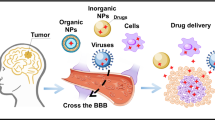
Abstract
At present, brain tumor is among the most challenging diseases to treat and the therapy is limited by the lack of effective methods to deliver anticancer agents across the blood-brain barrier (BBB). BBB is a selective barrier that separates the circulating blood from the brain extracellular fluid. In its neuroprotective function, BBB prevents the entry of toxins, as well as most of anticancer agents and is the main impediment for brain targeted drug delivery approaches. Nanotechnology-based delivery systems provide an attractive strategy to cross the BBB and reach the central nervous system (CNS). The incorporation of anticancer agents in various nanovehicles facilitates their delivery across the BBB. Moreover, a more powerful tool in brain tumor therapy has relied surface modifications of nanovehicles with specific ligands that can promote their passage through the BBB and favor the accumulation of the drug in CNS tumors. This review describes the physiological and anatomical features of the brain tumor and the BBB, and summarizes the recent advanced approaches to deliver anticancer drugs into brain tumor using nanobiotechnology-based drug carrier systems. The role of specific ligands in the design of functionalized nanovehicles for targeted delivery to brain tumor is reviewed. The current trends and future approaches in the CNS delivery of therapeutic molecules to tumors are also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a group of diseases that involves uncontrolled cell division, and resistance to cell death. Cancer cells grow into an abnormal cell mass called a tumor (Mitra et al. 2015). According to the National Cancer Institute (NCI), in 2016, an estimated 1,685,210 new cases of cancer will be diagnosed in the United States and 595,690 people will die from the disease (Siegel et al. 2016). It is estimated that by 2020, 15 million will be diagnosed with cancer. Brain tumor is an abnormal growth of tissue in the central nervous system (CNS) that can disrupt the proper brain function. In 2015, nearly 25,000 new cases of primary malignant and 53,000 non-malignant brain tumors were diagnosed (Ostrom et al. 2016). The mean survival time of brain tumor patients is only 3–16 months and depends on age, histology, molecular markers and tumor behavior (Liu and Lu 2012). Brain tumor-targeted drug delivery strategies remain particularly challenging for chemotherapy due to the inability of intravenously administered anticancer agents to reach the brain parenchyma. This is primarily due to the presence of several barriers in the CNS, which blocks the transport of anticancer drugs from the bloodstream to the brain tumor site. The above issues in delivering therapeutic molecules to the brain tumor can be overcome by understanding the physiology of these barriers, as well as the various transport mechanisms and receptors availability.
In order to efficiently deliver anticancer agents into the brain, several conventional strategies have been used. These strategies include the disruption of the CNS barriers using biochemical reagents, focused ultrasound and radiation exposure, and invasive delivery methods, such as intra-cerebrospinal fluid injection, intrathecal, and Intratumoral injections (Chacko et al. 2013; Azad et al. 2015). However, most of these conventional methods suffer from severe neurotoxic and neuropathological consequences. Recent research in nanobiotechnology instills new advances in the treatment of brain tumors (Bhaskar et al. 2010; Wong et al. 2012; Zhang et al. 2015; Karim et al. 2016). There are several advantages that nanotechnology can provide in the drug delivery research area. Nanovehicles facilitate the delivery of encapsulated therapeutic agents to the CNS. Moreover, surface modifications of nanovehicles with specific ligands have been shown to improve the targeting and crossing of the blood-brain barrier (BBB), which is one of the major barriers in brain targeted drug delivery approaches that favor the accumulation of the drugs in CNS tumors. The current nanobiotechnology based delivery strategies (combination of nano-, bio-, theranostic and imaging technologies) offer new options for the treatment of brain tumors. In that respect, the present review describes the physiological and anatomical features of the brain tumor and the BBB, and summarizes the recent advanced approaches to deliver anticancer drugs into brain tumors. The design of nanostructures, their advantages and limitations for targeted delivery to brain tumor are also reviewed. Current trends and future approaches in the CNS drug delivery to brain tumors are also discussed.
Physiological and Anatomical Features of the Brain Tumor
Brain Tumor
Brain tumors can be classified according to the type of cells involved in the tumor (meningioma, astrocytoma, lymphoma) or by the tumor location in the brain (Ohgaki and Kleihues 2013). Tumors that originate within brain tissue are known as primary brain tumors, whereas, tumors that originate from another part of the body are called secondary brain tumors. Glioma is the most common primary brain tumor that begins in the glial tissue and mainly includes astrocytoma, oligodendroglioma and ependymoma, based on the glial cells associated (Fig. 1a). According to their locations in the brain, the other common primary brain tumors are gliomas, meningioma, pituitary adenomas, and vestibular schwannoma.
As a rapidly growing tissue, the extracellular environment of brain tumor differs to the normal brain tissue (Engin et al. 1995). The extracellular pH of the tumor tissue is about 5.7–7.2 due to the high rate of glycolysis in tumor cells. With the deterioration of brain tumors, angiogenesis and vasculogenic mimicry occur from pre-existing blood vessels, as the growth and metastasis of tumor depend on an adequate supply of nutrients and oxygen (Nishida et al. 2006). Brain tumor tissue mainly consists of tumor cells with a relatively small number of stem cells. Normal tumor cells are incapable of tumorigenesis and can be easily killed by chemotherapeutic drugs. However, tumor stem cells have the ability to self-renew and produce new tumor cells, thereby inducing the chemotherapeutic drug resistance tumorigenesis and cancer spread (Wei et al. 2014) (Fig. 1b).
Anatomy and Physiology of the Physiochemical Barriers in Brain Tumor Therapy
One of the major obstacles in the development of therapeutic agents for the treatment of CNS diseases is to formulate an appropriate system that can effectively cross the CNS barriers, and reaching the diseased sites. These barriers mainly include the BBB, and the blood-brain tumor barrier (BBTB) (Wong et al. 2012; van Tellingen et al. 2015; Karim et al. 2016).
Blood-Brain Barrier (BBB)
The majority of small molecule drugs and macromolecule agents, such as proteins, peptides, and antibodies do not readily permeate into the brain parenchyma, which is one of the most significant challenges of an effective CNS drug delivery. This can be explained by the fact that before reaching the targeted sites in the CNS tumor, therapeutic agents have to pass through the BBB. The function of the BBB is to separate the brain extracellular fluid from the circulating blood, transport beneficial endogenous molecules and essential nutrients into the brain, and filter harmful compounds from the brain back to the bloodstream. The components of the BBB are monolayer of capillary endothelial cells, basement membrane, vascular endothelium, pericytes, astrocytes, and the intracellular space between the membranes (Fig. 2a). Transport of substances across the BBB is strictly limited through both physical specialized connections (tight junctions) and metabolic barriers (enzymes and transport systems). Unfortunately, over 95 % of substances never reach the brain in therapeutically relevant concentrations. The specific and selective molecular permeability of BBB is due to special features of the brain cells (Persidsky et al. 2006; Karim et al. 2016).
Brain capillary endothelial cells exhibit higher mitochondria numbers compared to those present in the normal circulatory cells (8–11 % versus 2–5 %, respectively) (Oldendorf et al. 1977; Ronaldson and Davis 2012). This excess metabolic capability of BBB tissue allows them maintaining the higher selective molecular permeability compared to normal cells. In addition, the brain capillary endothelial cells are tighter (50–100 times) than the normal circulation endothelial cells, which further restricts the diffusion of large hydrophilic molecules (Abbott 2002). The trans-endothelial electrical resistance (TEER) of the junctional complex that fuses the brain capillary endothelial cells is higher (1500–2000 Ω.cm2) (Wong et al. 2012) than that of the most non-cerebral capillaries (2–20 Ω.cm2), which severely impedes the paracellular diffusion of polar molecules (Butt et al. 1990). Moreover, the capillary endothelial cells of the BBB are surrounded by the astrocytes (star-shaped glial cells in the brain) projections called astrocytic feet, which is responsible for releasing several biochemicals and growth factors for the maintenance of the phenotype of BBB and the modulation of the permeability of the endothelial cells (Abbott 2002). In contrast to other vascular endothelial cells, the absence of fenestrations and the low activity of pinocytosis further limit the non-specific transport of small lipophilic molecules through BBB (Reese and Karnovsky 1967). In addition to the above physical barriers, the expression of several ion channels as well as the influx/efflux transporters at the BBB sites play a pivotal function in restricting the permeability of several molecules. The roles of these specific transporters are discussed later in this review.
Blood-Brain Tumor Barrier (BBTB)
BBTB is the barrier between the brain tumor and capillary vessels (Fig. 2b). BBTB prevents the delivery of most hydrophilic molecules and antitumor agents to brain tumor site. It is formed when BBB is damaged after the volume of the tumor reaches a certain level (> 0.2 mm3) (Liu and Lu 2012). At the early stage of malignant brain tumor, such as glioma or small brain metastasis, the growth of tumor cells depends on normal brain vascular systems before the formation of tumor neovessels, while the BBB remains intact. With the deterioration of brain tumor, tumor cells begin to invade the surrounding normal brain tissues. The volume of the tumor cell cluster must be large enough (>0.2 mm3) to damage the BBB and allow the formation of BBTB.
In low-grade gliomas, the function of the BBTB is similar to that of the BBB under normal conditions. However, high-grade gliomas are characterized by alterations of the normal vascular function resulting in leaky BBTB (Squire et al. 2001). In spite of that, the level of this disruption is not sufficient to allow the therapeutic quantity of drugs, and thus, BBTB remains an obstacle for brain-targeted drug delivery. As the tumor invades the surrounding normal brain tissues, astrocyte feet are displaced from the endothelial cells by tumor cells. The capillary endothelial cells are not able to maintain their BBB phenotype due to the lack of biochemical factors secreted by astrocyte end-feet, which leads to the breaching of the BBB (Watkins et al. 2014). As a result, the BBTB capillary vessels become continuous fenestrated micro-vessels, with variable permeability and pore size during the progress of tumors (Squire et al. 2001). Although, in the majority of cases, BBTB prevents the transport of hydrophilic molecules to the brain tumor. Nonetheless, the evaluation of BBTB targeted drug delivery holds hopes for the treatment of the brain tumor.
Drug Transport Mechanisms of BBB for Brain Tumor Targeting
The transport mechanisms at the BBB can be divided into three major categories: (i) passive diffusion; (ii) transporters-mediated transcytosis (TMT); (iii) fluid phase transport by vesicles, including receptor-mediated endocytosis (RME), and adsorptive mediated transcytosis (AMT) (Laschinger and Engelhardt 2000; Gabathuler 2010; Zhang et al. 2016b).
Passive Diffusion
Passive diffusion is a concentration gradient dependent process that allows molecules to move across cell membranes. The BBB allows the passage of water (H2O), oxygen (O2), carbon dioxide (CO2), and lipid-soluble small molecules by passive diffusion (Mikitsh and Chacko 2014) (Fig. 3a). Drugs that passively diffuse through the BBB are generally lipophilic small molecules with MW <500 Da, log P 1 to 5, number of hydrogen bond donors <5, and the number of hydrogen bond acceptors <10 (Lipinski 2004; Wager et al. 2010; Ghose et al. 2012). However, nearly all large molecules (MW >1 kDa), do not cross the BBB through passive diffusion (Pardridge 2005). The essential substances that the brain needs for metabolism and survival are glucose, insulin, growth hormone, low-density lipoproteins (LDLs), etc. These molecules can be recognized by specific receptors or transport mechanisms, resulting in their specific transport into the brain. A novel magnetic/ultrasound system was developed to enhance the passive transport of 1,2-bis(2-chloroethyl)-1-nitrosourea to rodent glioma by transient disruption of the BBB (Chen et al. 2010). The system was magnetically stimulated to enhance the delivery of drug to brain tumors. The suppression of tumor progression was verified by MRI and histological examination.
Transporters-Mediated Transcytosis (TMT)
TMT is extensively reviewed for peptides and small molecule drugs (Gabathuler 2010; Wei et al. 2014; Bhowmik et al. 2015). TMT mechanism uses the specific transporters expressed on the luminal and the basolateral side of the endothelial cells forming the BBB to cross into the brain. In this approach, the carrier facilitates the transport of molecules by influx and efflux transporters, which transport substances in and out of the CNS, respectively.
The influx transporters allow the entrance of essential endogenous substances including glucose, amino acids, nucleosides, vitamins, monocarboxylic acids, and purine/pyrimidine bases (Mikitsh and Chacko 2014; Bhowmik et al. 2015). These transporters, such as facilitated transporters, and ion-coupled transporters, generally ferry solutes without the help of ATP hydrolysis, therefore, they are also called solute carriers SLCs (Ohtsuki and Terasaki 2007; Gabathuler 2010; Bhowmik et al. 2015). SLC plays critical roles in various cellular physiological processes, such as importing/exporting nutrients, neurotransmitters, and metabolites. In the BBB, most of these transporters regulate the transport of anticancer drugs by hindering their entry into the tumor. However, the electrochemical or concentration gradients of solutes are essentially required for this type of transportation. SLCs can further be categorized as nucleoside, organic anion/cation, monocarboxylate and proton-coupled oligopeptide transporters.
Efflux transporters play an important role in maintaining the homeostasis of the brain. However, a large number of anticancer drugs that are able to cross the BBB are removed out of the CNS due to the extensive activity of the efflux transporter. Efflux transporters belong to the super-family named ATP-binding cassette (ABC). ABC transporters utilize the energy of ATP binding and hydrolysis to transport various substrates across cellular membranes from the CNS to blood circulation against the concentration gradient. The ABC efflux transporters include P-glycoprotein (P-gp), multidrug resistance-related proteins (MRP), and breast cancer resistance protein (BCRP) expressed at the apical side of the BBB (Ohtsuki and Terasaki 2007; Gabathuler 2010; Bhowmik et al. 2015). This process is one of the well-known underlying mechanisms of CNS anticancer drug resistance. BCRP also limits the BBB permeability of various substrates in the same way as P-gp. The MRPs are efflux pumps capable of transporting lipophilic anions (Ohtsuki and Terasaki 2007; Gabathuler 2010; Bhowmik et al. 2015).
These ABC transporters show broad substrate specificity and collectively impede brain uptake of lipophilic molecules, potentially toxic metabolites, and drugs (Bhowmik et al. 2015). Hence, enhanced drug uptake can be achieved by blocking these efflux transporters. Du et al. incorporated tamoxifen into the lipid bilayer membrane of the liposome. Tamoxifen was able to inhibit efflux of MRP in the brain tumor and BBB. As a result, the overall survival of the brain tumor-bearing rats was significantly improved (Du et al. 2009). Utilizing the TMT in drug delivery systems to the brain is a promising brain targeting strategy. However, since the TMT is substrate selective, only drugs that closely mimic the endogenous carrier substrates will be taken up and transported into the brain. Moreover, multiple factors affecting the transport mechanism must be considered. Those include the transport kinetics of molecules, the structural binding requirements of the transporter and the therapeutic compound manipulation so that the compound could bind and remain active in vivo.
Fluid Phase Transport by Vesicles
Fluid phase transport by vesicles, including adsorptive mediated transcytosis (AMT), and receptor-mediated endocytosis (RME) mainly involves three steps: endocytosis at the luminal (blood) side, intracellular movement, and exocytosis at the abluminal (brain) side (Laschinger and Engelhardt 2000; Liu and Lu 2012). A targeting molecule is bound to the membrane of the brain capillary endothelial cells due to the anionic charge or its interaction with specific receptors through AMT and RME mechanisms, respectively. Both RME and AMT allow the transport of large molecules; therefore, they are potentially useful for the delivery of anticancer biologics into the CNS (Begley and Brightman 2003; Pardridge 2003).
Adsorptive-Mediated Transcytosis (AMT)
AMT is triggered by nonspecific electrostatic interactions between the cationic delivery system and the anionic micro domains on the brain capillary endothelial cell membrane (Herve et al. 2008; Wei et al. 2014). AMT is mainly achieved using the cationic polymers, such as chitosan and gelatin, since most of these polymers possess amine groups in their polymer backbone and/or in their side chains (Upadhyay 2014). The cell-penetrating peptides (CCPs) made of cationic peptides (< 30 amino acids), capable of penetrating cell membrane and transporting the substances into cells. (Herve et al. 2008). According to their origin or sequences characteristics, CPPs can be divided into subgroups (Koren and Torchilin 2012). TAT, penetratin, and polyarginine belongs to the low amphipathic peptide class (Heitz et al. 2009). The cationic guanidine head group of arginine is able to form hydrogen bonds with the anionic sulfates and phosphates located on the surface of cell membrane. The high amphipathic peptide class CPP including model amphipathic peptide (MAP), Pep-1, and transportan (Heitz et al. 2009; Munyendo et al. 2012) do not have guanidine head group, and the charge contribution originates primarily from lysine residues which have less penetrating capacity. The other class of CPP includes vascular endothelial-cadherin (pVEC) and MPG peptides, where the hydrophobic residues and the charges are separated lengthwise on the chain (Koren and Torchilin 2012).
A novel TAT-modified liposome (TAT-LIP) was developed for overcoming the ineffective delivery of drug formulation to the brain (Qin et al. 2011). The results showed that the majority of TAT-LIP accumulated in the brain within 24 h of their administration and the positive charge of the TAT played an important role in increasing its brain targeting. CPPs have also been successfully employed for gene delivery in the brain. Yao et al., conjugated a cell-penetrating peptide, LIMK2 NoLS peptide (LNP), with dendrigraft poly-l-lysine (DGL) and poly-ethylene glycol (PEG) (Yao et al. 2015a). In addition, plasmid DNA encoding inhibitor of growth 4 (ING4) was used as therapeutic gene for glioma. The conjugate showed an enhanced BBB-crossing efficiency, cellular uptake, gene expression, apoptosis on the tumor site, and median survival time of glioma-bearing mice. Beside the CPP, other positively charged molecules, such as polysorbate 80 and cationic bovine serum albumin were also used to coat delivery systems. Most of these systems showed successful trans-BBB passage (Lu et al. 2006; Jain et al. 2015).
Receptor-Mediated Endocytosis (RME)
RME is considered as one of the most mature strategies and extensively applied for brain-targeted delivery with the characteristics of high specificity, selectivity, and affinity. Its mechanism is based on the interaction of specific targeting ligands with the receptors expressed in brain (Gabathuler 2010; Wei et al. 2014; Zhang et al. 2016b). There are several types of receptors, expressed on the capillary endothelium of the brain, such as transferrin (Tf), low-density lipoprotein (LDL), insulin, and nicotinic acetylcholine (nACh) receptors (Gabathuler 2010).
Tf receptor (TfR) has been found to be one of the most attractive targets for delivering CNS tumor therapies as indicated in recent studies (Daniels et al. 2012; Zong et al. 2014; Dixit et al. 2015a). TfR is a membrane glycoprotein highly expressed on endothelial cells in the BBB and is necessary to import iron in the brain (Recht et al. 1990). Drug targeting to the TfR can be achieved by using its endogenous ligand Tf. As the expression of TfR is regulated in response to intracellular iron, the in vivo application of Tf will be limited. Therefore, utilizing the targeted monoclonal antibody (mAb) against TfR provides better selectivity and specificity (Daniels et al. 2012).
LDL receptor-related protein (LRP) is a multifunctional endocytic receptor that mediates the internalization of multiple ligands involved in diverse metabolic pathways (Demeule et al. 2002; Bell et al. 2007). LRP is highly expressed in many tissues and in the CNS. It interacts with a broad range of secreted proteins and resident cell surface molecules (eq. apoE (apolipoprotein E), α2 M (α2 macroglobulin), tPA (tissue Plasminogen Activator), PAI-1 (Plasminogen Activator Inhibitor 1), APP (Amyloid Precursor Protein), Factor VIII, Lactoferrin), and mediates the endocytosis of multiple ligands across the BBB including lactoferrin (Lf) and melanotransferrin (Demeule et al. 2002; Bell et al. 2007). LRP is also over-expressed in human glioma cells, which makes it a potential targeted moiety for BBB penetration and glioma targeting as well (Maletinska et al. 2000).
Insulin is transported to the brain tissue from the systemic circulation by means of transcytotic mechanism, involving the insulin receptor (IR) present at the vascular endothelial cell surface (Laron 2009). Coloma et al., have extensively documented the use of IR for the targeted delivery of drugs to the brain using specific antibodies directed against the IR (Coloma et al. 2000). Ulbrich et al., conjugated an IR Ab (29B4) with drug-loaded human serum albumin nanoparticles (NPs), that are able to transport loperamide across the BBB in mouse model (Ulbrich et al. 2011). Other receptors, such as endothelial growth factors, and diphtheria toxin receptors are also expressed on the BBB (Uotani et al. 1999; Grapp et al. 2013). Beside the receptors on the brain capillary endothelial cells, various receptors expressed on tumor cells, tumor stem cells, and tumor-invaded endothelial cells are also identified and utilized for brain tumor-targeted drug delivery (Table 1).
Nanobiotechnology Based Strategies for Brain Tumor Targeting
Nanocarriers or nanovehicles are colloidal systems in the nanoscale size range and capable of encapsulating small molecules as well as macromolecule drugs. Nanocarriers provide several advantages, such as drug protection from in vivo/in vitro degradation, reduction of drug clearance and increase of the drug half-life in vivo, enhancement of the drug payload, controlled drug release, improved drug-solubility, and enhancement of the targeted delivery by incorporation of targeting ligands (Youm et al. 2014; Zhang et al. 2015; Karim et al. 2016).
The major properties that govern the in vivo characteristics of the brain targeted nanocarriers are their size, surface charge, and the presence of targeting ligands on their surface (Bhaskar et al. 2010; Yoo et al. 2010; Morachis et al. 2012; Ernsting et al. 2013). The nanocarriers are cleared mainly by the reticuloendothelial system (RES) consisting of phagocytic cells (monocytes and macrophages) which can engulf and remove the nanocarriers from the systemic circulation through the process of opsonization. In general, opsonization process involves the coating of nanocarriers with opsonin proteins, thus, marking them recognized by the immune system for phagocytosis (Owens and Peppas 2006; Sanhai et al. 2008; Riehemann et al. 2009; Agrahari et al. 2016a). The addition of hydrophilic polymers, such as PEG on the surface of nanocarriers causes a steric hindrance to the opsonins and thus, reduces the uptake by the RES system (van Vlerken et al. 2007; Davis et al. 2008; Salmaso and Caliceti 2013). Generally, opsonization and phagocytic uptake of nanocarriers increase with the size of the particles (Karim et al. 2016). The surface charge of the nanocarriers is also a critical parameter that can influence their biodistribution and interactions with RES cells. Commonly, nanocarriers with neutral surface charge have fewer chances to be phagocytosed by the RES system, whereas, nanocarriers with positive or negative surface charges are more prone to be engulfed by phagocytic cells. However, for brain targeted drug delivery systems, cationic nanocarriers are more attractive as they may cross the BBB through AMT. There are several approaches that have been applied to improve the brain tumor targeting of nanocarriers using the RME or AMT mechanisms (Table 1). In the following sub-sections, the advantages and limitations of several brain tumor targeted nanocarrier systems will be discussed (Fig. 4).
Liposomes
Liposomes have been comprehensively used for brain tumor therapeutics (Table 1) due to several advantages, such as their ability to cross the BBB through the inter-endothelial gaps of the highly vascularized leaky BBTB. Liposomes are lipid vesicles in the size range from 0.1 to 10 μm made of amphiphilic phospholipid bilayers surrounding an aqueous core. The aqueous core can encapsulate hydrophilic drugs, whereas hydrophobic drugs can be encapsulated in the phospholipid bilayers. Based on their size and number of phospholipid bilayers, liposomes are classified as small/large unilamellar and multilamellar vesicles. The surfaces of liposomes can be easily modified to make stealth and site-specific formulations (PEGylated liposomes: immunoliposomes) for targeted delivery to the brain tumors (Immordino et al. 2006; Laquintana et al. 2009).
Targeting approaches enable liposomes to cross the intact BBB by means of RME or AMT (Deshpande et al. 2013). Using the PEGylation strategy, in vivo circulation time of liposomes can be significantly extended to days (Immordino et al. 2006). The physiochemical properties of liposomes can be modified by mixing different lipids to control their size, surface charge, and functionalization. Although, liposomes exhibit numerous advantages, they present certain limitations, such as low stability and poor reproducibility in terms of size and lamellarity, low drug loading capacity for poorly soluble drugs, difficulties in sterilization, potential immunotoxicity, and limited control over drug release (Akbarzadeh et al. 2013).
Polymeric NPs
Polymeric NPs are defined as biodegradable colloidal systems in the size range of 10–1000 nm. Therapeutic molecules could be dissolved, encapsulated or chemically conjugated to the nanoparticulate system. Depending on the specific method of preparation, polymeric NPs can be formulated as nanospheres (matrix system, where drug is dispersed throughout the particles) or nanocapsules (reservoir system, where drug is confined to an aqueous or oily core surrounded by a polymeric membrane). Polymeric NPs are considered as one of the most promising systems to deliver the therapeutic drugs across the BBB and treat brain tumors as confirmed in several studies (Table 1). There are several advantages that polymeric NPs can provide. Those include the various routes of administration, flexibility of surface modification by targeting molecules, stimuli-responsive formulation development, encapsulation and delivery of multiple drugs in a single NP with adjustable size, shape, surface functionality. Besides their advantages, polymeric NPs also have several drawbacks, such as burst release of drug that may lead to potential drug toxicity, rapid phagocytic clearance, immunogenicity issues, scale-up of formulations, large surface area that may lead to particle aggregation, and non-uniformity in size distribution.
Polymersomes
Polymersomes are self-assembled vesicles of amphiphilic block copolymers containing hydrophilic and hydrophobic blocks which can effectively encapsulate both hydrophilic and hydrophobic drugs (Krishnamoorthy et al. 2014; Tuguntaev et al. 2016). Compared to the liposomes, polymersomes contain many advantages, such as adjustable amphiphilic polymer molecular weight and ratio, tunable physical and chemical properties, better colloidal/mechanical/storage stability, high drug loading capacity, long blood circulation time, and reduced drug leakage. Their surface functionalization with a targeting ligand can enhance the therapeutic effect by targeting the brain tumor site, thereby, reducing the unwanted toxicity in normal cells. However, the scale-up of polymerosome batch production continues to be a major challenges. Moreover, potential biocompatibility issues of polymersomes regarding long-term administration and presence of toxic residual organic solvents in the final formulation need to be addressed (Anajafi and Mallik 2015).
Nanomicelles
Nanomicelles are self-assembled systems from biodegradable and biocompatible amphiphilic block polymers in the nanoscale size range of ~10–100 nm. They can encapsulate poorly-water soluble drugs in the core and their hydrophilic shell allows the encapsulation of hydrophilic molecules (Oerlemans et al. 2010; Lu and Park 2013). The hydrophilic shell also provides the stability of the nanomicelles and long blood circulation time in vivo. Owing to their small size, nanomicelles can leave the blood circulation at the tumor site via the enhanced permeability and retention (EPR) effect (Biswas et al. 2016). Nanomicelles have wide advantages in drug delivery applications, such as easy and reproducible formulation, sterilization by simple filtration, possibilities of changing polymer block arrangements as per the requirements, and their small size provide longer blood circulation time by evading the mononuclear phagocyte system (MPS) also known as the RES or macrophage system. Therefore, nanomicelles could be used as a potential candidate in the development of brain-targeted delivery systems. Drawbacks of nanomicelles include low stability, premature drug release, immunogenicity, and lack of appropriate methods for formulation scale-up. In addition, they are willing to dissociate, especially at a concentration below their critical micelle concentration (CMC) (Oerlemans et al. 2010; Lu and Park 2013).
Dendrimers
Dendrimers are highly branched three-dimensional synthetic polymeric macromolecules (10–100 nm). Various dendrimers based on polyamidoamine (PAMAM), poly (propylenemine) (PPI), and poly-L-lysine (PLL) have been explored as delivery vehicles. The specific advantages of dendrimers in brain-targeted delivery systems include their uniform size distribution, availability of multiple locations for drug and ligand conjugation, high drug loading capacity, conjugation of multiple molecules at the same time, and high thermodynamic stability (Somani and Dufes 2014; Dwivedi et al. 2016). However, the complexity of formulation development, multistep synthesis, and toxicological issues mainly due to the presence of amino functional groups, limit the applicability of dendrimers in clinics.
Nanogels
Nanogels are basically NPs composed of hydrogels and emerged as a versatile hydrophilic platform for drug delivery applications (Kabanov and Vinogradov 2009; Agrahari et al. 2016b). They are formed by cross-linked swellable polymeric networks with a high water holding capacity. The characteristic properties of nanogels, such as size, surface charge, porosity, softness, and degradability can be modified by varying the chemical composition of the polymers used in their formulation (Kabanov and Vinogradov 2009). They are capable of holding small molecules, macromolecule drugs, and inorganic particles within their cross-linked networks, which allow them to be applied in therapy as well as imaging applications. Because of their hydrophilicity, nanogels are highly biocompatible, their softness and swelling properties allow to achieve a controlled or triggered response at the target site. However, burst release of drug may occur, and probably due to the high water content and soft nature of hydrogels thus causing a relative or substantial loss of the drug in the systemic circulation. Moreover, the complexity of the nanogel systems and their low scalable production, limit their applicability (Hoare and Kohane 2008).
Gold NPs
Gold NPs have the ability to permeate through the brain microvasculature due to their small size (Cheng et al. 2014c). The large surface of the gold NPs allows them to be coated with a variety of ligands and therapeutic agents for targeting approaches. Gold NPs have several characteristics that make them a promising carrier candidate for the drug transport across the BBB. Those include their small size, minimal or no tissue reactivity, low toxicity, and neutral surface charge of modified gold NPs (Shukla et al. 2005; Joh et al. 2013). Moreover, gold NPs have the ability of producing heat, which can kill the tumors in photodynamic therapy (Cabuzu et al. 2015). Despite their advantages, certain questions need to be addressed before their use in clinics. The most critical are: the toxicity and immunogenicity issues, the effect of ligand conjugation on the biodistribution, and consequent side effects (Arvizo et al. 2010).
Carbon Nanotubes (CNTs)
CNTs are graphene sheets rolled into a seamless cylinder that can be open ended or capped, having a high aspect ratio with diameters as small as one nanometer and a length of several micrometers (Ji et al. 2010; Zhang et al. 2011). There are two broad categories of CNTs: single-walled CNTs (SWCNTs) and multi-walled CNTs (MWCNTs). SWCNTs are made of single graphene layer wrapped in a hexagonal close-packed cylindrical structure. Whereas, MWCNTs consist of several coaxial cylinders made of a single graphene sheet surrounding a hollow core (Ji et al. 2010; Zhang et al. 2011). CNTs have the ability to penetrate the BBB like a needle due to their unit structure. CNTs can be covalently or non-covalently functionalized with the targeting ligands. However, CNTs are insoluble in most of the solvents which generate some toxicity and biosafety issues. Nevertheless, chemical modifications may transform them in water-soluble carriers, and reduce their toxicity (Beg et al. 2011; Zhang et al. 2011). In addition, the pharmacokinetic data of CNTs are severely challenged because of their low reproducibility (Beg et al. 2011; Zhang et al. 2011).
Magnetic NPs
Magnetic NPs have gained great attention and are being explored for various applications including the brain tumor targeting and imaging (Wankhede et al. 2012; Mahmoudi and Hadjipanayis 2014; Liu et al. 2016). The ability of magnetic NPs to cross the BBB makes them an attractive system for brain tumor therapy (Wankhede et al. 2012). Magnetic NPs are commonly comprised of a core-shell morphology with an iron oxide core coated with a biocompatible material, such as polysaccharide, polymer, lipid, or protein. They can be systemically administered into the blood stream and targeted by applying an external magnetic field for therapy, imaging or diagnostic applications. The uptake of magnetic NPs by malignant brain tumor cells has been demonstrated in several studies (Table 1). However, the aggregation, instability, non-specific uptake by the RES system, difficulty in penetrating the tissue, as well as the toxicity and potential immunogenicity of magnetic NPs needs to be well determined before their clinical application in brain tumor targeting approaches (Wankhede et al. 2012).
Exosomes
Exosomes are nanosized vesicles with a lipid bilayer membrane surrounding an aqueous inner core. Exosomes can be loaded with both hydrophilic and hydrophobic drug molecules (Braccioli et al. 2014; Katakowski and Chopp 2016). Exosomes are composed of different types of lipids, such as cholesterol, sphingolipids, phosphoglycerides, ceramides, and saturated fatty acid chains. They are naturally produced in body cells and can be found in several body fluids, including blood, saliva, and urine (Keller et al. 2011). They are naturally stable and have inherent targeting properties depending on the composition of the exosomes. The capacity of exosomes to serve as a system to encapsulate nucleic acids, proteins, and lipids, and their role in intercellular communication, make them a versatile platform for drug delivery applications (Johnsen et al. 2014).
Exosomes have many desirable features to become an ideal drug delivery system. Those include long circulating half-life, intrinsic ability to target tissues, minimal off-target effects, higher biocompatibility and minimal or no inherent toxicity issues (when self-derived exosomes are used). One of the significant advantages of these drug delivery vehicles is their ability to cross the BBB. However, the characterization of exosomes of different sources needs further understanding of their transport mechanisms across various barriers. Currently, there is no purification technique available for the isolation of exosomes with high efficiency (Kooijmans et al. 2012; Yang et al. 2015). In addition, the isolation and large scale production of exosomes for clinical studies are costly and remain an area of investigation.
Conclusions and Future Perspectives
Drug delivery to the brain tumor has seen significant progress in recent years and nanocarriers systems have been considered as one of the promising approaches in brain tumor targeted therapy. This is due to the higher stability of nanocarriers in biological fluids, long circulation in vivo and a good bioavailability. In addition, nanocarriers can provide a versatile and easy surface functionalization, homogenous size distributions, high drug loading, flexibility in drug release characteristics (stimuli-responsive or controlled), and a possibility of co-encapsulation of more than one anticancer drug in one system. Although, nanocarrier drug delivery systems are very promising for the treatment of brain cancers, no nanocarriers with active targeting have been approved by the U.S. Food and Drug Administration (FDA). The clinical failure of delivery systems is mainly due to the presence of BBB and BBTB barriers. In addition to these barriers, there are numerous other challenges that need to be solved, such as the low therapeutic efficiency of nanocarriers inside the tumor, specificity of targeting ligands to deliver them at the diseased site, and release of drugs in a controlled or stimuli-responsive manner.
The physicochemical parameters (size, shape, surface charge, and functionality) of nanocarriers play an important role in their in vivo therapeutic efficacy. Thus, the screening of physicochemical parameters of nanocarriers must be performed to develop a pharmaceutical formulation that has a better therapeutic efficacy. Currently, the design of experiment approach is a valuable tool for the pre-formulation screening design and the optimization of the process and formulation parameters of the nanocarriers system Zhang et al. 2013; Agrahari et al. 2014; Youm et al. 2014; Meng et al. 2014). This initial screening is very important to identify an optimized system that may provide a potential way for an efficient CNS delivery of anticancer therapeutics. Although, there are promising but posing challenges to develop a successful brain tumor targeted system, the nanotechnology approach may provide a way to develop a system that can be efficiently targeted to the brain tumor sites.
In addition to the above, developing a stimuli-responsive nanocarrier system is an attractive area in drug delivery research (Mura et al. 2013; Torchilin 2014). The concept of stimuli-responsive systems are widely used for anticancer therapy and other diseases (Cheng et al. 2013, 2014a; Maya et al. 2013; Agrahari et al. 2014; Bagherifam et al. 2015). These novel systems are able to respond to their environment and enhance/trigger the release of therapeutic molecules within a particular site of interest. However, nothing much has been explored in the stimuli-responsive systems intended for brain-tumor target drug delivery. There are a number of triggering signals (pH, temperature, enzymes, oxidative stress, magnetic field, etc.) that can be used as a stimulus in cancer therapeutics. The intrinsic environmental differences in brain tumor sites (compared to normal cells), such as low pH and differences in the enzyme and glutathione (GSH) levels, can be utilized to develop a stimuli-responsive system (Fang Liu et al. 2016) that will be able to enhance the overall efficacy of the nanocarriers to the brain tumor. External stimuli, such as magnetic field, light, and heat are also promising options to be applied to control the release of therapeutic molecules in a spatial and temporal manner. In a recent publication, a theranostic agent for brain tumor MRI, paramagnetic, pH and temperature-sensitive polymeric particles (PPPs) with a high drug release rate have been synthesized. The results showed that the PPPs have potential in diagnosing and treating glioma (Ruiqing Liu et al. 2015).
The incorporation of nano-, bio-, theranostic and imaging technologies in drug delivery systems (multi-functional nanocarriers) has shown great promises for the diagnosis and therapy of brain tumor (He et al. 2013; Meyers et al. 2013; Cheng et al. 2014b; Yao et al. 2015b). These systems can be used to visualize cancer cells through in vivo imaging techniques, and monitor the effects of the drug-loaded nanocarrier treatment in real time. However, potential neurotoxicity and related systemic toxicity of such particles should be evaluated in clinics. Moreover, the long-term distribution, biodegradation, and the elimination mechanism of these particles require further investigation. Despite numerous challenges, these nanobiotechnology based particles could lead to exciting breakthroughs in brain tumor therapeutics.
Overall, in this review, a brief overview of targeting approaches and development of nanocarrier systems for brain tumor has been provided. The nanobiotechnological drug delivery methods provide exciting opportunities combining the targeting, therapeutic and imaging agents together and create innovative nanomedicines for brain tumor-targeted therapy. Further studies are warranted to explore the promising application of nanobiotechnology in tumor diagnosis at earlier stage, as well as the monitoring of brain tumors over the duration of a treatment regime. This will facilitate the development of effective medicine for curing patients with brain tumors.
References
Abakumov MA, Nukolova NV, Sokolsky-Papkov M, et al. (2015) VEGF-targeted magnetic nanoparticles for MRI visualization of brain tumor. Nanomedicine 11:825–833
Abbott NJ (2002) Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat 200:629–638
Agrahari V, Zhang C, Zhang T, et al. (2014) Hyaluronidase-sensitive nanoparticle templates for triggered release of HIV/AIDS microbicide in vitro. AAPS J 16:181–193
Agrahari V, Agrahari V, Mitra AK (2016a) Nanocarrier fabrication and macromolecule drug delivery: challenges and opportunities. Ther Deliv 7:257–278
Agrahari V, Agrahari V, Hung WT, et al. (2016b) Composite nanoformulation therapeutics for long-term ocular delivery of macromolecules. Mol Pharm. doi:10.1021/acs.molpharmaceut.5b00828
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, et al. (2013) Liposome: classification, preparation, and applications. Nanoscale Res Lett 8:102
Anajafi T, Mallik S (2015) Polymersome-based drug-delivery strategies for cancer therapeutics. Ther Deliv 6:521–534
Arvizo R, Bhattacharya R, Mukherjee P (2010) Gold nanoparticles: opportunities and challenges in nanomedicine. Expert Opin Drug Deliv 7:753–763
Azad TD, Pan J, Connolly ID, et al. (2015) Therapeutic strategies to improve drug delivery across the blood-brain barrier. Neurosurg Focus 38:E9
Bagherifam S, Skjeldal FM, Griffiths G, et al. (2015) pH-responsive nano carriers for doxorubicin delivery. Pharm Res 32:1249–1263
Bai CZ, Choi S, Nam K, et al. (2013) Arginine modified PAMAM dendrimer for interferon beta gene delivery to malignant glioma. Int J Pharm 445:79–87
Baklaushev VP, Nukolova NN, Khalansky AS, et al. (2015) Treatment of glioma by cisplatin-loaded nanogels conjugated with monoclonal antibodies against Cx43 and BSAT1. Drug Deliv 22:276–285
Beg S, Rizwan M, Sheikh AM, et al. (2011) Advancement in carbon nanotubes: basics, biomedical applications and toxicity. J Pharm Pharmacol 63:141–163
Begley DJ, Brightman MW (2003) Structural and functional aspects of the blood-brain barrier. Prog Drug Res 61:39–78
Bell RD, Sagare AP, Friedman AE, et al. (2007) Transport pathways for clearance of human Alzheimer's amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab 27:909–918
Bhaskar S, Tian F, Stoeger T, et al. (2010) Multifunctional Nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: perspectives on tracking and neuroimaging. Part Fibre Toxicol 7:3
Bhowmik A, Khan R, Ghosh MK (2015) Blood brain barrier: a challenge for effectual therapy of brain tumors. Biomed Res Int 2015:320941
Biswas S, Kumari P, Lakhani PM, et al. (2016) Recent advances in polymeric micelles for anti-cancer drug delivery. Eur J Pharm Sci 83:184–202
Braccioli L, van Velthoven C, Heijnen CJ (2014) Exosomes: a new weapon to treat the central nervous system. Mol Neurobiol 49:113–119
Butt AM, Jones HC, Abbott NJ (1990) Electrical resistance across the blood-brain barrier in anaesthetized rats: a developmental study. J Physiol 429:47–62
Byeon HJ, Thao le Q, Lee S, et al. (2016) Doxorubicin-loaded nanoparticles consisted of cationic- and mannose-modified-albumins for dual-targeting in brain tumors. J Control Release 225:301–313
Cabuzu D, Cirja A, Puiu R, et al. (2015) Biomedical applications of gold nanoparticles. Curr Top Med Chem 15:1605–1613
Chacko AM, Li C, Pryma DA, et al. (2013) Targeted delivery of antibody-based therapeutic and imaging agents to CNS tumors: crossing the blood-brain barrier divide. Expert Opin Drug Deliv 10:907–926
Chen PY, Liu HL, Hua MY, et al. (2010) Novel magnetic/ultrasound focusing system enhances nanoparticle drug delivery for glioma treatment. Neuro-Oncology 10:1050–1060
Chen YC, Chiang CF, Chen LF, et al. (2014) Polymersomes conjugated with des-octanoyl ghrelin and folate as a BBB-penetrating cancer cell-targeting delivery system. Biomaterials 35:4066–4081
Chen B, He XY, Yi XQ, et al. (2015) Dual-peptide-functionalized albumin-based nanoparticles with ph-dependent self-assembly behavior for drug delivery. ACS Appl Mater Interfaces 7:15148–15153
Cheng R, Meng F, Deng C, et al. (2013) Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 34:3647–3657
Cheng W, Gu L, Ren W, et al. (2014a) Stimuli-responsive polymers for anti-cancer drug delivery. Mater Sci Eng C Mater Biol Appl 45:600–608
Cheng Y, Morshed RA, Auffinger B, et al. (2014b) Multifunctional nanoparticles for brain tumor imaging and therapy. Adv Drug Deliv Rev 66:42–57
Cheng Y, Dai Q, Morshed RA, et al. (2014c) Blood-brain barrier permeable gold nanoparticles: an efficient delivery platform for enhanced malignant glioma therapy and imaging. Small 10:5137–5150
Chowdhury SM, Surhland C, Sanchez Z, et al. (2015) Graphene nanoribbons as a drug delivery agent for lucanthone mediated therapy of glioblastoma multiforme. Nanomedicine 11:109–118
Coloma MJ, Lee HJ, Kurihara A, et al. (2000) Transport across the primate blood-brain barrier of a genetically engineered chimeric monoclonal antibody to the human insulin receptor. Pharm Res 17:266–274
Cui Y, Xu Q, Chow PK, et al. (2013) Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials 34:8511–8520
Daniels TR, Bernabeu E, Rodriguez JA, et al. (2012) The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim Biophys Acta 1820:291–317
Davis ME, Chen ZG, Shin DM (2008) Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov 7:771–782
Demeule M, Poirier J, Jodoin J, et al. (2002) High transcytosis of melanotransferrin (P97) across the blood-brain barrier. J Neurochem 83:924–933
Deshpande PP, Biswas S, Torchilin VP (2013) Current trends in the use of liposomes for tumor targeting. Nanomedicine (London) 8:1509–1528
Dixit S, Novak T, Miller K, et al. (2015a) Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale 7:1782–1790
Dixit S, Miller K, Zhu Y, et al. (2015b) Dual receptor-targeted theranostic nanoparticles for localized delivery and activation of photodynamic therapy drug in glioblastomas. Mol Pharm 12:3250–3260
Du J, Lu WL, Ying X, et al. (2009) Dual-targeting topotecan liposomes modified with tamoxifen and wheat germ agglutinin significantly improve drug transport across the blood-brain barrier and survival of brain tumor-bearing animals. Mol Pharm 6:905–917
Dwivedi N, Shah J, Mishra V, et al. (2016) Dendrimer-mediated approaches for the treatment of brain tumor. J Biomater Sci Polym Ed 27:557–580
Engin K, Leeper DB, Cater JR, et al. (1995) Extracellular pH distribution in human tumours. Int J Hyperth : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group 11:211–216
Ernsting MJ, Murakami M, Roy A, et al. (2013) Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J Control Release : official journal of the Controlled Release Society 172:782–794
Fang Liu XL, Li-Yuan Z, Qing-Ru S, et al. (2016) Stimuli-responsive nanocarriers for drug delivery to the central nervous system. Curr Nanosci 12:14
Fang JH, Lai YH, Chiu TL, et al. (2014) Magnetic core-shell nanocapsules with dual-targeting capabilities and co-delivery of multiple drugs to treat brain gliomas. Adv Healthcare Mater 3:1250–1260
Fang JH, Chiu TL, Huang WC, et al. (2016) Dual-targeting lactoferrin-conjugated polymerized magnetic polydiacetylene-assembled nanocarriers with self-responsive fluorescence/magnetic resonance imaging for in vivo brain tumor therapy. Adv Healthcare Mater 5:688–695
Gabathuler R (2010) Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol Dis 37:48–57
Gao HL, Pang ZQ, Fan L, et al. (2010) Effect of lactoferrin- and transferrin-conjugated polymersomes in brain targeting: in vitro and in vivo evaluations. Acta Pharmacol Sin 31:237–243
Gao JQ, Lv Q, Li LM, et al. (2013) Glioma targeting and blood-brain barrier penetration by dual-targeting doxorubincin liposomes. Biomaterials 34:5628–5639
Ghose AK, Herbertz T, Hudkins RL, et al. (2012) Knowledge-based, central nervous system (CNS) lead selection and lead optimization for CNS drug discovery. ACS Chem Neurosci 3:50–68
Grapp M, Wrede A, Schweizer M, et al. (2013) Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat Commun 4:2123
Gu G, Hu Q, Feng X, et al. (2014) PEG-PLA nanoparticles modified with APTEDB peptide for enhanced anti-angiogenic and anti-glioma therapy. Biomaterials 35:8215–8226
Guo J, Gao X, Su L, et al. (2011) Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials 32:8010–8020
He H, Li Y, Jia XR, et al. (2011) PEGylated Poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials 32:478–487
He H, David A, Chertok B, et al. (2013) Magnetic nanoparticles for tumor imaging and therapy: a so-called theranostic system. Pharm Res 30:2445–2458
Heitz F, Morris MC, Divita G (2009) Twenty years of cell-penetrating peptides: from molecular mechanisms to therapeutics. Br J Pharmacol 157:195–206
Herve F, Ghinea N, Scherrmann JM (2008) CNS delivery via adsorptive transcytosis. AAPS J 10:455–472
Hoare TR, Kohane DS (2008) Hydrogels in drug delivery: progress and challenges. Polymer 49:1993–2007
Huang R, Ke W, Han L, et al. (2011a) Targeted delivery of chlorotoxin-modified DNA-loaded nanoparticles to glioma via intravenous administration. Biomaterials 32:2399–2406
Huang S, Li J, Han L, et al. (2011b) Dual targeting effect of Angiopep-2-modified, DNA-loaded nanoparticles for glioma. Biomaterials 32:6832–6838
Immordino ML, Dosio F, Cattel L (2006) Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine 1:297–315
Jain A, Jain A, Garg NK, Tyagi RK, et al. (2015) Surface engineered polymeric nanocarriers mediate the delivery of transferrin-methotrexate conjugates for an improved understanding of brain cancer. Acta Biomater 24:140–151
Ji SR, Liu C, Zhang B, et al. (2010) Carbon nanotubes in cancer diagnosis and therapy. Biochim Biophys Acta 1806:29–35
Jiang W, Xie H, Ghoorah D, et al. (2012) Conjugation of functionalized SPIONs with transferrin for targeting and imaging brain glial tumors in rat model. PLoS One 7:e37376
Jiang L, Zhou Q, Mu K, et al. (2013) pH/temperature sensitive magnetic nanogels conjugated with Cy5.5-labled lactoferrin for MR and fluorescence imaging of glioma in rats. Biomaterials 34:7418–7428
Jiang X, Xin H, Ren Q, et al. (2014) Nanoparticles of 2-deoxy-D-glucose functionalized poly(ethylene glycol)-co-poly(trimethylene carbonate) for dual-targeted drug delivery in glioma treatment. Biomaterials 35:518–529
Joh DY, Sun L, Stangl M, et al. (2013) Selective targeting of brain tumors with gold nanoparticle-induced radiosensitization. PLoS One 8:e62425
Johnsen KB, Gudbergsson JM, et al. (2014) A comprehensive overview of exosomes as drug delivery vehicles - endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta 1846:75–87
Kabanov AV, Vinogradov SV (2009) Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed Eng 48:5418–5429
Kafa H, Wang JT, Rubio N, et al. (2016) Translocation of LRP1 targeted carbon nanotubes of different diameters across the blood-brain barrier in vitro and in vivo. J Control Release 225:217–229
Karim R, Palazzo C, Evrard B, et al. (2016) Nanocarriers for the treatment of glioblastoma multiforme: Current state-of-the-art. J Control Release 227:23–37
Katakowski M, Chopp M (2016) Exosomes as tools to suppress primary brain tumor. Cell Mol Neurobiol 36:343–352
Keller S, Ridinger J, Rupp AK, et al. (2011) Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med 9:86
Kooijmans SA, Vader P, van Dommelen SM, et al. (2012) Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine 7:1525–1541
Koren E, Torchilin VP (2012) Cell-penetrating peptides: breaking through to the other side. Trends Mol Med 18:385–393
Krishnamoorthy B, Karanam V, Chellan VR, et al. (2014) Polymersomes as an effective drug delivery system for glioma--a review. J Drug Target 22:469–477
Kuang Y, An S, Guo Y, et al. (2013) T7 peptide-functionalized nanoparticles utilizing RNA interference for glioma dual targeting. Int J Pharm 454:11–20
Kuo YC, Lee CH (2016) Dual targeting of solid lipid nanoparticles grafted with 83-14 MAb and anti-EGF receptor for malignant brain tumor therapy. Life Sci 146:222–231
Laquintana V, Trapani A, Denora N, et al. (2009) New strategies to deliver anticancer drugs to brain tumors. Expert Opin Drug Deliv 6:1017–1032
Laron Z (2009) Insulin and the brain. Arch Physiol Biochem 115:112–116
Laschinger M, Engelhardt B (2000) Interaction of alpha4-integrin with VCAM-1 is involved in adhesion of encephalitogenic T cell blasts to brain endothelium but not in their transendothelial migration in vitro. J Neuroimmunol 102:32–43
Li Y, He H, Jia X, et al. (2012) A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials 33:3899–3908
Li AJ, Zheng YH, Liu GD, et al. (2015) Efficient delivery of docetaxel for the treatment of brain tumors by cyclic RGD-tagged polymeric micelles. Mol Med Rep 11:3078–3086
Li L, Di X, Zhang S, et al. (2016) Large amino acid transporter 1 mediated glutamate modified docetaxel-loaded liposomes for glioma targeting. Colloids Surf B: Biointerfaces 141:260–267
Lipinski CA (2004) Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol 1:337–341
Liu Y, Lu W (2012) Recent advances in brain tumor-targeted nano-drug delivery systems. Expert Opin Drug Deliv 9:671–686
Liu Y, Ran R, Chen J, et al. (2014) Paclitaxel loaded liposomes decorated with a multifunctional tandem peptide for glioma targeting. Biomaterials 35:4835–4847
Liu Y, Mei L, Yu Q, et al. (2015) Multifunctional tandem peptide modified paclitaxel-loaded liposomes for the treatment of vasculogenic mimicry and cancer stem cells in malignant glioma. ACS Appl Mater Interfaces 7:16792–16801
Liu H, Zhang J, Chen X, et al. (2016) Application of iron oxide nanoparticles in glioma imaging and therapy: from bench to bedside. Nanoscale 8:7808–7826
Locatelli E, Naddaka M, Uboldi C, et al. (2014) Targeted delivery of silver nanoparticles and alisertib: in vitro and in vivo synergistic effect against glioblastoma. Nanomedicine (London) 9:839–849
Lu Y, Park K (2013) Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int J Pharm 453:198–214
Lu W, Sun Q, Wan J, et al. (2006) Cationic albumin-conjugated pegylated nanoparticles allow gene delivery into brain tumors via intravenous administration. Cancer Res 66:11878–11887
Lu YJ, Wei KC, Ma CC, et al. (2012) Dual targeted delivery of doxorubicin to cancer cells using folate-conjugated magnetic multi-walled carbon nanotubes. Colloids Surf B: Biointerfaces 89:1–9
Mahmoudi K, Hadjipanayis CG (2014) The application of magnetic nanoparticles for the treatment of brain tumors. Front Chem 2:109
Maletinska L, Blakely EA, Bjornstad KA, et al. (2000) Human glioblastoma cell lines: levels of low-density lipoprotein receptor and low-density lipoprotein receptor-related protein. Cancer Res 60:2300–2303
Maya S, Sarmento B, Nair A, et al. (2013) Smart stimuli sensitive nanogels in cancer drug delivery and imaging: a review. Curr Pharm Des 19:7203–7218
Meng J, Zhang T, Agrahari V, et al. (2014) Comparative biophysical properties of tenofovir-loaded, thiolated and nonthiolated chitosan nanoparticles intended for HIV prevention. Nanomedicine (London) 9:1595–1612
Meyers JD, Doane T, Burda C, et al. (2013) Nanoparticles for imaging and treating brain cancer. Nanomedicine (London) 8:123–143
Meyers JD, Cheng Y, Broome AM, et al. (2015) Peptide-targeted gold nanoparticles for photodynamic therapy of brain cancer. Part Part Syst Charact 32:448–457
Mikitsh JL, Chacko AM (2014) Pathways for small molecule delivery to the central nervous system across the blood-brain barrier. Perspect Medicin Chem 6:11–24
Mitra AK, Agrahari V, Mandal A, et al. (2015) Novel delivery approaches for cancer therapeutics. J Control Release 219:248–268
Miura Y, Takenaka T, Toh K, et al. (2013) Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood-brain tumor barrier. ACS Nano 7:8583–8592
Morachis JM, Mahmoud EA, Almutairi A (2012) Physical and chemical strategies for therapeutic delivery by using polymeric nanoparticles. Pharmacol Rev 64:505–519
Mu K, Zhang S, Ai T, et al. (2015) Monoclonal antibody-conjugated superparamagnetic iron oxide nanoparticles for imaging of epidermal growth factor receptor-targeted cells and gliomas. Mol Imaging 14:1–12.
Munyendo WL, Lv H, Benza-Ingoula H, et al. (2012) Cell penetrating peptides in the delivery of biopharmaceuticals. Biomolecules 2:187–202
Mura S, Nicolas J, Couvreur P (2013) Stimuli-responsive nanocarriers for drug delivery. Nat Mater 12:991–1003
Ni D, Zhang J, Bu W, et al. (2014) Dual-targeting upconversion nanoprobes across the blood-brain barrier for magnetic resonance/fluorescence imaging of intracranial glioblastoma. ACS Nano 8:1231–1242
Nishida N, Yano H, Nishida T, et al. (2006) Angiogenesis in cancer. Vasc Health Risk Manag 2:213–219
Niu J, Wang A, Ke Z, et al. (2014) Glucose transporter and folic acid receptor-mediated Pluronic P105 polymeric micelles loaded with doxorubicin for brain tumor treating. J Drug Target 22:712–723
Oerlemans C, Bult W, Bos M, et al. (2010) Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res 27:2569–2589
Ohgaki H, Kleihues P (2013) The definition of primary and secondary glioblastoma. Clin Cancer Res 19:764–772
Ohtsuki S, Terasaki T (2007) Contribution of carrier-mediated transport systems to the blood-brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm Res 24:1745–1758
Oldendorf WH, Cornford ME, Brown WJ (1977) The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann Neurol 1:409–417
Ostrom QT, Gittleman H, de Blank PM, et al. (2016) American brain tumor association adolescent and young adult primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro-Oncology 18(Suppl 1):i1–i50
Owens DE 3rd, Peppas NA (2006) Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm 307:93–102
Pang Z, Feng L, Hua R, et al. (2010) Lactoferrin-conjugated biodegradable polymersome holding doxorubicin and tetrandrine for chemotherapy of glioma rats. Mol Pharm 7:1995–2005
Pang Z, Gao H, Yu Y, et al. (2011) Enhanced intracellular delivery and chemotherapy for glioma rats by transferrin-conjugated biodegradable polymersomes loaded with doxorubicin. Bioconjug Chem 22:1171–1180
Pardridge WM (2003) Blood-brain barrier drug targeting: the future of brain drug development. Mol Interv 3:90–105, 151
Pardridge WM (2005) The blood-brain barrier: bottleneck in brain drug development. NeuroRx 2:3–14
Persidsky Y, Ramirez SH, Haorah J, et al. (2006) Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J NeuroImmune Pharmacol 1:223–236
Qin Y, Chen H, Yuan W, et al. (2011) Liposome formulated with TAT-modified cholesterol for enhancing the brain delivery. Int J Pharm 419:85–95
Recht L, Torres CO, Smith TW, et al. (1990) Transferrin receptor in normal and neoplastic brain tissue: implications for brain-tumor immunotherapy. J Neurosurg 72:941–945
Reese TS, Karnovsky MJ (1967) Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol 34:207–217
Ren J, Shen S, Wang D, et al. (2012) The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials 33:3324–3333
Riehemann K, Schneider SW, Luger TA, et al. (2009) Nanomedicine--challenge and perspectives. Angew Chem 48:872–897
Ronaldson PT, Davis TP (2012) Blood-brain barrier integrity and glial support: mechanisms that can be targeted for novel therapeutic approaches in stroke. Curr Pharm Des 18:3624–3644
Ruan S, Yuan M, Zhang L, et al. (2015a) Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials 37:425–435
Ruan S, Chen J, Cun X, et al. (2015b) Noninvasive in vivo diagnosis of brain glioma using rgd-decorated fluorescent carbonaceous nanospheres. J Biomed Nanotechnol 11:2148–2157
Ruiqing Liu SL, Cun J, Xin W, et al. (2015) Paramagnetic, pH and temperature-sensitive polymeric particles for anticancer drug delivery and brain tumor magnetic resonance imaging. RSC Adv 5:9
Salmaso S, Caliceti P (2013) Stealth properties to improve therapeutic efficacy of drug nanocarriers. J Drug Deliv 2013:374252
Sanhai WR, Sakamoto JH, Canady R, et al. (2008) Seven challenges for nanomedicine. Nat Nanotechnol 3:242–244
Shevtsov MA, Nikolaev BP, Yakovleva LY, et al. (2014) Superparamagnetic iron oxide nanoparticles conjugated with epidermal growth factor (SPION-EGF) for targeting brain tumors. Int J Nanomedicine 9:273–287
Shevtsov MA, Nikolaev BP, Yakovleva LY, et al. (2015) Recombinant interleukin-1 receptor antagonist conjugated to superparamagnetic iron oxide nanoparticles for theranostic targeting of experimental glioblastoma. Neoplasia 17:32–42
Shukla R, Bansal V, Chaudhary M, et al. (2005) Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: a microscopic overview. Langmuir 21:10644–10654
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30
Somani S, Dufes C (2014) Applications of dendrimers for brain delivery and cancer therapy. Nanomedicine (London) 9:2403–2414
Somani S, Blatchford DR, Millington O, et al. (2014) Transferrin-bearing polypropylenimine dendrimer for targeted gene delivery to the brain. J Control Release 188:78–86
Squire JM, Chew M, Nneji G, et al. (2001) Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J Struct Biol 136:239–255
Torchilin VP (2014) Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov 13:813–827
Tuguntaev RG, Okeke CI, Xu J, et al. (2016) Nanoscale polymersomes as anti-cancer drug carriers applied for pharmaceutical delivery. Curr Pharm Des 22:2857–2865
Ulbrich K, Knobloch T, Kreuter J (2011) Targeting the insulin receptor: nanoparticles for drug delivery across the blood-brain barrier (BBB). J Drug Target 19:125–132
Uotani S, Bjorbaek C, Tornoe J, et al. (1999) Functional properties of leptin receptor isoforms: internalization and degradation of leptin and ligand-induced receptor downregulation. Diabetes 48:279–286
Upadhyay RK (2014) Drug delivery systems, CNS protection, and the blood brain barrier. BioMed Res Int 2014:869269
van Tellingen O, Yetkin-Arik B, de Gooijer MC, et al. (2015) Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat 19:1–12
van Vlerken LE, Vyas TK, Amiji MM (2007) Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm Res 24:1405–1414
Wager TT, Chandrasekaran RY, Hou X, et al. (2010) Defining desirable central nervous system drug space through the alignment of molecular properties, in vitro ADME, and safety attributes. ACS Chem Neurosci 1:420–434
Wang F, Zhang W, Shen Y, et al. (2015) Efficient RNA delivery by integrin-targeted glutathione responsive polyethyleneimine capped gold nanorods. Acta Biomater 23:136–146
Wankhede M, Bouras A, Kaluzova M, et al. (2012) Magnetic nanoparticles: an emerging technology for malignant brain tumor imaging and therapy. Expert Rev Clin Pharmacol 5:173–186
Watkins S, Robel S, Kimbrough IF, et al. (2014) Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun 5:4196
Wei X, Chen X, Ying M, et al. (2014) Brain tumor-targeted drug delivery strategies. Acta Pharm Sin B 4:193–201
Wei X, Gao J, Zhan C, et al. (2015) Liposome-based glioma targeted drug delivery enabled by stable peptide ligands. J Control Release 218:13–21
Wong HL, Wu XY, Bendayan R (2012) Nanotechnological advances for the delivery of CNS therapeutics. Adv Drug Deliv Rev 64:686–700
Yang Y, Yan Z, Wei D, et al. (2013) Tumor-penetrating peptide functionalization enhances the anti-glioblastoma effect of doxorubicin liposomes. Nanotechnology 24:405101
Yang ZZ, Li JQ, Wang ZZ, et al. (2014) Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials 35:5226–5239
Yang T, Martin P, Fogarty B, et al. (2015) Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio Rerio. Pharm Res 32:2003–2014
Yao H, Wang K, Wang Y, et al. (2015a) Enhanced blood-brain barrier penetration and glioma therapy mediated by a new peptide modified gene delivery system. Biomaterials 37:345–352
Yao J, Hsu CH, Li Z, et al. (2015b) Magnetic resonance nano-theranostics for glioblastoma multiforme. Curr Pharm Des 21:5256–5266
Yoo JW, Chambers E, Mitragotri S (2010) Factors that control the circulation time of nanoparticles in blood: challenges, solutions and future prospects. Curr Pharm Des 16:2298–2307
Youm I, Agrahari V, Murowchick JB, et al. (2014) Uptake and cytotoxicity of docetaxel-loaded hyaluronic acid-grafted oily core nanocapsules in MDA-MB 231 cancer cells. Pharm Res 31:2439–2452
Zhang W, Zhang Z, Zhang Y (2011) The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res Lett 6:555
Zhang P, Hu L, Yin Q, et al. (2012) Transferrin-conjugated polyphosphoester hybrid micelle loading paclitaxel for brain-targeting delivery: synthesis, preparation and in vivo evaluation. J Control Release 159:429–434
Zhang T, Zhang C, Agrahari V, et al. (2013) Spray drying tenofovir loaded mucoadhesive and pH-sensitive microspheres intended for HIV prevention. Antiviral Res 97:334–346
Zhang F, Lin YA, Kannan S, et al. (2015) Targeting specific cells in the brain with nanomedicines for CNS therapies. J Control Release. doi:10.1016/j.jconrel.2015.12.013
Zhang J, Chen N, Wang H, et al. (2016a) Dual-targeting superparamagnetic iron oxide nanoprobes with high and low target density for brain glioma imaging. J Colloid Interface Sci 469:86–92
Zhang TT, Li W, Meng G, et al. (2016b) Strategies for transporting nanoparticles across the blood-brain barrier. Biomater Sci 4:219–229
Zhao YZ, Lin Q, Wong HL, et al. (2016) Glioma-targeted therapy using Cilengitide nanoparticles combined with UTMD enhanced delivery. J Control Release 224:112–125
Zhou Q, Mu K, Jiang L, et al. (2015) Glioma-targeting micelles for optical/magnetic resonance dual-mode imaging. Int J Nanomedicine 10:1805–1818
Zong T, Mei L, Gao H, et al. (2014) Synergistic dual-ligand doxorubicin liposomes improve targeting and therapeutic efficacy of brain glioma in animals. Mol Pharm 11:2346–2357
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing financial interest.
Additional information
Jianing Meng and Vivek Agrahari contributed equally.
Rights and permissions
About this article
Cite this article
Meng, J., Agrahari, V. & Youm, I. Advances in Targeted Drug Delivery Approaches for the Central Nervous System Tumors: The Inspiration of Nanobiotechnology. J Neuroimmune Pharmacol 12, 84–98 (2017). https://doi.org/10.1007/s11481-016-9698-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-016-9698-1