals, we reported that CD14+CD16+, but not CD14lowCD16+, monocyte transmigration across BBB cocultures was increased in response to CCL2 (Williams et al. 2014a; Williams et al. 2015).
Dopamine Increases the Transmigration of Cultured CD14+CD16+ Monocytes across the BBB but Has No Effect on the Transmigration of T Cells from HIV Seronegative Donors
Dopamine does not cross the BBB (Abbott et al. 2006), yet the addition of dopamine to the bottom of our BBB model increased baseline transmigration of CD14+CD16+ monocytes from HIV infected individuals after their addition to the top of cocultures. Baseline transmigration is mediated by low level constitutive chemokine production by BBB endothelial cells and astrocytes, both components of our BBB model (Weiss et al. 1998; Weiss et al. 1999) and brain microvessels in vivo (Williams et al. 2014c). Since the BMVEC and astrocytes used for all the cocultures in this study were derived from the same primary cells, dopamine-mediated effects on the cells of the BBB should be similar in all the experiments performed. Therefore, increased transmigration in response to dopamine is not due to effects on cells of different primary origins forming the barrier. Rather, the increase in transmigration is likely to result, at least in part, from specific effects of dopamine on CD14+CD16+ monocytes that do not occur with T cells. Studies report that during peripheral blood monocyte transmigration across vascular endothelium, monocytes elongate as they pass through the junctions between endothelial cells (Hyun et al. 2012; Tsubota et al. 2013). This suggests that as CD14+CD16+ monocytes traverse BBB endothelium in response to low level constitutive chemokine expression, the leading edge of transmigrating cells can interact with dopamine on the CNS side of the barrier before the cell has completed the process of transmigration. As a consequence, dopamine-mediated effects on transmigrating CD14+CD16+ monocytes may contribute to an increase in the passage of these cells through the BBB.
To determine the mechanisms by which dopamine mediates increased CD14+CD16+ monocyte transmigration across the BBB, we examined biochemical changes involved in cellular migration that are induced by dopamine in CD14+CD16+ monocytes. These experiments were not feasible using blood from HIV infected individuals from the MHBB or WIHS cohorts. The number of CD14+CD16+ monocytes in PBMC from the restricted volume of blood samples we were able to obtain from individuals in the two cohorts was not sufficient for these experiments. Therefore, we obtained HIV seronegative leukopaks from which we could isolate large numbers of PBMC. We purified CD14+ monocytes from these PBMC’s and cultured them non-adherently, as described previously (Buckner et al. 2011; Williams et al. 2013; Coley et al. 2015), to produce mature monocyte cultures with increased numbers of CD14+CD16+ monocytes. We developed this method of culturing peripheral blood monocytes to obtain sufficient numbers of mature CD14+CD16+ monocytes for studies of the ability of these cells to transmigrate across the BBB in response to CCL2, and of their increased movement and adhesion in response to dopamine. While the percentage of peripheral blood monocytes that are CD14+CD16+ in healthy individuals is less than 10% (Ziegler-Heitbrock et al. 1993), the average percentage of CD14+CD16+ monocytes in the mature monocyte cultures used in this study was 64% ± 15% (n = 26). Thus, we were able to examine the effects of dopamine on monocytes in cultures that were composed of predominately CD14+CD16+ monocytes.
We first determined whether CD14+CD16+ monocytes obtained from our mature monocyte cultures transmigrated across the BBB in response to dopamine, as occurred with CD14+CD16+ monocytes in the PBMC from HIV infected individuals. T cell transmigration in response to dopamine was also examined using PBMC isolated from HIV seronegative leukopaks. CXCL12 was included in these studies because this chemokine is both a T cell and monocyte chemoattractant (Bleul et al. 1996) that is expressed constitutively at low levels in the CNS (Stumm et al. 2002), with increased expression during the neuropathogenesis of HIV (Langford et al. 2002; Rostasy et al. 2003). In addition, we have previously shown that CD14+CD16+ monocytes transmigrate across BBB cocultures in response to CXCL12 (Williams et al. 2014b). Thus, CXCL12 may be involved in the recruitment of T cells and/or CD14+CD16+ monocytes into the HIV infected CNS. Additionally, since CXCL12 is expressed by endothelial cells and astrocytes (Salvucci et al. 2002; Calderon et al. 2006), baseline transmigration in our BBB cocultures may be mediated, at least in part, by this chemokine. Therefore, we determined whether there were interactive effects of CXCL12 and dopamine on the transmigration of CD14+CD16+ monocytes across the BBB.
After the addition of mature monocytes to the top of cocultures, CD14+CD16+ monocyte transmigration in response to dopamine or CXCL12 was expressed as fold change compared to the baseline transmigration elicited by the addition of media plus 0.1% BSA (CXCL12 diluent) to the bottom of cocultures, with baseline transmigration (ranging from 209 to 8143 transmigrated CD14+CD16+ monocytes) set to one as indicated by the dotted line (Fig. 2a). Dopamine (DA), at 10 and 20 μM, significantly increased CD14+CD16+ monocyte transmigration (n = 15 for DA 10 μM and n = 13 for DA 20 μM, p < 0.001). CXCL12 at 25 ng/ml also increased CD14+CD16+ monocyte transmigration (n = 14, p < 0.001). There was no additive effect with CXCL12 and dopamine as transmigration in response to the addition of both of these molecules to the bottom of cocultures was not greater than the transmigration elicited by dopamine alone. Similar results were seen with CXCL12 at 50 ng/ml (data not shown). Mature monocyte cultures contained very low levels of CD14lowCD16+ monocytes (Williams et al. 2013) and dopamine did not increase the transmigration of these cells across the BBB (data not shown).
Cultured CD14+CD16+ monocytes, but not CD3+ T cells, transmigrate across the BBB in response to dopamine. a Transmigration experiments were performed with mature monocyte cultures derived from HIV seronegative leukopaks. CD14+CD16+ monocyte transmigration across the BBB in response to dopamine (DA) and/or CXCL12 was expressed as fold increase over baseline (media plus 0.1% BSA in PBS), which was set to one (dotted line) for each independent donor. Data are expressed as mean ± SEM. Significance was determined by two-tailed Wilcoxon signed-rank test comparing medians for each treatment group to a hypothetical value of one. ***p < 0.001 (DA 10 μM compared to baseline, n = 15; DA 20 μM compared to baseline, n = 13; CXCL12 25 ng/ml compared to baseline, n = 14). Transmigration in response to CXCL12 + DA was not significant compared to DA alone. b Transmigration experiments performed with PBMC from HIV seronegative leukopaks to quantify CD3+ T cell transmigration. Data are expressed as mean ± SEM. Significance was determined by two-tailed Wilcoxon signed-rank test comparing medians for each treatment group to a hypothetical value of one. *p < 0.05 (CXCL12 25 and 50 ng/ml compared to baseline); n = 6 each
Increased transmigration of CD14+CD16+ monocytes in response to dopamine was not due to disruption of BBB impermeability as determined by assaying cocultures after transmigration for the passage of albumin coupled Evans blue dye from the top to the bottom of cocultures (data not shown). Transmigration experiments in response to dopamine in the presence of ascorbic acid were also performed as ascorbic acid has been shown to inhibit reactive oxygen species produced by auto-oxidation of dopamine (Pedrosa and Soares-da-Silva 2002). These experiments were designed to determine whether CD14+CD16+ monocyte transmigration was increased specifically by dopamine or was a response to a byproduct produced by the oxidative breakdown of dopamine. Similar results were obtained with dopamine alone or with dopamine plus ascorbic acid (data not shown), indicating that increased transmigration is mediated specifically by dopamine.
PBMC were added to the top of cocultures and T cell transmigration in response to dopamine or CXCL12 was quantified. Unlike CD14+CD16+ monocytes, T cell transmigration was not increased by dopamine at 10 or 20 μM (Fig. 2b). In contrast, CXCL12, at both 25 and 50 ng/ml, significantly increased T cell transmigration (p < 0.05) (n = 6 each for CXCL12 25 ng/ml and 50 ng/ml), indicating that the T cells were capable of chemokine-induced transmigration across the BBB. The fact that dopamine does not increase T cell transmigration indicates that dopamine does not significantly disrupt the integrity of our BBB cocultures, resulting in indiscriminant leukocyte transmigration.
Dopamine Does Not Increase CCL2 or CXCL12 in BBB Cocultures
To identify mechanisms by which dopamine increases CD14+CD16+ monocyte transmigration across the BBB, we examined whether the addition of dopamine to the bottom of cocultures increased the expression of chemokines by cocultured BMVEC and/or astrocytes, which both express dopamine receptors (Bal et al. 1994; Zanassi et al. 1999; Sarkar et al. 2004), and/or by mature monocytes that transmigrated to the bottom of cocultures. For these studies, media plus dH2O (untreated) or media containing 10 μM dopamine was added to the bottom of BBB cocultures and mature monocytes were added to the top. After 24 h, media was collected from the bottom of cocultures, transmigrated monocytes were removed by centrifugation, and the media was analyzed by ELISA for the chemokines CCL2 and CXCL12 that have been demonstrated to increase CD14+CD16+ monocyte transmigration across the BBB (Buckner et al. 2011; Williams et al. 2013; Williams et al. 2014a; Williams et al. 2014b; Williams et al. 2015). CCL2 was detected in untreated cocultures (29.64 ± 4.13 ng/ml, n = 4) and this chemokine contributes to low level baseline transmigration (Weiss et al. 1998; Weiss et al. 1999). After the addition of dopamine, CCL2 did not increase significantly above the levels produced under baseline conditions (23.89 ± 2.84 ng/ml, n = 4). A low level of CXCL12 (0.06 ± 0.01 ng/ml, n = 3) was detected in the media from untreated cocultures and did not increase with the addition of dopamine (0.84 ± 0.71 ng/ml, n = 3). Therefore, dopamine did not increase CCL2 or CXCL12 production from cocultured BMVEC or astrocytes, or from transmigrated mature monocytes within the 24 h time frame of our transmigration experiments. Future studies will determine whether dopamine induces or increases the production of other chemokines, and/or has effects on BBB cells that may contribute specifically to increased CD14+CD16+ monocyte transmigration across the BBB.
Dopamine Induces a Chemotactic Phenotype in Cultured CD14+CD16+ Monocytes, but Not in T Cells
We determined whether dopamine increased the chemotactic phenotype of CD14+CD16+ monocytes or T cells. Monocyte movement necessary for transmigration across the BBB is associated with cytoskeletal rearrangements that result in actin and tubulin reorganization during the formation of membrane protrusions indicative of cellular motility (Mitchison and Cramer 1996). Mature monocytes were treated with media plus dH2O (Untreated) or with 20 μM dopamine for 15 min and then stained for actin and tubulin. The stained cells were examined by confocal microscopy for cell membrane protrusions containing both actin and tubulin. In a microscopic field from a representative experiment using mature monocytes from one donor, untreated cells maintained a spherical morphology (Fig. 3a). Dopamine treated cells exhibited altered actin and tubulin staining localized to membrane protrusions characteristic of a chemotactic phenotype as indicated by the arrows. In experiments with mature monocyte cultures from 6 independent donors, the percentage of cells with membrane protrusions in each treatment group was quantified. The data were then expressed as fold change compared to untreated cells, which was set to one (dotted line) for each independent donor (Fig. 3b). Dopamine significantly increased the fold change in cells exhibiting membrane protrusions when compared to untreated cells (p < 0.05). These results demonstrate that dopamine increases the chemotactic phenotype of CD14+CD16+ monocytes and this may contribute to the increased transmigration of these cells across the BBB.
Dopamine increases cell membrane protrusions in CD14+CD16+ monocytes. a Cells from a mature monocyte culture were treated with media plus dH2O (Untreated) or 20 μM dopamine (DA) for 15 min and stained for actin (Texas Red®-X phalloidin) (red staining), tubulin (FITC) (green staining) and nuclei (DAPI) (blue staining). Cell membrane protrusions with colocalized actin and tubulin staining, indicative of a chemotactic phenotype and indicated by the arrows, were identified by confocal microscopy. b The percentage of mature monocytes with membrane protrusions in untreated and dopamine (DA) treated cultures was quantified. The data were expressed as fold change compared to untreated cells, which was set to one for each independent donor as indicated by the dotted line. Data are expressed as mean ± SD. Significance was determined by two-tailed Wilcoxon signed-rank test comparing medians for each treatment group to a hypothetical value of one. *p < 0.05 (DA 20 μM compared to untreated); n = 6
In contrast to CD14+CD16+ monocytes, the chemotactic phenotype of T cells did not increase in response to dopamine. T cells were treated with media plus 0.1% BSA (Untreated) or 20 μM dopamine for 15, 30 or 45 min and then stained for actin and tubulin. Untreated and dopamine treated T cells (15 min) in a microscopic field from one representative experiment exhibited a circular shape lacking prominent membrane protrusions (Fig. 4a). Because dopamine did not increase membrane protrusions, T cells were treated with 50 ng/ml CXCL12 as a positive control to assure their ability to form membrane protrusions under these experimental conditions. After 15 min of treatment with CXCL12, the cells exhibited membrane protrusions with co-localized actin and tubulin as indicated by the arrows. The time point of maximal induction of membrane protrusion formation with CXCL12 treatment was variable with T cells from different donors. Therefore, we quantified protrusion formation at the time point that resulted in the maximal increase in each experiment and expressed the data as fold increase compared to untreated cells (Fig. 4b). CXCL12 significantly increased T cell membrane protrusions (n = 6, p < 0.05) but dopamine treatment did not. Thus, the inability of dopamine to increase T cell transmigration across the BBB correlates with the lack of membrane protrusion formation in T cells exposed to dopamine.
Dopamine does not increase cell membrane protrusions in T cells. a Purified T cells from one representative donor treated with media plus 0.1% BSA (Untreated), 20 μM dopamine (DA), or 50 ng/ml CXCL12 for 15 min were fixed and stained for actin (Texas Red®-X phalloidin), tubulin (FITC) and nuclei (DAPI). Membrane protrusions were detected by colocalized actin and tubulin staining as indicated by the arrows. b T cells were treated with 20 μM dopamine (DA) or 50 ng/ml CXCL12 for 15, 30 or 45 min. The percentage of T cells with membrane protrusions was quantified at the time point of optimal membrane protrusion formation with CXCL12 in each experiment. The data were then expressed as fold change compared to untreated cells, which was set to one for each independent donor as indicated by the dotted line. Data are expressed as mean ± SD. Significance was determined by two-tailed Wilcoxon signed-rank test comparing medians for each treatment group to a hypothetical value of one. *p < 0.05 (CXCL12 50 ng/ml compared to untreated); n = 6
Dopamine Increases Active ADAM17 in Cultured CD14+CD16+ Monocytes, but Not in T Cells
Activation of the metalloproteinase ADAM17 in monocytes contributes to the transmigration of these cells across the vascular endothelium (Tsubota et al. 2013; Rzeniewicz et al. 2015). As the lagging edge of monocytes binds to the apical surface of endothelial cells, monocytes elongate through interendothelial junctions (Hyun et al. 2012; Tsubota et al. 2013). ADAM17-mediated shedding of L-selectin at the leading edge of transmigrating monocytes, localized specifically to monocyte pseudopods that have reached the basolateral side of the endothelium, regulates the polarity and directional movement of the cells as they transmigrate across endothelium (Rzeniewicz et al. 2015). Detachment of the lagging edge of monocytes bound to the apical endothelial surface is facilitated by ADAM17-mediated cleavage of CD11b/CD18 (Mac-1) on monocytes bound to ICAM-1 on endothelial cells, which completes the diapedesis of monocytes through interendothelial junctions (Tsubota et al. 2013). Dopamine increases general ADAM activity in neural precursor cells (Iwakura et al. 2011). We therefore determined whether dopamine increased active ADAM17 in CD14+CD16+ monocytes and not in T cells, indicating a potential mechanism for increased transmigration of CD14+CD16+ monocytes across the BBB in response to dopamine.
Mature monocytes and T cells were treated with media plus dH2O, dopamine, or PMA, a positive control for ADAM17 activation (Althoff et al. 2000), for times ranging from 5 to 240 min, and cell lysates were analyzed by Western blot with an antibody to the active site of ADAM17. A representative Western blot of total cell lysates from mature monocytes and T cells from the same donor treated for 5 min with media plus dH2O (Un), 10 μM dopamine (DA), or 100 ng/ml PMA is shown in Fig. 5. ADAM17 is glycosylated as the protein progresses from the endoplasmic reticulum to the plasma membrane (Schlondorff et al. 2000). With Western blot analysis, the molecular size of the immature, prodomain form of ADAM17 is approximately 120 to 135 kD while the proteolytically cleaved, active form of ADAM17 is approximately 95 to 98 kD (Takamune et al. 2008; Adrain et al. 2012). In Fig. 5, a broad diffuse protein band of approximately 95 kD represents active ADAM 17 in mature monocytes. Dopamine treatment for 5 min increases active ADAM 17 1.4 fold when compared to untreated cells as determined by densitometric analysis of active ADAM 17 normalized to β-actin. PMA did not increase active ADAM 17 in mature monocytes at this time point. In contrast, dopamine does not increase active ADAM 17 in T cells but a 1.4 fold increase is induced by PMA treatment for 5 min. The protein bands greater than 135 kD may represent active ADAM17 dimers (Xu et al. 2012). Increased active ADAM17 was also detected when mature monocytes from this donor were treated with dopamine or PMA for time periods ranging from 10 to 240 min, while dopamine did not increase active ADAM17 in T cells from the same donor at any time point (data not shown). Similar results were obtained with mature monocytes and T cells obtained from two additional donors (data not shown), indicating that active ADAM17 is increased in CD14+CD16+ monocytes after exposure to dopamine for time points ranging from a few minutes to a few hours. These data suggest that during baseline transmigration, the leading edge of elongated CD14+CD16+ monocytes in interendothelial junctions is exposed to dopamine on the CNS side of the BBB, resulting in the activation of ADAM17 within 5 min, which contributes to the accelerated completion of diapedesis. Thus, this may be one mechanism by which the transmigration of CD14+CD16+ monocytes across the BBB in response to low level constitutive chemokine expression is increased by dopamine.
Dopamine increases active ADAM17 in CD14+CD16+ monocytes, but not in T cells. Mature monocytes and T cells from the same leukopak were treated with media plus dH2O (Un), 10 μM dopamine (DA), or 100 ng/ml PMA for 5 min. Cell lysates prepared from these cells were then analyzed by Western blot for active ADAM17 and β-actin
D1-like Dopamine Receptor Expression Is higher on Cultured CD14+CD16+ Monocytes as Compared to T Cells
Dopamine can interact with both CD14+CD16+ monocytes and T cells once they have penetrated the BBB. Therefore, differential expression of dopamine receptors by CD14+CD16+ monocytes as compared to T cells may contribute to the specificity of dopamine for increasing CD14+CD16+ monocyte transmigration. Thus, we examined dopamine receptor surface expression on mature monocytes and T cells. Dopamine receptors are members of the family of seven transmembrane domain G-protein coupled receptors. There are two groups of dopamine receptors, the D1-like dopamine receptors, comprised of D1R and D5R, and the D2-like dopamine receptors, comprised of D2R, D3R and D4R (Beaulieu and Gainetdinov 2011). Mature monocytes and T cells were incubated with dopamine receptor antibodies followed by PE-labeled secondary antibodies. Dopamine receptor staining that was greater than staining with the appropriate negative control antibody or serum was quantified.
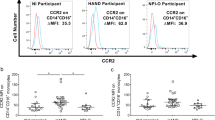
We were able to detect D1R, D5R and D4R surface expression on mature monocytes and T cells by flow cytometry. D1R was absent on T cells (n = 10) and was expressed on mature monocytes (n = 19) as indicated by the mean fluorescence intensity (MFI) (p < 0.001) (Fig. 6). D5R was not expressed by T cells from most donors (n = 6) while T cells from other donors expressed low levels of this receptor (n = 3). In contrast, mature monocytes (n = 17) expressed significantly higher levels of D5R (p < 0.01). D4R was highly expressed by T cells (n = 9) while mature monocytes (n = 18) expressed significantly lower levels of this receptor (p < 0.001). Thus, mature monocytes expressed significantly higher levels of D1R and D5R, and less D4R, when compared to T cells. Since CD14+CD16+ monocytes, but not T cells, transmigrate across the BBB in response to dopamine, the D1-like dopamine receptors, D1R and/or D5R, on CD14+CD16+ monocytes may be involved, at least in part, in the dopamine-mediated increase in transmigration of this mature monocyte subpopulation. Very few antibodies target extracellular epitopes of dopamine receptors and with several of those specific for D2R and D3R, we detected no signal for surface expression of these receptors above the appropriate negative control antibody or serum on mature monocytes or T cells.
CD14+CD16+ monocytes express greater surface D1R and D5R, and less D4R, than T cells. Mature monocytes and T cells from independent donors were incubated with antibodies to D1R, D5R, D4R or negative control rabbit serum or goat IgG. After incubation in primary antibody, cells were incubated with PE-labeled anti-rabbit or anti-goat IgG secondary antibody. Stained cells were analyzed by flow cytometry and the MFI was calculated by subtracting the appropriate negative control antibody or serum staining from the staining obtained with each dopamine receptor antibody. Significance was determined by two-tailed unpaired Mann-Whitney test. **p < 0.01 (D5R on mature monocytes, n = 17, compared to T cells, n = 9); ***p < 0.001 (D1R on mature monocytes, n = 19, compared to T cells, n = 10) (D4R on mature monocytes, n = 18, compared to T cells, n = 9)
A D1-like Dopamine Receptor Agonist Increases Cultured CD14+CD16+ Monocyte Transmigration across the BBB
To determine whether activation of D1-like dopamine receptors may be involved in CD14+CD16+ monocyte transmigration across the BBB in response to dopamine, we performed transmigration experiments using the D1-like dopamine receptor agonist SKF38393. CD14+CD16+ monocyte transmigration significantly increased with 1, 10 and 50 μM SKF38393 (Fig. 7) (n = 6 each, p < 0.05 for SKF38393 1 μM and 50 μM; n = 8, p < 0.01 for SKF38393 10 μM). CD14+CD16+ monocyte transmigration was also significantly increased with the same concentrations of dopamine (n = 11, p < 0.05 for DA 1 μM; n = 10, p < 0.01 for DA 10 μM; n = 8, p < 0.01 for DA 50 μM). These data suggest that dopamine induced transmigration of CD14+CD16+ monocytes across the BBB involves the activation of D1-like dopamine receptors on CD14+CD16+ monocytes. BMVEC and astrocytes also express D1-like dopamine receptors (Hansson and Ronnback 1988; Bacic et al. 1991). The possible contribution of activation of D1-like dopamine receptors on the cells of the BBB in dopamine induced CD14+CD16+ monocyte transmigration will be examined in future studies.
CD14+CD16+ monocytes transmigrate across the BBB in response to the D1-like dopamine receptor agonist, SKF38393. Quantification of CD14+CD16+ monocyte transmigration across the BBB in response to different concentrations of SKF38393 or dopamine (DA) expressed as fold increase over baseline (media plus dH2O), which was set to one as indicated by the dotted line. Data are expressed as mean ± SEM. Significance was determined by two-tailed Wilcoxon signed-rank test comparing medians for each treatment group to a hypothetical value of one. *p < 0.05 (SKF38393 1 μM and 50 μM compared to baseline, n = 6 each) (DA 1 μM compared to baseline, n = 11); **p < 0.01 (SKF38393 10 μM and DA 50 μM compared to baseline, n = 8 each) (DA 10 μM compared to baseline, n = 10)
Discussion
Substance abuse is prevalent in the HIV-infected population (Bing et al. 2001; Mathers et al. 2008). In New York City, for example, injection drug use is a risk factor for infection in 6.5% of new HIV cases, with rates increasing up to 16.8% in specific areas of the city (Amesty et al. 2011), and in a study of non-injection illicit drug users, 16% were positive for HIV infection (Des Jarlais et al. 2014). In a study of injection and non-injection drug users in Spain, the percentage of HIV infected individuals was 15% (Reyes-Uruena et al. 2015). Thus, there is a significant association between substance abuse and HIV, and the mechanisms by which HIV associated neurocognitive impairments may be exacerbated by drug use are not completely understood. In this study, we examined peripheral blood CD16+ monocytes in HIV infected substance abusers from the MHBB cohort to determine whether the frequency of this mature monocyte subpopulation is increased with active substance use. In a large number of individuals from this cohort, poly drug use was detected. Therefore, this study does not correlate the frequency of peripheral blood CD16+ monocytes with the active use of a specific drug, including those not assayed for by urine toxicology. Within the parameters of this study, we found that peripheral CD14+CD16+ and CD14lowCD16+ monocytes were increased significantly in frequency in HIV infected substance abusers as compared to those without active substance use. However, we do not yet know whether this increase is a direct result of active substance use, as the individuals in this study have several other comorbidities or unknown factors that may contribute to an increase in peripheral CD16+ monocytes. Future studies will address this question.
In the general population of HIV infected substance abusers, a significant number of HIV positive CD14+CD16+ monocytes will be present in the peripheral circulation of individuals when they first become infected and have not yet been diagnosed and prescribed cART. In addition, during later stages of infection, a lack of strict adherence to cART (Arnsten et al. 2002; Hinkin et al. 2007) may result in an increase in the number of peripheral HIV positive CD14+CD16+ monocytes. Thus, an increase in the frequency of this mature monocyte subpopulation in HIV infected substance abusers establishes a larger pool of uninfected, and likely HIV positive, CD14+CD16+ monocytes in the peripheral circulation capable of transmigrating across the BBB into the CNS.
In SIV infected monkeys, administration of methamphetamine increased CD14+CD16+ macrophages in the brain (Marcondes et al. 2010), suggesting that substance abuse increases the influx of peripheral CD14+CD16+ monocytes into the SIV infected CNS in vivo. The mechanisms by which peripheral CD14+CD16+ monocytes accumulate in the CNS have not been completely characterized. Previously, we showed that CD14+CD16+ monocytes transmigrate across the BBB in response to CCL2 (Buckner et al. 2011; Williams et al. 2013; Williams et al. 2015), a chemokine elevated in the CNS in response to HIV infection (Cinque et al. 1998). We now report that dopamine increases the transmigration of CD14+CD16+ monocytes across the BBB mediated by low level constitutive chemokine expression. Thus, in conjunction with the observed increase in the frequency of peripheral CD14+CD16+ monocytes in HIV infected active substance users, the entry of these cells into the CNS may be enhanced after drug use by a common mechanism involving increased extracellular dopamine in areas of the brain adjacent to microvessels.
Using in vivo microdialysis in rats and rhesus macaques, basal extracellular levels of dopamine in different areas of the brain are reported to range from 0.31 to 12.9 nM (Calipari et al. 2014; Jedema et al. 2014; Matsumoto et al. 2014). In a study using in vivo low-flow push-pull perfusion sampling in rats, basal extracellular levels of dopamine range from 0.4 nM to 11 nM in different regions of the brain (Slaney et al. 2013). In animal model studies, extracellular dopamine in the brain was shown to be increased 1460% by methamphetamine (Matsumoto et al. 2014), 5807% by amphetamine (Jedema et al. 2014), and 700% by cocaine (Calipari et al. 2014). In our study, dopamine at 1 μM significantly increased CD14+CD16+ monocyte transmigration, with a trend toward increased transmigration with 100 nM dopamine. Thus, efflux of dopamine from synaptic spaces after the use of different types of drugs, particularly those that increase extracellular dopamine the most, may result in levels of this neurotransmitter in areas in close proximity to the brain microvasculature that are sufficient to increase CD14+CD16+ monocyte transmigration across the BBB.
In chronic substance abusers, lower levels of dopamine in the CNS are produced after drug use when compared to individuals who are new or intermittent drug users (Espana and Jones 2013; Willuhn et al. 2014). In addition, HIV infection of the CNS for extended periods of time can result in significant damage to dopaminergic neurons, resulting in decreased overall levels of dopamine in the brain (Sardar et al. 1996; Kumar et al. 2009). This suggests that the levels of extracellular dopamine in the CNS that are sufficient to increase CD14+CD16+ monocyte transmigration are more likely to occur in new or intermittent substance abusers during the acute and early stages of chronic HIV infection. The fact that drug use in some individuals will not produce a high enough concentration of extracellular dopamine in the CNS to increase the transmigration of CD14+CD16+ monocytes across the BBB may be one reason why some studies report that substance abuse does not have a significant effect on the severity of cognitive deficits in HIV infected individuals (Byrd et al. 2011; Levine et al. 2014). In individuals in whom drug-induced extracellular dopamine levels are sufficient to increase the entry of CD14+CD16+ monocytes into the CNS, initial viral seeding of the brain right after infection and subsequent replenishment of HIV reservoirs during the early stages of infection may be increased in response to repeated drug use. In addition, CNS accumulation of both infected and uninfected CD14+CD16+ monocytes may contribute to chronic neuroinflammation. As a result, HIV associated cognitive impairments may develop earlier after initial infection, with increases in the magnitude of these impairments throughout the course of infection in some HIV infected substance abusers.
We report that activation of D1-like dopamine receptors is involved in increased CD14+CD16+ monocyte transmigration across the BBB. Previous studies have examined the role of dopamine receptor activation in the migration of other cell types. A D1-like dopamine receptor agonist induces cytoskeletal rearrangements in a human hematopoietic progenitor cell, resulting in a highly polarized phenotype indicative of cell motility (Spiegel et al. 2007). D1R activation also induces rat astrocyte and mouse neuron and neural progenitor cell migration (Crandall et al. 2007; Hiramoto et al. 2008; Huang et al. 2012). These studies support our findings that activation of D1-like dopamine receptors contributes to the specificity of dopamine in increasing the transmigration of CD14+CD16+ monocytes across the BBB. Although we were not able to demonstrate D2R and D3R on mature monocytes or T cells by flow cytometry, there may still be low level expression of these receptors that is below the limits of detection of our assay. In previous studies in our laboratory, we detected mRNA for all five dopamine receptors in mature monocyte cultures (Coley et al. 2015). The role of D2R and D3R in dopamine induced CD14+CD16+ monocyte transmigration across the BBB will be the focus of future studies.
This study identifies potential mechanisms by which active substance use contributes to the development of HIV associated cognitive impairments. The frequency of CD14+CD16+ monocytes is increased significantly in the periphery of HIV infected substance abusers as compared to infected individuals without active drug use. Drug-induced elevations of extracellular dopamine in areas of the CNS with dopaminergic neurons in close proximity to the brain microvasculature may increase the transmigration of peripheral CD14+CD16+ monocytes across the BBB. We propose that increased transmigration is mediated by dopamine activation of D1R/D5R receptors on the leading edge of CD14+CD16+ monocytes traversing the BBB in response to basal levels of chemokines, including CCL2. D1R/D5R activation increases active ADAM 17 on the monocyte surface, resulting in the cleavage of monocyte MAC-1 bound to endothelial ICAM-1 and the accelerated completion of monocyte diapedesis through BBB interendothelial junctions, as illustrated in the schematic shown in Fig. 8. These events will most likely occur repeatedly over time with each episode of drug use, resulting in the accumulation of uninfected and infected CD14+CD16+ monocytes in dopaminergic regions of the CNS. Once within the CNS parenchyma, these monocytes will mature into macrophages, with subsequent infection of additional CNS cells resulting from HIV infected macrophage accumulation within the brain. This will contribute to the establishment and maintenance of viral reservoirs within the CNS. We previously reported that dopamine increases HIV entry and replication in human macrophages, a mechanism by which substance abuse may increase macrophage specific viral replication in the HIV infected CNS (Gaskill et al. 2009; Gaskill et al. 2014). Thus, the accumulation of infected newly matured macrophages within dopaminergic regions of the CNS may result in increased viral replication and protein production after drug-induced increases in extracellular dopamine. As a consequence, nearby uninfected macrophages and other CNS cells may be infected and/or activated, and chronic viral protein production and neuroinflammation may increase, contributing to increased cognitive impairments. Therefore, inhibiting the dopamine mediated influx of CD14+CD16+ monocytes into the CNS by antagonizing D1-like dopamine receptors may be a therapeutic strategy to decrease ongoing viral seeding of the CNS, chronic neuroinflammation, and cognitive impairments in HIV infected substance abusers.
Schematic representation of the proposed mechanism by which dopamine increases chemokine induced CD14+CD16+ monocyte transmigration across the BBB in HIV infected substance abusers. In areas of the CNS with dopaminergic neuronal synapses in close proximity to BBB microvasculature, active substance use will increase the concentration of dopamine, resulting in the activation D1R/D5R receptors on the leading edge of CD14+CD16+ monocytes transmigrating across the BBB in response to chemokines, including CCL2. Upon dopamine receptor activation, ADAM 17 on the cell surface of the lagging edge of the monocyte is activated, resulting in cleavage of monocyte Mac-1 bound to endothelial ICAM-1 and the accelerated completion of transmigration
References
Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7:41–53
Adrain C, Zettl M, Christova Y, Taylor N, Freeman M (2012) Tumor necrosis factor signaling requires iRhom2 to promote trafficking and activation of TACE. Science 335:225–228
Althoff K, Reddy P, Voltz N, Rose-John S, Mullberg J (2000) Shedding of interleukin-6 receptor and tumor necrosis factor alpha. Contribution of the stalk sequence to the cleavage pattern of transmembrane proteins. Eur J Biochem 267:2624–2631
Amesty S, Rivera AV, Fuller CM (2011) Overview of HIV among injection drug users in New York City: critical next steps to eliminate racial/ethnic disparities. Subst Use Misuse 46:285–294
Anthony IC, Crawford DH, Bell JE (2003) B lymphocytes in the normal brain: contrasts with HIV-associated lymphoid infiltrates and lymphomas. Brain 126:1058–1067
Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, Schoenbaum EE (2002) Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med 17:377–381
Bacic F, Uematsu S, McCarron RM, Spatz M (1991) Dopaminergic receptors linked to adenylate cyclase in human cerebromicrovascular endothelium. J Neurochem 57:1774–1780
Bal A, Bachelot T, Savasta M, Manier M, Verna JM, Benabid AL, Feuerstein C (1994) Evidence for dopamine D2 receptor mRNA expression by striatal astrocytes in culture: in situ hybridization and polymerase chain reaction studies. Brain Res Mol Brain Res 23:204–212
Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J (1998) The Women's interagency HIV study. WIHS Collaborative Study Group Epidemiology 9:117–125
Beaulieu JM, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217
Bell JE, Busuttil A, Ironside JW, Rebus S, Donaldson YK, Simmonds P, Peutherer JF (1993) Human immunodeficiency virus and the brain: investigation of virus load and neuropathologic changes in pre-AIDS subjects. J Infect Dis 168:818–824
Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitiello B, Morton SC, Orlando M, Bozzette SA, Ortiz-Barron L, Shapiro M (2001) Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry 58:721–728
Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA (1996) A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med 184:1101–1109
Buch S, Yao H, Guo M, Mori T, Mathias-Costa B, Singh V, Seth P, Wang J, Su TP (2012) Cocaine and HIV-1 interplay in CNS: cellular and molecular mechanisms. Curr HIV Res 10:425–428
Buckner CM, Calderon TM, Willams DW, Belbin TJ, Berman JW (2011) Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: implications for NeuroAIDS. Cell Immunol 267:109–123
Burdo TH, Lackner A, Williams KC (2013) Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev 254:102–113
Byrd DA, Fellows RP, Morgello S, Franklin D, Heaton RK, Deutsch R, Atkinson JH, Clifford DB, Collier AC, Marra CM, Gelman B, McCutchan JA, Duarte NA, Simpson DM, McArthur J, Grant I, Group C (2011) Neurocognitive impact of substance use in HIV infection. J Acquir Immune Defic Syndr 58:154–162
Calderon TM, Eugenin EA, Lopez L, Kumar SS, Hesselgesser J, Raine CS, Berman JW (2006) A role for CXCL12 (SDF-1alpha) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J Neuroimmunol 177:27–39
Calipari ES, Ferris MJ, Jones SR (2014) Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J Neurochem 128:224–232
Chan P, Hellmuth J, Spudich S, Valcour V (2016) Cognitive impairment and persistent CNS injury in treated HIV. Curr HIV/AIDS Rep 13:209–217
Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, Transidico P, Vagani A, Sozzani S, Mantovani A, Lazzarin A, Poli G (1998) Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS 12:1327–1332
Clay CC, Rodrigues DS, Ho YS, Fallert BA, Janatpour K, Reinhart TA, Esser U (2007) Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection. J Virol 81:12040–12048
Coley JS, Calderon TM, Gaskill PJ, Eugenin EA, Berman JW (2015) Dopamine increases CD14 + CD16+ monocyte migration and adhesion in the context of substance abuse and HIV neuropathogenesis. PLoS One 10:e0117450
Crandall JE, McCarthy DM, Araki KY, Sims JR, Ren JQ, Bhide PG (2007) Dopamine receptor activation modulates GABA neuron migration from the basal forebrain to the cerebral cortex. J Neurosci 27:3813–3822
Crowe S, Zhu T, Muller WA (2003) The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol 74:635–641
Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA (1992) Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology 42:1736–1739
Des Jarlais DC, McKnight C, Arasteh K, Feelemyer J, Perlman DC, Hagan H, Dauria EF, Cooper HL (2014) A perfect storm: crack cocaine, HSV-2, and HIV among non-injecting drug users in New York City. Subst Use Misuse 49:783–792
El-Bassel N, Shaw SA, Dasgupta A, Strathdee SA (2014) Drug use as a driver of HIV risks: re-emerging and emerging issues. Curr Opin HIV AIDS 9:150–155
Ellery PJ, Tippett E, Chiu YL, Paukovics G, Cameron PU, Solomon A, Lewin SR, Gorry PR, Jaworowski A, Greene WC, Sonza S, Crowe SM (2007) The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol 178:6581–6589
Espana RA, Jones SR (2013) Presynaptic dopamine modulation by stimulant self-administration. Front Biosci (Schol Ed) 5:261–276
Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW (2006) CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci 26:1098–1106
Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L'Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J (2001) CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol 7:528–541
Fischer-Smith T, Croul S, Adeniyi A, Rybicka K, Morgello S, Khalili K, Rappaport J (2004) Macrophage/microglial accumulation and proliferating cell nuclear antigen expression in the central nervous system in human immunodeficiency virus encephalopathy. Am J Pathol 164:2089–2099
Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW (2009) Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. Am J Pathol 175:1148–1159
Gaskill PJ, Yano HH, Kalpana GV, Javitch JA, Berman JW (2014) Dopamine receptor activation increases HIV entry into primary human macrophages. PLoS One 9:e108232
Gonzalez-Scarano F, Martin-Garcia J (2005) The neuropathogenesis of AIDS. Nat Rev Immunol 5:69–81
Gras G, Kaul M (2010) Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection. Retrovirology 7:30
Hansson E, Ronnback L (1988) Neurons from substantia nigra increase the efficacy and potency of second messenger arising from striatal astroglia dopamine receptor. Glia 1:393–397
Hauser KF, El-Hage N, Stiene-Martin A, Maragos WF, Nath A, Persidsky Y, Volsky DJ, Knapp PE (2007) HIV-1 neuropathogenesis: glial mechanisms revealed through substance abuse. J Neurochem 100:567–586
Heaton RK et al (2010) HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology 75:2087–2096
Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, Myers HF, Longshore D (2007) Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav 11:185–119
Hiramoto T, Satoh Y, Takishima K, Watanabe Y (2008) Induction of cell migration of neural progenitor cells in vitro by alpha-1 adrenergic receptor and dopamine D1 receptor stimulation. Neuroreport 19:793–797
Hong S, Banks WA (2015) Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun 45:1–12
Huang C, Wu J, Liao R, Zhang W (2012) SKF83959, an agonist of phosphatidylinositol-linked D(1)-like receptors, promotes ERK1/2 activation and cell migration in cultured rat astrocytes. PLoS One 7:e49954
Hyun YM, Sumagin R, Sarangi PP, Lomakina E, Overstreet MG, Baker CM, Fowell DJ, Waugh RE, Sarelius IH, Kim M (2012) Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J Exp Med 209:1349–1362
Iwakura Y, Wang R, Abe Y, Piao YS, Shishido Y, Higashiyama S, Takei N, Nawa H (2011) Dopamine-dependent ectodomain shedding and release of epidermal growth factor in developing striatum: target-derived neurotrophic signaling (part 2). J Neurochem 118:57–68
Jaworowski A, Kamwendo DD, Ellery P, Sonza S, Mwapasa V, Tadesse E, Molyneux ME, Rogerson SJ, Meshnick SR, Crowe SM (2007) CD16+ monocyte subset preferentially harbors HIV-1 and is expanded in pregnant Malawian women with plasmodium falciparum malaria and HIV-1 infection. J Infect Dis 196:38–42
Jedema HP, Narendran R, Bradberry CW (2014) Amphetamine-induced release of dopamine in primate prefrontal cortex and striatum: striking differences in magnitude and timecourse. J Neurochem 130:490–497
Kramer-Hammerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R (2005) Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res 111:194–213
Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, Kumar M (2009) Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J Neurovirol 15:257–274
Kusao I, Shiramizu B, Liang CY, Grove J, Agsalda M, Troelstrup D, Velasco VN, Marshall A, Whitenack N, Shikuma C, Valcour V (2012) Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiatry Clin Neurosci 24:71–80
Langford D, Sanders VJ, Mallory M, Kaul M, Masliah E (2002) Expression of stromal cell-derived factor 1alpha protein in HIV encephalitis. J Neuroimmunol 127:115–126
Levine AJ, Reynolds S, Cox C, Miller EN, Sinsheimer JS, Becker JT, Martin E, Sacktor N, Neuropsychology Working Group of the Multicenter ACS (2014) The longitudinal and interactive effects of HIV status, stimulant use, and host genotype upon neurocognitive functioning. J Neurovirol 20:243–257
Li W, Li G, Steiner J, Nath A (2009) Role of tat protein in HIV neuropathogenesis. Neurotox Res 16:205–220
Marcondes MC, Burudi EM, Huitron-Resendiz S, Sanchez-Alavez M, Watry D, Zandonatti M, Henriksen SJ, Fox HS (2001) Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J Immunol 167:5429–5438
Marcondes MC, Flynn C, Watry DD, Zandonatti M, Fox HS (2010) Methamphetamine increases brain viral load and activates natural killer cells in simian immunodeficiency virus-infected monkeys. Am J Pathol 177:355–361
Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, Wodak A, Panda S, Tyndall M, Toufik A, Mattick RP, Reference Group to the UNoHIV, Injecting Drug U (2008) Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet 372:1733–1745
Matsumoto T, Maeno Y, Kato H, Seko-Nakamura Y, Monma-Ohtaki J, Ishiba A, Nagao M, Aoki Y (2014) 5-hydroxytryptamine- and dopamine-releasing effects of ring-substituted amphetamines on rat brain: a comparative study using in vivo microdialysis. Eur Neuropsychopharmacol 24:1362–1370
Meade CS, Towe SL, Skalski LM, Robertson KR (2015) Independent effects of HIV infection and cocaine dependence on neurocognitive impairment in a community sample living in the southern United States. Drug Alcohol Depend 149:128–135
Meyer VJ, Rubin LH, Martin E, Weber KM, Cohen MH, Golub ET, Valcour V, Young MA, Crystal H, Anastos K, Aouizerat BE, Milam J, Maki PM (2013) HIV and recent illicit drug use interact to affect verbal memory in women. J Acquir Immune Defic Syndr 63:67–76
Mitchison TJ, Cramer LP (1996) Actin-based cell motility and cell locomotion. Cell 84:371–379
Morgello S, Estanislao L, Simpson D, Geraci A, DiRocco A, Gerits P, Ryan E, Yakoushina T, Khan S, Mahboob R, Naseer M, Dorfman D, Sharp V, Manhattan HIVBB (2004) HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: the Manhattan HIV brain Bank. Arch Neurol 61:546–551
Nockher WA, Bergmann L, Scherberich JE (1994) Increased soluble CD14 serum levels and altered CD14 expression of peripheral blood monocytes in HIV-infected patients. Clin Exp Immunol 98:369–374
Otani I, Akari H, Nam KH, Mori K, Suzuki E, Shibata H, Doi K, Terao K, Yosikawa Y (1998) Phenotypic changes in peripheral blood monocytes of cynomolgus monkeys acutely infected with simian immunodeficiency virus. AIDS Res Hum Retrovir 14:1181–1186
Pedrosa R, Soares-da-Silva P (2002) Oxidative and non-oxidative mechanisms of neuronal cell death and apoptosis by L-3,4-dihydroxyphenylalanine (L-DOPA) and dopamine. Br J Pharmacol 137:1305–1313
Peng H, Erdmann N, Whitney N, Dou H, Gorantla S, Gendelman HE, Ghorpade A, Zheng J (2006) HIV-1-infected and/or immune activated macrophages regulate astrocyte SDF-1 production through IL-1beta. Glia 54:619–629
Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS (1997) Unique monocyte subset in patients with AIDS dementia. Lancet 349:692–695
Reyes-Uruena J, Brugal MT, Majo X, Domingo-Salvany A, Cayla JA (2015) Cross sectional study of factors associated to self-reported blood-borne infections among drug users. BMC Public Health 15:1122
Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I, Group H (2004) Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc 10:1–14
Rostasy K, Egles C, Chauhan A, Kneissl M, Bahrani P, Yiannoutsos C, Hunter DD, Nath A, Hedreen JC, Navia BA (2003) SDF-1alpha is expressed in astrocytes and neurons in the AIDS dementia complex: an in vivo and in vitro study. J Neuropathol Exp Neurol 62:617–626
Rzeniewicz K, Newe A, Rey Gallardo A, Davies J, Holt MR, Patel A, Charras GT, Stramer B, Molenaar C, Tedder TF, Parsons M, Ivetic A (2015) L-selectin shedding is activated specifically within transmigrating pseudopods of monocytes to regulate cell polarity in vitro. Proc Natl Acad Sci U S A 112:E1461–E1470
Sadagopal S, Lorey SL, Barnett L, Basham R, Lebo L, Erdem H, Haman K, Avison M, Waddell K, Haas DW, Kalams SA (2008) Enhancement of human immunodeficiency virus (HIV)-specific CD8+ T cells in cerebrospinal fluid compared to those in blood among antiretroviral therapy-naive HIV-positive subjects. J Virol 82:10418–10428
Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G (2002) Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood 99:2703–2711
Sardar AM, Czudek C, Reynolds GP (1996) Dopamine deficits in the brain: the neurochemical basis of parkinsonian symptoms in AIDS. Neuroreport 7:910–912
Sarkar C, Chakroborty D, Mitra RB, Banerjee S, Dasgupta PS, Basu S (2004) Dopamine in vivo inhibits VEGF-induced phosphorylation of VEGFR-2, MAPK, and focal adhesion kinase in endothelial cells. Am J Physiol Heart Circ Physiol 287:H1554–H1560
Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC (2016) HIV-associated neurocognitive disorder - pathogenesis and prospects for treatment. Nat Rev Neurol 12:234–248
Schlondorff J, Becherer JD, Blobel CP (2000) Intracellular maturation and localization of the tumour necrosis factor alpha convertase (TACE). Biochem J 347(Pt 1):131–138
Schrier RD, Hong S, Crescini M, Ellis R, Perez-Santiago J, Spina C, Letendre S, Group H (2015) Cerebrospinal fluid (CSF) CD8+ T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND). PLoS One 10:e0116526
Shiramizu B, Gartner S, Williams A, Shikuma C, Ratto-Kim S, Watters M, Aguon J, Valcour V (2005) Circulating proviral HIV DNA and HIV-associated dementia. AIDS 19:45–52
Slaney TR, Mabrouk OS, Porter-Stransky KA, Aragona BJ, Kennedy RT (2013) Chemical gradients within brain extracellular space measured using low flow push-pull perfusion sampling in vivo. ACS Chem Neurosci 4:321–329
Spiegel A, Shivtiel S, Kalinkovich A, Ludin A, Netzer N, Goichberg P, Azaria Y, Resnick I, Hardan I, Ben-Hur H, Nagler A, Rubinstein M, Lapidot T (2007) Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol 8:1123–1131
Spiller MW, Broz D, Wejnert C, Nerlander L, Paz-Bailey G (2015) HIV infection and HIV-associated behaviors among persons who inject drugs −20 cities, United States, 2012. MMWR Morb Mortal Wkly Rep 64:270–275
Spudich S, Gonzalez-Scarano F (2012) HIV-1-related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harb Perspect Med 2:a007120
Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Hollt V, Schulz S (2002) A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci 22:5865–5878
Takamune Y, Ikebe T, Nagano O, Shinohara M (2008) Involvement of NF-kappaB-mediated maturation of ADAM-17 in the invasion of oral squamous cell carcinoma. Biochem Biophys Res Commun 365:393–398
Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N (1995) CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol 25:3418–3424
Tomlinson GS, Simmonds P, Busuttil A, Chiswick A, Bell JE (1999) Upregulation of microglia in drug users with and without pre-symptomatic HIV infection. Neuropathol Appl Neurobiol 25:369–379
Tsubota Y, Frey JM, Tai PW, Welikson RE, Raines EW (2013) Monocyte ADAM17 promotes diapedesis during Transendothelial migration: identification of steps and substrates targeted by Metalloproteinases. J Immunol 190:4236–4244
Valcour VG, Shiramizu BT, Shikuma CM (2010) HIV DNA in circulating monocytes as a mechanism to dementia and other HIV complications. J Leukoc Biol 87:621–626
Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, Suwanwela NC, Jagodzinski L, Michael N, Spudich S, van Griensven F, de Souza M, Kim J, Ananworanich J, Group RSS (2012) Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 206:275–282
Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F (2009) Imaging dopamine's role in drug abuse and addiction. Neuropharmacology 56(Suppl 1):3–8
Wang T, Rumbaugh JA, Nath A (2006) Viruses and the brain: from inflammation to dementia. Clin Sci (Lond) 110:393–407
Weber E, Morgan EE, Iudicello JE, Blackstone K, Grant I, Ellis RJ, Letendre SL, Little S, Morris S, Smith DM, Moore DJ, Woods SP, Group T (2013) Substance use is a risk factor for neurocognitive deficits and neuropsychiatric distress in acute and early HIV infection. J Neurovirol 19:65–74
Weiss JM, Downie SA, Lyman WD, Berman JW (1998) Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood-brain barrier. J Immunol 161:6896–6903
Weiss JM, Nath A, Major EO, Berman JW (1999) HIV-1 tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes. J Immunol 163:2953–2959
Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA (2001) Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med 193:905–915
Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG (2005) Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J Clin Invest 115:2534–2545
Williams DW, Eugenin EA, Calderon TM, Berman JW (2012) Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol 91:401–415
Williams DW, Calderon TM, Lopez L, Carvallo-Torres L, Gaskill PJ, Eugenin EA, Morgello S, Berman JW (2013) Mechanisms of HIV entry into the CNS: increased sensitivity of HIV infected CD14 + CD16+ monocytes to CCL2 and key roles of CCR2, JAM-A, and ALCAM in diapedesis. PLoS One 8:e69270
Williams DW, Byrd D, Rubin LH, Anastos K, Morgello S, Berman JW (2014a) CCR2 on CD14(+)CD16(+) monocytes is a biomarker of HIV-associated neurocognitive disorders. Neurol Neuroimmunol Neuroinflamm 1:e36
Williams DW, Veenstra M, Gaskill PJ, Morgello S, Calderon TM, Berman JW (2014b) Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Curr HIV Res 12:85–96
Williams JL, Holman DW, Klein RS (2014c) Chemokines in the balance: maintenance of homeostasis and protection at CNS barriers. Front Cell Neurosci 8:154
Williams DW, Anastos K, Morgello S, Berman JW (2015) JAM-A and ALCAM are therapeutic targets to inhibit diapedesis across the BBB of CD14 + CD16+ monocytes in HIV-infected individuals. J Leukoc Biol 97:401–412
Willuhn I, Burgeno LM, Groblewski PA, Phillips PE (2014) Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nat Neurosci 17:704–709
Xu P, Liu J, Sakaki-Yumoto M, Derynck R (2012) TACE activation by MAPK-mediated regulation of cell surface dimerization and TIMP3 association. Sci Signal 5:ra34
Zanassi P, Paolillo M, Montecucco A, Avvedimento EV, Schinelli S (1999) Pharmacological and molecular evidence for dopamine D(1) receptor expression by striatal astrocytes in culture. J Neurosci Res 58:544–552
Ziegler-Heitbrock HW, Fingerle G, Strobel M, Schraut W, Stelter F, Schutt C, Passlick B, Pforte A (1993) The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol 23:2053–2058
Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116:e74–e80
Acknowledgements
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) with the Bronx WIHS principal investigator, Dr. Kathryn Anastos. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. The authors thank Dr. Brad Poulos (Fetal Tissue Repository), the New York Blood Center, Dr. Lydia Tesfa (Flow Cytometry Core, Albert Einstein College of Medicine, Montefiore Medical Center), and the patients and staff of the MHBB and WHIS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by U.S. NIH Grants DA025567 (to J.W.B. and T.M.C.), MH075679 and MH090958 (to J.W.B.), DA029476 (to P.J.G.), MH096625 (to E.A.E.), Al035004 and Al142590 (to K.A.), and MH083501 and MH100931 (to S.M.); Mount Sinai Institute for NeuroAIDS Disparities Pilot Funds (MH080663 to D.W.W); United Negro College Fund/Merck Graduate Science Dissertation Fellowship (to D.W.W.); and the Center for AIDS Research at the Albert Einstein College of Medicine, Montefiore Medical Center (CFAR/AI051519).
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Calderon, T.M., Williams, D.W., Lopez, L. et al. Dopamine Increases CD14+CD16+ Monocyte Transmigration across the Blood Brain Barrier: Implications for Substance Abuse and HIV Neuropathogenesis. J Neuroimmune Pharmacol 12, 353–370 (2017). https://doi.org/10.1007/s11481-017-9726-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-017-9726-9