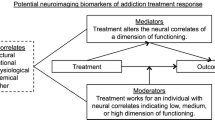
Abstract
Neuroimaging techniques to measure the function and biochemistry of the human brain such as positron emission tomography (PET), proton magnetic resonance spectroscopy (1H MRS), and functional magnetic resonance imaging (fMRI), are powerful tools for assessing neurobiological mechanisms underlying the response to treatments in substance use disorders. Here, we review the neuroimaging literature on pharmacological and behavioral treatment in substance use disorder. We focus on neural effects of medications that reduce craving (e.g., naltrexone, bupropion hydrochloride, baclofen, methadone, varenicline) and that improve cognitive control (e.g., modafinil, N-acetylcysteine), of behavioral treatments for substance use disorders (e.g., cognitive bias modification training, virtual reality, motivational interventions) and neuromodulatory interventions such as neurofeedback and transcranial magnetic stimulation. A consistent finding for the effectiveness of therapeutic interventions identifies the improvement of executive control networks and the dampening of limbic activation, highlighting their values as targets for therapeutic interventions in substance use disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Contents
Abstract
-
1.
Introduction
-
2.
Neuroimaging techniques
-
3.
Neural effects of pharmacological interventions
-
3.1.
Pharmacological treatment studies that aim to reduce drug craving
-
3.2.
Pharmacological treatment studies that aim to increase cognitive control
-
3.1.
-
4.
Neural effects of behavioral interventions
-
4.1.
Cognitive Behavioral Therapy
-
4.2.
Motivational Interventions
-
4.3.
Cue-exposure treatment
-
4.4.
Cognitive bias modification training
-
4.5.
Virtual Reality
-
4.1.
-
5.
Neuromodulatory techniques for treatment
-
5.1.
Neurofeedback
-
5.2.
Transcranial magnetic stimulation
-
5.3.
Transcranial direct current stimulation
-
5.1.
-
6.
Summary and Outlook
Introduction
Addiction is a disease that cycles through steps of intoxication, craving, bingeing, and withdrawal and is mainly characterized by the continuation of drug taking despite awareness of negative consequences (Goldstein and Volkow 2002). Differing models have been presented to account for the shift from controlled use to the cycle of addiction, and to investigate why this only happens in certain individuals. That is, it has been theorized that addiction is a deficit in bottom-up motivational processes (i.e., increased motivational reactions to drugs and drug cues) as well as a lack of top-down cognitive control over prepotent responses, which lead to increasing drug taking (Bechara 2005; Volkow et al. 2003) At a neural level, drugs of abuse directly produce rewarding effects from increased dopamine release in the mesolimbic pathway (Nestler 2001) and the subsequent stimulation of dopamine D1 and D2 receptors in the nucleus accumbens (NAc) (Volkow and Morales 2015). This pathway includes dopaminergic neurons from the ventral tegmental area to the NAc, which is located in the ventral striatum, and which fire in response to rewarding cues (Koob 1992). In addition, other neurotransmitters, such as glutamate and GABA, are indirectly affected by different drugs, further altering the brain’s reward pathway (Parvaz et al. 2011). Importantly, prefrontal cortical (PFC) areas are less active in substance users, a decrease associated with impaired self-control (Goldstein and Volkow 2011; Volkow et al. 1991, 1992, 1988).
Treatments currently available for addiction include both pharmacological interventions as well as behavioral methods. With the variety of brain regions available as targets from treatment, most of these pharmacological interventions aim to accomplish three things: reduce drug craving, increase cognitive control and/or decrease the enhanced stress reactivity and negative moods. Medications that are aimed at attenuating the drug cravings include naltrexone, methadone, varenicline, bupropion, baclofen, amisulpride, and aripiprazole (Konova et al. 2013; Young et al. 2014; Hermann et al. 2006; Myrick et al. 2010). Treatments that aim to increase cognitive control in order to normalize performance include N-acetylcysteine and modafinil (Schmaal et al. 2012; Schmaal et al. 2014). Some of these medications, specifically varenicline and modafinil, were shown to both attenuate craving and improve cognitive performance (Wheelock et al. 2014; Goudriaan et al. 2013). Additionally, antidepressants are used to improve negative moods which are associated with addiction and which significantly contribute to relapse. Behavioral approaches to abstinence include motivation enhancement,, cognitive bias modification and virtual reality trainings(Wiers et al. 2015b; Son et al. 2015). Another recently investigated treatment includes the use of real time fMRI neurofeedback where participants are shown the activation of certain regions and asked to manipulate the activation in a certain direction (Li et al. 2013). The use of neuroimaging techniques in capturing treatment effects may provide insight into neurobiological mechanisms of the treatment in the pathology of addiction, which is a unique benefit over behavioral outcome measures of treatment. In addition, neuroimaging may be more sensitive than behavioral measures in detecting effects of treatment (Linden 2012).
After introducing neuroimaging techniques, we will review the literature on pharmacological treatments effects, which we separated for treatments that target the increase of control versus those that target attenuation of limbic activation to overcome craving and stress reactivity. We then summarize behavioral interventions including cognitive bias modification training, virtual reality, cognitive-behavioral therapy, and motivational interventions. Lastly, we present information on the use of neuroimaging as a treatment method, covering recent studies investigating the benefits of neurofeedback and transcranial magnetic stimulation in addiction. For this review, we searched PubMed for clinical studies done in drug-dependent populations that investigated treatment efficacy, with neuroimaging available for the post-treatment condition. Various studies have investigated neuroimaging mechanisms as predictors of relapse (e.g., Mann et al. 2014) or used neuroimaging methods as the treatment itself assessing its efficacy with behavioral measures (e.g., Alba-Ferrara et al. 2014; Batista et al. 2015; Gorelick et al. 2014). However, this review only concentrates on studies that used neuroimaging to evaluate treatment efficacy in drug addiction.
Neuroimaging Techniques
Over the last two decades neuroimaging techniques have been used to study neural effects of pharmacological and behavioral treatment in substance use disorders. We discuss studies using Positron Emission Tomography (PET), a technique that utilizes positron-emitting compounds, known as radiotracers, to measure various physiological functions. The radioisotope decays over time releasing positrons that collide with electrons. These collisions create gamma rays, which are recorded by the PET scanner, providing information on the uptake of the tracer by location and over time (Michaelides et al. 2012). The radiotracers that are utilized can include ones that mimic endogenous compounds, such as [18F]-flouro-2-deoxyglucose (FDG) which allows for the measurement of glucose metabolism, and others that bind to specific receptors in the brain, such as [11C]raclopride, which binds primarily to the D2 and D3 receptors and allows for the study of the dopamine pathway (Thanos et al. 2008). Studies utilizing PET have been used to study the effects of different substances of abuse (Heinz et al. 2004; Volkow et al. 2009).
A second technique that has been used for the study of treatment effects is proton magnetic resonance spectroscopy (1H MRS). MRS allows for the measurement of brain metabolites, with the differing levels signaling a variety of potential changes (Chang et al. 2013). The measurement of endogenous compounds, such as glutamate, myoinositol and N-acetyl aspartate (NAA), allows for clinical applications in investigating psychiatric disorders, including substance dependence (Lyoo and Renshaw 2002).
Third, we discuss functional magnetic resonance imaging (fMRI) in combination with a drug cue reactivity task, the most frequently used imaging technique to study treatment effects. Participants are shown drug-related cues (often pictorial) that are known to induce craving and reward-related brain activation patterns inside the fMRI. The fMRI scanner captures the blood oxygenation level-dependent (BOLD) response, a measure of brain activation (e.g., Courtney et al. 2016). Key brain areas that are activated in drug-users in cue reactivity paradigms are the NAc, medial PFC (mPFC), basolateral amygdala and other PFC areas (for reviews, see Courtney et al. 2016; Schacht et al. 2013). The NAc, mPFC and amygdala have been associated with bottom-up motivational aspects of cue reactivity (Barros-Loscertales et al. 2011), reward processing (Koob and Volkow 2010), subjective drug craving and relapse (Beck et al. 2012; Sinha 2012; Volkow et al. 2004), whereas the dorsolateral PFC (dlPFC) is involved in top-down control over motivational reactions to drug cues in addiction (Baler and Volkow 2006; Bechara 2005; Sinha 2012). Since there is evidence that PFC regions are hypoactive in drug-dependent individuals at baseline when not intoxicated and when performing cognitive tasks (e.g., Volkow et al. 2004; Goldstein and Volkow 2002, 2011), it has been proposed that drug-cue induced activation measured with functional MRI may correspond to an immediate effort to regulate limbic reward responses (Goldstein and Volkow 2011; Hayashi et al. 2013; Lubman et al. 2004). Interestingly, however, PET studies measuring changes in regional brain glucose metabolism (marker of brain function) to cues show reductions in frontal brain areas (i.e., PFC and ACC) in female cocaine abusers following the exposure to cocaine cues (Volkow et al. 2011). This apparent discrepancy in results is likely to reflect the different temporal resolution for PET metabolic measures (30 min) and fMRI (2–5 min) and are consistent with impairment in control capacity following exposure to cues for which females appear to be more vulnerable than males (Volkow et al. 2011). Various studies have focused on cue reactivity and relapse (recently reviewed in Courtney et al. 2016). For example, alcohol cue-induced activation in the mPFC was elevated in alcoholics versus controls (Beck et al. 2012). A recent meta-analysis by Konova et al. (2013) on targets for addiction therapies emphasized the relevance of the ventral striatum (VS), inferior frontal gyrus (IFG), orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), middle frontal gyrus, (MFG) and precuneus in pharmacological and behavioral interventions.
In contrast to task-based fMRI, resting state MRI (rsMRI) measures the spontaneous activity across different regions during a resting scan. The common activation of certain regions is referred to as the functional connectivity, providing an organizational overview of the connections between different brain regions (Lu and Stein 2014). Resting state functional connectivity is altered in numerous psychiatric and neurological disorders, including in addiction (Ma et al. 2010; Meunier et al. 2012; Tomasi et al. 2010). In particular addicted individuals show decreased functional connectivity in executive control networks, which are responsible for self-regulation and enhanced connectivity in the default mode network (DMN), which is responsible for interoceptive awareness (Pariyadath et al. 2016).
Last, electroencephalography (EEG) is a noninvasive technique that involves the measurement of electrical signals from the brain (Stewart and May 2016). Endogenous event-related potentials (ERPs) are signals that are temporally linked to specific events. A frequently used component of ERPs is the P300, which is an ERP deflection seen between 300 and 600 ms after the initiation of an intervention (Fabiani et al. 2000). P300 has been recognized as a phenotypic marker for drug addiction, as it was altered in individuals with substance use disorder (SUD) on several tasks including reward processing (Goldstein et al. 2008; Parvaz et al. 2012), and was predictive of relapse (reviewed in Stewart and May 2016). As such, the P300 has been used as a potential target for capturing treatment effects of SUDs (Stotts et al. 2006).
Neural Effects of Pharmacological Interventions
Pharmacological Treatment Studies That aim to Reduce Drug Craving
Individuals who are addicted to a drug often report strong cravings in response to drug-related cues or to negative emotions (i.e. stress, boredom, depression). However, self-reported craving is a subjective measure. Researchers are using neuroimaging to objectively quantify brain activation that is the result of drug cues. Neuroimaging can be used to evaluate the treatment utility of different pharmacological agents. Several studies involving different drugs of abuse, including alcohol, nicotine and cocaine, have shown consistent brain areas of increased activation in drug abusers when they view cues of their drug. In addition, pharmacological agents that block dopamine release can erase the increased activation of these cue-craving areas (Courtney et al. 2016).
Naltrexone, an opioid antagonist, is one of the medications currently approved to treat alcohol as well as opioid use disorders. Naltrexone blocks the NAc-activated release of dopamine in response to alcohol (Gonzales and Weiss 1998) and it is expected that serotonin 3 antagonists, such as ondansetron, might have a similar action (Grant 1995; Johnson et al. 2000). Previous behavioral studies showed that these medications, in combination with psychotherapy, reduced craving, reduced number of drinks per drinking day, and increased time to relapse (Anton et al. 1999; Johnson et al. 2000). A study by Weerts et al. (2008) utilized PET to study the availability of two of the opioid receptor (OR) subtypes, μ- and δ-OR, before and during a standard naltrexone treatment using [11C]carfentanil and [11C]methylnaltrindole, respectively. Alcohol dependent subjects scanned during treatment (after their fourth dose) showed complete inhibition of the μ-OR, which was hypothesized to be responsible for the craving reduction seen with the medication. The δ-OR, however, was only partially inhibited by naltrexone. The degree of inhibition was highly variable between subjects and correlated with blood levels of naltrexone. This variability in δ-OR inhibition may explain the differences in treatment success with this medication (Weerts et al. 2008). In an additional study, Myrick et al. (2008) measured brain activation in craving areas after alcohol dependent subjects were given two acute pharmacological treatments: naltrexone and ondansetron hydrochloride. Subjects treated with naltrexone showed reduced VS activation compared to placebo-treated controls. However, subjects treated with only ondansetron showed a non-significant reduction in VS activation compared to placebo-treated controls. The acute combination of naltrexone and ondansetron not only significantly reduced subjective craving in alcoholics, but also decreased VS cue-related activation during fMRI as compared to placebo-treated alcoholics. In addition, subjective craving significantly correlated with VS activation (Myrick et al. 2008). These results provide evidence supporting the effectiveness of an acute combination treatment for reducing alcohol-related cravings in alcohol use disorders (AUD
Studies have also been done with an extended-release naltrexone (XRNTX) injection, which improves compliance over that of oral medication. Drug cue reactivity was evaluated before and between 10 and 14 days after a single XRNTX injection, in both heroin and alcohol dependent patients (Langleben et al. 2014; Lukas et al. 2013). Both of the study populations reported decreases in drug craving. In the heroin dependent subjects, the post treatment scans showed decreased activation in the amygdala, caudate, precentral gyrus, and cuneus, which are related to drug conditioning, self-regulation and craving. Increased activation was seen in MFG and precuneus, which are involved in “self-referential processes”. These changes suggest that the XRNTX regulates the drug cue responses by increasing inhibitory control (Langleben et al. 2014). The alcohol-dependent patients in the other study were exposed to both visual and olfactory cues. The XRNTX decreased activation in cue related regions known to be involved in cognition, reward processing, and memory, altering the responsiveness to drug cues (Lukas et al. 2013). XRNTX has been shown to have similar results as oral naltrexone as far as treatment potential, but with the added advantage of facilitating treatment compliance.
Two antipsychotics, amisulpride and aripiprazole, have been considered for addiction treatment due to their dopamine-D2/D3 receptor blocking effects. The utility of these treatments has also been studied through the imaging of cue-induced craving. An fMRI study by Hermann et al. (2006) looked at amisulpride, a dopamine D2/D3 receptor antagonist, and reported reduced activation in the right thalamus during alcohol cues compared to placebo. Further, Myrick et al. (2010) found that in alcoholics, aripiprazole reduced activation in VS compared to placebo. For the alcoholics given aripiprazole, right VS activity and heavy drinking during the 14-day medication period significantly decreased compared to alcoholics given placebo. However, there were no significant differences in subjective cravings between aripiprazole and placebo (Myrick et al. 2010). In another study, the combination of aripiprazole and escitalopram given to alcoholics with co-morbid major depressive disorder increases activation of the left ACC on a cue reactivity task compared to escitalopram alone (Han et al. 2013). Increases in left ACC activation were negatively associated with subjective craving. Because the ACC is a mediator of depressive symptoms, the authors suggest that the negative association may be due to the co-morbidity of depression in these subjects.
Baclofen has also been proposed as a treatment for addiction. One study reported that cocaine-dependent individuals given baclofen had reduced activation of VS, ventral pallidum, amygdala, midbrain, and OFC in response to cocaine cues compared to cocaine-dependent individuals given a placebo (Young et al. 2014). This study used subliminal cues—the cocaine stimuli were presented for 33 ms only and were followed by longer masking stimuli to prevent visual processing of the cocaine cues. The brain areas where activation decreased after treatment (i.e., ventral pallidum, amygdala, and OFC), were previously reported to be activated during subliminal cue tasks in drug abusers at baseline before treatment (Childress et al. 2008). This study reveals a potential target for evaluating pharmacotherapies by utilizing subliminal cues to test unconscious drug cue processing.
Varenicline is a partial agonist to nicotinic acetylcholine receptors that is used as a smoking cessation medication. It has an indirect action on mesolimbic dopamine (DA) release, reducing the rewarding effects of nicotine. Functional neuroimaging studies in smokers revealed reduced activation of the VS and medial OFC, and increased activation of the ACC, lateral OFC, posterior cingulate cortex (PCC), superior frontal gyrus (SFG), and dorsolateral PFC (dlPFC) (Franklin et al. 2011). The combination of varenicline and naltrexone reduced activation in bilateral ACC compared to a placebo in heavy drinking smokers (Ray et al. 2015). Varenicline also reduced cue-induced OFC activation bilaterally in alcohol-dependent individuals, although it had no effect on reducing drinking behavior (Schacht et al. 2014). A preliminary study by Wheelock et al. (2014) utilized both 1H-MRS and fMRI with the Stroop task, a color-naming paradigm that can be used to measure cognitive control, on smokers before and after treatment with varenicline. MRS showed a reduction in glutamine and glutamate levels in the ACC after treatment, with the baseline levels associated with craving and nicotine dependence. Decreases in BOLD signal in fMRI occurred in the rostral ACC, medial OFC, and precuneus/PCC. Functional connectivity analysis revealed changes between the dorsal ACC and the DMN, specifically in the rostral ACC and PCC (Wheelock et al. 2014) suggesting that varenicline exerts its actions through changes in glutamine/glutamate decrease and alterations of the functional connectivity of the dorsal ACC and the DMN.
Another smoking cessation medication, bupropion hydrochloride modulates DA and norepinephrine levels by blocking reuptake transporters. This action may reduce nicotine reward and withdrawal (Foley et al. 2006). A study using PET-FDG in the presence of cigarette-related cues showed decreased glucose metabolism in the perigenual and ventral ACC in bupropion-treated smokers compared to non-treated smokers (Brody et al. 2004). An fMRI study by Culbertson et al. (2011) reported reduced self-reported craving and cue-activation in limbic and PFC areas in bupropion-treated smokers. Reduced activation in bilateral medial OFC and left ACC correlated with self-reported craving (Culbertson et al. 2011). There was also decreased cue-induced activation in left VS in bupropion-treated subjects, consistent with the studies of other craving-reducing medications (e.g., Courtney et al. 2016).
Methadone, an opioid receptor agonist, is effective in the treatment of opioid use disorder and is the most widely used pharmacological treatment for heroin addiction (Wang et al. 2014). Methadone prevents withdrawal symptoms and reduces craving upon cessation of heroin use (Shi et al. 2008). Multiple studies have utilized fMRI and a heroin-related cue paradigm to investigate the effects of methadone maintenance treatment (MMT). A study by Wang et al. (2014) investigated differences in brain activations in heroin users with different treatment durations of MMT (less than a year vs treatment for at least 2 years) and also compared them with healthy controls. MMT patients, regardless of treatment length, had increased activity in both the mesolimbic reward and the visuospatial attention circuits compared to controls, consistent with results from a previous study (Wang et al. 2011). The MMT patients with longer treatments exhibited a reduction in activity compared to patients with shorter treatment lengths in the bilateral caudate, supporting the idea that long term MMT decreases the risk of relapse in heroin dependent patients by reducing the activation in reward-related regions (Wang et al. 2014). Further, Tabatabaei-Jafari et al. (2014) studied heroin users undergoing MMT, heroin users undergoing opiate abstinence, and healthy volunteers. Their results suggest that while both opiate abstinence and MMT both decrease craving, the craving reductions seemed to be caused by different patterns of activations. Although a number of regions were activated in both conditions, opiate abstinence led to an additional increase in activation in ACC and IFG, suggesting that these regions are involved in drug processing and aversion. MMT patients had higher activation in cerebellum and right lingual gyrus, implicating this enhanced activation as a medication effects (Tabatabaei-Jafari et al. 2014). In a study by Langleben et al. (2008), heroin dependent patients on MMT were scanned before and after their daily methadone dose. At the post-dose scanning sessions, MMT patients had increased activation to drug-related cues in OFC, insula, and left hippocampal complex. Pre-dose scans showed similar significant responses in these same regions, to an even greater degree, as well as increased response in ACC and the amygdala. This difference between the pre-dose and post-dose conditions suggests that MMT patients are most vulnerable in the time just before their dose (Langleben et al. 2008). These increases in activation persist even though the self-reported craving scores are not significantly different between any of the groups, a result also replicated by other studies (Tabatabaei-Jafari et al. 2014; Wang et al. 2011; Wang et al. 2014).
Resting state functional connectivity (RFC) has also been used to study MMT’s effects. Ma et al. (2010) used fMRI to investigate RFC changes in chronic heroin users (majority undergoing treatment with methadone) compared to healthy controls. The heroin users had increased connectivity in regions related to reward and craving including between NAcc and ACC, NAcc and OFC, and amygdala and OFC. Reduced connectivity between the PFC and both the ACC and OFC suggests impaired cognitive control (Ma et al. 2010). The combination of increased activation in reward and craving related regions with decreased cognitive restraint provides evidence of the shifts in functional connectivity that occur in chronic heroin users, even when undergoing methadone treatment or controlled heroin-maintenance.
Studies have also utilized PET and MRS to learn more about other aspects of MMT. It was proposed that chronic opiates disrupt dopamine transporters (DAT) in brain and that methadone counteracts this, accounting for the anti-craving effects. To test this hypothesis, a study used PET and [11C]-2β-carbomethoxy-3β-aryltropane ([11C]CFT), a DAT radioligand, to compare DAT between abstinent heroin abusers on methadone treatment, heroin abusers in prolonged abstinence (no methadone), and controls (Shi et al. 2008). The results revealed that subjects on MMT had lower than normal DAT in caudate and putamen. Abstinent (with or without methadone) heroin users had even lower DAT in caudate when compared to controls. The lower DAT in the caudate was associated with subjective anxiety only in MMT subjects. Further, MMT subjects had lower DAT levels in the putamen compared to the prolonged abstinence subjects, illustrating a restoration of DAT levels for the subjects who were cleared from all types of opioids, both heroin and methadone. This study concluded that long-term heroin use (presumably also methadone) can reduce DAT in striatum. Although methadone does reduce craving for heroin, sustained abstinence from the opioids in general can facilitate DAT restoration to a greater extent (Shi et al. 2008). In another PET study using FDG, Galynker et al. (2007) studied three similar groups: opiate-abstinent former opiate-dependent patients, MMT patients, and controls. While both experimental groups showed atypical relative activity patterns in similar regions, the opiate abstinent group showed decreased activity in a greater number of regions including the bilateral perigenual ACC, left mid-cingulate, left insula, and right SFG. Some of these regions, specifically the left perigenual ACC and mid-cingulate cortex, play an important role in mood processing. These findings suggest that MMT may normalize activity in mood processing regions, even in non-depressed patients, decreasing the probability of relapse (Galynker et al. 2007). A 1H MRS study measured glutamate levels in the ACC in heroin-dependent subjects, at both high (100 mg/day) and low (10–25 mg/day) dose MMT. Subjects first received a high dose and where then tapered off to a lower dose. The shift from high to low MMT led to an increase in ACC glutamate in the heroin-dependent subjects. This shift in glutamate levels, which approached control levels, suggests that the lower dose MMT might help normalize glutamate levels in ACC (Greenwald et al. 2015).
A study in patients undergoing protracted heroin-maintenance also showed increased RFC of the putamen, which was associated with the reported effects of the drug (Schmidt et al. 2015a). Another study by the same group investigated changes in connectivity with fMRI in response to fearful faces to test differences in stress reactivity. Heroin-maintained participants increased amygdala connectivity in response to the task after placebo administration, whereas the connectivity after heroin administration was similar to that of controls (Schmidt et al. 2015b). The combination of increased activation in reward and craving related regions with decreased cognitive restraint provides evidence of the shifts in functional connectivity that occur in chronic heroin users, even during MMT or controlled heroin-maintenance. A structural imaging study also on patients undergoing heroin-maintenance treatment revealed decreased gray matter volume in frontal areas. Decreased gray matter volume was associated with lower perfusion, an index of cerebral blood flow (CBF), which serves as marker of brain function. Reduced frontal CBF is consistent with impaired executive function in heroin addicted subjects (Denier et al. 2013).
Pharmacological Treatment Studies That aim to Increase Cognitive Control
Modafinil, N-acetylcysteine, methylphenidate and amphetamine and others are cognition enhancing drugs that aim to boost control processes in SUD. Modafinil is a mild stimulant that is marketed for the treatment of narcolepsy, to inhibit bouts of daytime drowsiness. Modafinil modulates DA signaling in the human brain (Volkow et al. 2009). In a study by Ghahremani et al. (2011), a learning task performed in the fMRI was used to evaluate the effect of modafinil in methamphetamine (METH) dependent patients. METH dependent patients and healthy controls underwent two scanning sessions, with modafinil or placebo in random order. Modafinil increased performance during the task in METH group, reaching levels similar to those seen in controls. Activation in ACC and bilateral insula/ventrolateral PFC also increased after modafinil administration, possibly reflecting the increased activation of these regions in the learning and cognitive processes activated during the MRI task (Ghahremani et al. 2011). The normalization of performance after one dose of modafinil supports its use as a therapy for METH dependence.
In AUD, modafinil improves impulsive decision making on a delay discounting task measured using an fMRI paradigm (Schmaal et al. 2014). Alcohol dependent (AD) patients and healthy controls underwent two fMRI sessions, either with a placebo or modafinil administration. For the AD patients, activation in frontoparietal regions was enhanced whereas ventromedial PFC activation was reduced after modafinil administration. Modafinil also enhanced functional connectivity between the SFG and VS and this was associated with behavioral improvement in task performance (Schmaal et al. 2014). This study supports the suggestion that modafinil enhances coupling of PFC control regions and reward areas thereby decreasing impulsive decision-making.
In another publication, Schmaal et al. (2013b) explored whether modafinil could play a role in enhancing response inhibition, which is typically compromised in AUD. Similar to their previous study, AD patients and healthy controls completed two fMRI sessions with either a placebo or modafinil. Response inhibition performance was measured from stop signal reaction times during a stop signal task performed during the scans. For AD subjects, modafinil enhanced response inhibition only for those subjects whose inhibition was already significantly compromised at baseline. In contrast, modafinil actually worsened performance for those AD patients with more regular response inhibition at baseline. For healthy controls with adept baseline response inhibition abilities, the drug caused no significant modulation in the task across the two conditions. Modafinil did, however, improve response inhibition for healthy controls with a more deficient baseline measurements. For the AD patients who improved their response inhibition as a result of modafinil, a larger activation of the thalamus and supplementary motor area was observed in parallel with diminished communication between the thalamus and the primary motor cortex (Schmaal et al. 2013a). This indicates that modafinil can improve response inhibition for AD patients, although baseline measurements of the ability should be taken into consideration before relying on modafinil as a means of treatment. A follow up study investigated the effect the dose of modafinil had on functional connectivity in the DMN in AD patients and controls as they completed the Stroop task. Modafinil increased functional coupling between DMN and both the salience network and the central executive network in AD patients. These two networks are involved in cognitive tasks, with the increased association providing a potential mechanism by which modafinil improved cognitive performance (Schmaal et al. 2013a).
Modafinil is also used for reducing craving in cocaine addicts upon exposure to drug-related cues. A study by Goudriaan et al. (2013) sought to investigate the modulations in cerebral activity, if any, that are present in response to substance-related cues under the influence of modafinil. Cocaine-dependent patients and healthy controls participated in two fMRI scans separated by 1 week, receiving either a placebo or modafinil. While in the scanner observing cocaine-related cues, the cocaine-dependent individuals exhibited large activations of the “MFG, ACC, angular gyrus, left OFC, and ventral tegmental area” during the baseline condition compared to controls. There were no activation differences for these reward and motivation centers between groups with treated with modafinil. In addition, in cocaine-dependent subjects, there was less activation in the ventral tegmental area and more activation in the right ACC and putamen with modafinil than with placebo. This variation did not occur in healthy controls. The overall findings indicate that modafinil plays a role in dampening the activity of motivational and subjective craving centers of the brain for cocaine-dependent individuals upon exposure to cocaine-related stimuli (Goudriaan et al. 2013)
In a placebo-controlled trial by Volkow et al. (2009), Fig. 1, the effects of oral modafinil on DA release and DAT occupancy were measured in healthy subjects using PET with [11C]raclopride and [11C]cocaine, respectively. PET results revealed that DA was significantly increased in the VS after modafinil administration due to the ability of modafinil to block DAT. Moreover modafinil decreases the binding of [11C]cocaine which is evidence that both modafinil and cocaine bind to the same site. The results of this study suggest that modafinil might hold some abuse potential due to the DA increases in VS following the blockade of DAT, similar to other drugs of abuse such as cocaine (Volkow et al. 2009). However modafinil is much less potent than cocaine in its ability to block DAT, which is likely to explain its low abuse potential. A recent study with [11C]-N-(3-iodopro-2E-enyl)-2beta-carbomethoxy-3beta-(4′-methylphenyl) nortropane ([11C]-PE2I), a DAT radiotracer, measured DAT availability in cocaine-dependent subjects administered either modafinil or placebo who underwent two PET scans during a 17-day period. While the modafinil treated patients showed a significant decrease in binding of the tracer, the medication failed to show greater abstinence levels than the placebo. This argues against the use of modafinil to treat cocaine abuse (Karila et al. 2016).
Adapted from Volkow et al. (2009). Modafinil blocks DAT ([C-11]cocaine) and increases dopamine release ([C-11]raclopride)
Addictive disorders have been associated with disruptions in the homeostasis of glutamate, an excitatory neurotransmitter in the brain, which may underlie vulnerability to drug-seeking behavior, such as craving and impulsivity (see Reissner and Kalivas (2010) for a review). For example, studies using 1H MRS found decreased levels of glutamate in the rostral ACC in patients with cocaine dependence (Yang et al. 2009), whereas increased levels of glutamate were found in the putamen of monkeys on long-term cocaine treatment (Liu et al. 2011). The administration of the drug N-acetylcysteine normalized glutamate levels in cocaine-treated rats (Baker et al. 2003). Whether this result is consistent in drug dependent humans was the focus of a study by Schmaal et al. (2012) that recruited cocaine dependent individuals as well as healthy controls to undergo two 1H MRS scans, with each individual receiving either no drug or N-acetylcysteine 1 h before the scan. Study results confirmed that baseline glutamate levels in the dorsal ACC of cocaine dependent individuals are significantly higher than in healthy controls. Following N-acetylcysteine, however, a significant reduction of dorsal ACC glutamate was observed in cocaine dependent subjects while no change was seen in the brains of healthy controls. These results imply that N-acetylcysteine is responsible for the normalization of abnormal glutamate levels seen in cocaine dependent individuals (Schmaal et al. 2012) and suggests an alternative treatment method for cocaine dependent individuals. Whether N-acetylcysteine is effective for reducing impulsivity and craving for cocaine-dependent individuals, as well as potentially benefiting abusers of other drugs, requires additional investigation.
Neural Effects of Behavioral Interventions
Cognitive Behavioral Therapy
Cognitive Behavioral Therapy (CBT) aims to change unhelpful thinking and behavior, and has been shown to decrease drug consumption in various SUD (Dutra et al. 2008). However, surprisingly few studies have investigated the effects of CBT with neuroimaging. A preliminary study by DeVito et al. (2012) evaluated the effect of CBT using an fMRI Stroop task (task that measures cognitive control) and compared BOLD response before and after treatment for subjects with SUD. After treatment, the patients exhibited decreased activation of ACC, IFG, dlPFC, and midbrain during the Stroop task. Compared to healthy controls who also underwent two scans, patients had a greater signal change in the subthalamic nucleus and midbrain after the second scan. These regions are involved in cognitive processes and impulse control, providing a mechanism by which this intervention method may help treat SUD (DeVito et al. 2012). A second study in heavy smokers compared the effects of CBT vs placebo on glucose metabolism using FDG PET. CBT, but not placebo, reduced FDG uptake in the posterior cingulate cortex (PCC), an area involved in value-based decision making (Costello et al. 2010).
Motivational Interventions
Motivational intervention is another treatment option available for SUD. Through a variety of techniques, including public service announcements (PSAs), personally tailored messages, or motivational interviewing, motivational interventions can increase the chances of drug use cessation (Zilverstand et al. 2016). An fMRI study by Feldstein Ewing et al. (2011) in AUD subjects investigated two different types of client speech during motivational interviewing and their effectiveness at altering neural activation following alcohol cue presentation.. Change talk (CT), where the participant addresses the need for modified behavior, was compared with counterchange talk (CCT), where participants believed they did not have to alter their behavior regarding drinking. After CCT and alcohol cue presentation, activation was seen in OFC, insula, NAcc, caudate, and putamen. However, there were no significant increases in activation following CT supporting the importance of CT during motivational interviewing as well as other behavioral interventions (Feldstein Ewing et al. 2011). Another study by Wilson et al. (2013) investigated differences in coping strategies during cue exposure in smokers: self-focused (with the focus being on personal benefit) versus other-focused (benefits presented in terms of another individual). Self-focused strategies, in contrast to other-focused strategies, produced an increase in activation in medial PFC, precuneus, and insula, all regions involved in self-referential processes (Wilson et al. 2013). These studies support the careful selection of specific strategies for therapies, with the best results seen with strategies that are more self-focused.
Two studies investigated the use of PSAs, frequently viewed on television, as a form of motivational intervention. Langleben et al. (2009) studied the brain responses of regular smokers to two types of anti-tobacco PSAs, those with high message sensation value (MSV) compared to those with low MSV, as well as neutral cues. MSV is a measure of intensity of the response to the different features of the video, such as visual and audio components. Frames from the PSAs, both high and low MSV, were more accurately identified during a “Frame Recognition Test” compared to those from the neutral videos, with the low MSV frames being those most accurately recognized. The activation elicited by the low MSV PSAs in the PFC and temporal cortex was greater than that of the high MSV PSAs, suggesting that higher intensity videos may not directly lead to greater retention (Langleben et al. 2009). A second study by Wang et al. (2013), investigated anti-smoking PSAs using fMRI in smokers while in the scanner. Smokers viewed PSAs with either high or low “argument strength (AS)”, i.e., the strength of the content and its persuasiveness with the audience. Smokers’ brain responses to AS and MSV strength interacted in the inferior parietal lobule, left IFG, left fusiform gyrus, right dorsomedial PFC (dmPFC), and precuneus. These results show the importance of processing the PSAs in these regions, with activation in the dmPFC also correlating to nicotine metabolite levels after a 1 month follow-up (Wang et al. 2013). These studies show the differences in brain activation by the varying types of PSAs, helping elucidate which types may be more beneficial for anti-tobacco PSAs.
Three studies by the same group investigated the use of tailored messages as a motivational intervention for smoking, by measuring brain responses to high-tailored, low-tailored, and generic smoking cessation messages. High-tailored messages produced greater activation of rostral mPFC and precuneus/PCC compared to low-tailored response, while the two tailored conditions produced greater activation in these regions compared to the generic messages (Chua et al. 2009a). A second study investigated the difference between neutral messages and three types of tailored messages: personalization/feedback, motivational, and instructional. The 3 types of messages each activated different areas: instructional messages activated the dlPFC, the personalized/feedback messages activated the mPFC and precuneus/PCC, and the motivational messages activated the ventral mPFC. The areas activated are involved in instructional processing, self-referential processes, and reward expectation, providing options for targets for the messages (Chua et al. 2009b). A more recent study investigated the neural changes seen with the tailored messages and its association with continued smoking cessation (Chua et al. 2011). After viewing tailored messages, participants showed increased activation in dmPFC, precuneus, and angular gyrus. The activations of dmPFC and precuneus were correlated with smoking cessation after a 4 month follow-up visit (Chua et al. 2011). The increased activation in mPFC and precuneus in the 3 studies in response to the tailored messages and its association with smoking cessation provides support for the use of tailored messages for smoking cessation treatments.
Cue-Exposure Treatment
Cue-exposure treatment (CET) aims at reducing conditioned responses towards drugs in individuals with SUD, which may reduce their risk of relapse (Drummond et al. 1990). A meta-analysis by Conklin and Tiffany (2002) showed no evidence for efficacy of CET for SUD, whereas Loeber et al. (2006) showed that CET decreased craving in AUD. Vollstädt-Klein et al. (2011) investigated the effects of CET on alcohol cue reactivity using fMRI in abstinent AUD patients. After treatment, the CET group exhibited stronger cue-reactivity reductions in VS, dorsal striatum, ACC, precentral gyrus, and insula compared to controls, which correlated with subjective craving.
Cognitive Bias Modification Training
Automatic processes may play a large role in drug addiction, and drug users have shown behavioral cognitive biases for drug cues, i.e., selective attention (attentional bias) and the tendency to approach drug cues faster than to avoid them (drug approach bias). The attentional bias can be measured with the addiction Stroop task (Cox et al. 2006), in which individuals with a SUD respond slower to drug-related words compared to neutral words, suggesting distraction by drug cues and the visual probe task; SUD participants generally fixate longer on a drug picture than a neutral picture (Field et al. 2013). The drug approach bias can be assessed with the Stimulus–Response Compatibility (SRC) task and a joystick Approach Avoidance Task (AAT). In the SRC, drug users moved a manikin faster towards drug cues than towards neutral cues (Bradley et al. 2008). In the AAT, participants push and pull pictorial cues that appear on a computer screen (drug-related/neutral) with a joystick in response to the format of the cue. The behavioral approach bias is calculated as the reaction time of pushing – pulling cues, with higher approach bias scores corresponding to stronger tendencies to pull than push certain cues. Patients with AUD (Wiers et al. 2014), cannabis use disorders (Cousijn et al. 2011), opiate use disorder (Zhou et al. 2012) and tobacco smokers (Wiers et al. 2013), have an approach bias for drug cues compared to control groups on the AAT; i.e., faster approach than avoidance. Attentional and approach biases for drugs correlated positively with explicit craving scores (Mogg et al. 2003; Mogg et al. 2005; Watson et al. 2013), and predicted relapse (Janes et al. 2010) and drug consumption (Volkow et al. 2005). These studies highlight the clinical importance of automatic biases in drug addiction.
Automatic biases can be “re-trained” by means of cognitive bias modification (CBM) training. The first study investigating CBM adapted a visual probe task for anxiety (MacLeod et al. 2002), in which probes follow neutral cues in the majority of cases, therefore disengaging attention from anxious cues and reducing attentional bias. CBM also decreased attentional biases for drug cues compared to neutral cues in heavy drinkers (Fadardi and Cox 2009), alcohol-dependent patients (Schoenmakers et al. 2010) and smokers (Field et al. 2009). Some of these studies found generalization to new stimuli, i.e., attention was not only disengaged from the drug cues that were used for CBM training, but reduced attentional biases also occurred for drug cues that participants hadn’t seen before (Fadardi and Cox 2009). This suggests that CBM may affect attentional biases for drug-related cues in general, which may influence other behavioral processes such as drug craving and drug seeking behavior.
In the AAT-based version of CBM participants systematically but implicitly push away drug cues with a joystick to decrease the drug approach bias. Typically a CBM group pushes away drug cues in 90 % of trials and pulls 10 % of alcohol cues towards them. Some study designs use a sham training group that pushes and pulls alcohol in 50 %/50 % of the cases. In two recent randomized-controlled trials, CBM reduces relapse rates up to 13 % in patients with AUD, compared with a placebo-training group (Wiers et al. 2011) and compared with a non-training group (Eberl et al. 2013), showing its clinical potential in alcohol addiction.
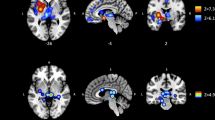
Two studies have investigated the neural effects of CBM in addicted populations. First, Wiers et al. (2015b) investigated recently detoxified patients with AUD on a blocked design alcohol cue reactivity task (Fig. 2). After the task, patients were randomly assigned to either a CBM group or a placebo group, and performed six sessions of CBM/sham training for 3 weeks. Before training, alcohol cue-evoked activation was observed in bilateral amygdala and right NAc, which correlated with craving and arousal ratings of alcohol stimuli. After training, the CBM group showed greater reductions in cue-evoked activation in amygdala and in behavioral arousal ratings of alcohol pictures, compared with the placebo training group. Decreases in right amygdala activity correlated with decreases in craving in the CBM but not the placebo training group.
Adapted from Wiers et al. (2015b). Cue-induced amygdala reactivity reduced after 3 weeks of CBM training compared to the placebo training group (p < 0.05 family-wise error corrected). Decreases in amygdala cue reactivity covaried with decreases in craving in the CBM group, but not in the placebo group
The patients also performed the AAT in a 3 T fMRI scanner pre and post training (Wiers et al. 2015a). The relevant neuroimaging contrast for the alcohol approach bias was the difference between approaching versus avoiding alcohol cues relative to soft drink cues: [(alcohol pull > alcohol push) > (soft drink pull > soft drink push)]. Before training, both groups showed significant alcohol approach bias-related activation in mPFC (Wiers et al. 2014). After training, patients in the CBM group showed stronger reductions in mPFC activation compared with the placebo group. Moreover, these reductions correlated with reductions in approach bias scores (but not with craving) in the CBM group only. Together, these findings provide evidence that CBM affects alcohol cue-induced mesolimbic brain activity and neural mechanisms involved in the automatic alcohol approach bias. This may be an important underlying mechanism of the therapeutic effectiveness of this training. Interestingly, two studies investigating the effects of CBM in anxious individuals also found effects of the amygdala for fear processing (Britton et al. 2015; Taylor et al. 2014). Since protocols for the neural effects of CBM have already been published (Attwood et al. 2014), more studies on the neural effects of CBM in the drug-dependent populations are to be expected.
Virtual Reality
Virtual reality (VR) therapy allows for the immersion into a three dimensional world, which can be altered to show any type of environment. When compared to the previous techniques, using videos or pictures, VR provides a different approach to cue exposure treatment (CET) for addiction. As it aims at cue extinction, the VR system can produce environments containing alcoholic drinks, drugs, advertisements, and even other people consuming drugs (Moon and Lee 2009). In recent years, this technology has been most effectively used to treat patients suffering from anxiety disorders including a range of phobias and PTSD (Bohil et al. 2011).
Recent studies utilized VR technology as a therapy for SUD in conjunction with fMRI. Using a VR approach that was previously shown to produce greater levels of craving than still photographs (Lee et al. 2003), VR was paired with 6 sessions of CET for smoking with MRI scanning before and after treatment completion. The combined treatment method, while not impacting craving, did result in a decrease in the urge to smoke. Craving reductions were also associated with a decreased activation of regions in PFC, specifically IFG and SFG (Moon and Lee 2009). In a recent preliminary study by Son et al. (2015), AUD subjects received 5 weeks of VR therapy, undergoing FDG-PET before and after treatment. At baseline prior to starting VR treatment, AUD subjects exhibited elevated metabolism in basal ganglia compared to controls. This finding is consistent with previous studies suggesting that elevated limbic activation in AUD subjects is associated with increased sensitivity to drug cues (Lee et al. 2013). After VR treatment completion, the metabolism in basal ganglia decreased suggesting a stabilization of the cue responses. This study was preliminary, however, since a small number of subjects was used (N = 12 AUD patients) and the control group did not undergo treatment, only a baseline scan (Son et al. 2015). Future expansion of this VR therapy into a larger number of subjects as well as inclusion of a healthy control group in the VR treatment would provide further evidence into its potential for AUD treatment. Nevertheless, the study stresses the potential of VR for addiction therapy. Since VR is a computer-based intervention, it also has strong potential for combination with neuroimaging techniques since VR can be done within the fMRI environment, whereas other behavioral treatments such as CBT cannot.
Neuromodulatory Techniques for Treatment
Neurofeedback
Neurofeedback through the use of fMRI has been investigated in nicotine-dependent smokers. In a study by Li et al. (2013), real time fMRI (rtfMRI) neurofeedback was tested on a nicotine-dependent population. Utilizing changes in BOLD response during rtfMRI in specific regions, the ACC and PFC, participants were trained to attempt to regulate the response during two separate states: a “reduce craving” state and an “increase resistance” state, respectively. Participants were shown a bar referred to as a “thermometer”, which monitored BOLD responses in either the ACC or the PFC, whose level they had to decrease or increase respectively, during separate sessions. The results suggest that the participants were able to possess control over the ACC and reduce its activation, a reduction that was also correlated with decreased craving. Canterberry et al. (2013) also investigated neurofeedback in nicotine-dependent cigarette smokers, in the ACC. A study by Hanlon et al. (2013), used a similar approach, except they merged the two instructions of reducing craving and increasing resistance into the same neurofeedback session, utilizing 2 thermometers with opposing instructions. Both the Canterberry et al. (2013) and the Hanlon et al. (2013) studies had similar findings as the Li et al. (2013). In a recently published study, Hartwell et al. (2016) further studied the aforementioned rtfMRI neurofeedback paradigm. A matched sample of smokers that did not receive neurofeedback but underwent scanning allowed for direct interpretation of the results of the treatment. Personalized ROI neurofeedback for each participant increased the potential benefit. After completing the 3 treatment visits, the rtfMRI neurofeedback group exhibited both lower craving scores and ROI activation, as observed in the previous studies (Li et al. 2013; Hanlon et al. 2013). The decrease in ACC activity found in all three rtfMRI studies discussed above is similar to those observed with nicotine-dependent patients receiving bupropion. This provides further validation for these findings and its treatment potential (Brody et al. 2004; Culbertson et al. 2011). The question of whether this approach would apply to normal, daily life has to still be answered before the true potential of this treatment for nicotine-dependent smokers or other SUD populations can be assessed.
Neurofeedback has recently been used in conjunction with rtfMRI to investigate its potential as a treatment for AUD. In a study by Kirsch et al. (2015), neurofeedback was used in heavy social drinkers to investigate its potential in reducing the response to alcohol stimuli. Compared to both no feedback and sham feedback control groups, the real feedback group had reduced activation of VS after treatment. These results were similar to those reported by Karch et al. (2015), who utilized two different control groups, one with false feedback and another with no feedback. The real feedback group exhibited lower activation in ACC, dlPFC, inferior temporal gyrus, MFG, and insula. The decrease in ACC activation in heavy drinkers is similar to that seen in the studies involving nicotine-dependent smokers (Hanlon et al. 2013; Hartwell et al. 2016; Li et al. 2013). Although both studies are preliminary and questions remain on the right type of control groups to be used, regarding if no feedback or false feedback are adequate control conditions, the results suggest a potential benefit in using neurofeedback treatment for AUD.
Neurofeedback as a treatment for SUD has also been investigated through EEG. Neurofeedback using EEG was utilized as treatment for AUD by Peniston and Kulkosky (1989). The AUD participants in the experimental group were able to increase their alpha and theta brain rhythms after treatment completion. In addition, depression scores and relapse percentage were lower in the biofeedback group compared to the control groups. Lastly, the biofeedback group failed to show the elevated beta-endorphin serum levels, which are related to stress, that was seen in AUD patients undergoing traditional treatment (Peniston and Kulkosky 1989). A study by Horrell et al. (2010) investigated a combined intervention of neurofeedback with motivational interviewing in cocaine abusers. The neurofeedback training aimed to increase sensorimotor rhythm (SMR) amplitude and/or decrease theta band frequency. Following treatment completion, the participants showed increased SMR amplitude while managing to maintain the theta band levels at a stable level. In a cue reactivity task after neurofeedback, participants decreased EEG gamma activation (Horrell et al. 2010). In a second study also using a combination of EEG neurofeedback and motivational interviewing in cocaine abusers, the treatment group showed the typical P300 amplitude decrease seen in retest (Stotts et al. 2006). Since the healthy controls had higher P300 in the baseline condition, this stabilization of the P300 indicates a difference in engagement during the visual task. The combined beneficial effects of neurofeedback and motivational interviewing provide support for a combination of treatments.
In a study combining two different approaches previously described, Sokunbi et al. (2014) present a “motivational neurofeedback” approach with an approach/avoidance visual feedback system. Using food to study the motivational neurofeedback paradigm, subjects were instructed to decrease the size of the image presented by decreasing the activation in an individualized target brain area using a localizer (e.g., amygdala, putamen and caudate), measured through fMRI. When compared to a passive session, the neurofeedback session lowered the region activation, providing evidence to the usefulness of this approach. This motivational neurofeedback approach can be further developed into one similar to the AAT where images increase or decrease in size as they are pulled towards or pushed away, respectively. This approach may be beneficial in the addiction field, as it adds a sense of direct involvement in the treatment. However, whether the approach works for addiction has not yet been explored.
Transcranial Magnetic Stimulation
Transcranial magnetic stimulation (TMS) is another neuromodulatory technique that stimulates the brain.. TMS is noninvasive technique using a high intensity magnetic pulse to alter the excitability of a specific brain area. Repetitive TMS (rTMS), which utilizes multiple currents, can provide a more lasting modulation (Bellamoli et al. 2014). While a number of studies have investigated TMS and shown its treatment potential for SUD, the majority had non-imaging outcome measures, such as craving scores or urine drug screen results (Amiaz et al. 2009; Mishra et al. 2010; Terraneo et al. 2016; Trojak et al. 2015). A recent study investigated differences in RFC in control and motivational networks after rTMS in AUD patients and controls. Participants received rTMS to the right dlPFC or sham stimulation. Overall, AUD patients showed greater functional connectivity within the left fronto-parietal cognitive control network, a change which was further increased after receiving rTMS. AUD patients also exhibited higher connectivity within the fronto-striatal motivational network and between the left and right fronto-parietal networks compared to controls. These findings suggest that rTMS may help increase connectivity in control-related regions, which can help in the cognitive processes needed to resist relapse (Jansen et al. 2015). Herremans et al. (2016) investigated accelerated high-frequency rTMS (HF-rTMS) in AUD patients using an fMRI cue-exposure paradigm. Patients received a total of 15 sessions of HF-rTMS over a 5 day period. While the majority of AUD patients relapsed, those who abstained had greater dACC activation during the cue-exposure paradigm. Those who abstained at the end of the treatment period showed decreased dACC activation after HF-rTMS while those who relapsed had an inverse relationship (Herremans et al. 2016). A preliminary study on one AUD patient utilized rTMS on the dACC to attempt to reduce severe cravings. Following the TMS, craving-related increases in EEG ACC and PCC connectivity in both beta and gamma bands as well as fMRI activation of the NAc, ACC, mPFC, and PCC were normalized. At a follow-up visit after the patient relapsed, the fMRI again showed increases in NAc, ACC, and PCC. The EEG increases in ACC and PCC connectivity also returned, but only in the gamma bands (De Ridder et al. 2011). While this provides evidence for the treatment potential of TMS in some patients, it suggests the need for continued study. A study by Pripfl et al. (2014) compared HF-rTMS using EEG on the left dlPFC with a sham condition in smokers. After the treatment sessions, both craving and resting state EEG delta power, which is related to the activity of the reward system, decreased (Pripfl et al. 2014). This reduction in a short term condition provides evidence supporting the use of HF-rTMS for treatment of cigarette smoking.
Transcranial Direct Current Stimulation
Transcranial direct current stimulation (tDCS) utilizes currents to alter the resting membrane potential of specific brain areas. This alteration effects neuronal excitability (da Silva et al. 2013). For addiction, it has been shown that stimulation of the dlPFC with tDCS decreased craving in heavy drinkers (den Uyl et al. 2015) and patients with AUD (Boggio et al. 2008) who were exposed to alcohol cues. Den Uyl, however, did not find evidence for changes in cognitive biases for alcohol. Another study by Conti et al. (2014) utilized this method in crack-cocaine dependent individuals. Patients either received 5 sessions of bilateral tCDS over the dlPFC or sham tDCS, with brain activity measured through P3 event related potentials (ERP) collected through EEG and low-resolution brain electromagnetic tomography (LORETA). Following one session, the P3 activity was already altered differently compared to those in the sham group. After the 5 treatment sessions, the subjects in the tDCS group showed increased intensity during drug-cue presentation in a variety of PFC areas, which included dlPFC, OFC, ACC, and frontopolar cortex. These results indicate that changes in neural activity are already present after a single session, expanding as the number of tCDS applications is increased (Conti et al. 2014). Another study by Conti and Nakamura-Palacios (2014) also studied tDCS on cocaine-addicted individuals. Increased activity in ACC was seen in the sham group while the tDCS group showed a decrease in activity. These results show that tDCS can alter activity in the ACC, an area important in drug-cue processing, suggesting its potential application in treatment (Conti and Nakamura-Palacios 2014). A study by da Silva et al. (2013) also investigated the effects of tDCS using ERPs in AUD patients, randomized into either a real tDCS group or a sham group. The sham group showed increased activation in response to alcohol-cues, a change not seen in the tCDS group. While the tDCS group also exhibited reduced craving and depression symptoms compared to the sham group, the tDCS group had a greater number of relapsers (da Silva et al. 2013). Nevertheless, tDCS has shown potential as being a valuable treatment strategy for drug dependence.
Summary and Conclusions
In summary, neuroimaging techniques have advanced our understanding of neural processes involved in the treatment of SUD as seen in Table 1. Some techniques, such as the virtual reality and neurofeedback, are still in early stages of clinical treatment investigations and further studies are needed. The expansion of the studies mentioned above to incorporate the follow-up of some of the populations studied at later time points is needed to determine whether some of the treatments, such as the neurofeedback training (Hanlon et al. 2013; Li et al. 2013), retain their effect following the initial study visits. In addition, pharmacological treatments, as the recent XRNTX, have been studied with neuroimaging only after a single injection (Langleben et al. 2014; Lukas et al. 2013). There remains a need to investigate its potential in the long term for both alcohol and heroin dependence. Other drugs such as bupropion hydrochloride, have been established as treatments for certain SUD, i.e., tobacco smoking.
In addition, there are other potential treatments for SUD that can be explored further. The brain can be stimulated using and deep brain stimulation (DBS), which also has potential in the treatment of SUDs (reviewed by Kravitz et al. 2015).
References
Alba-Ferrara L, Fernandez F, Salas R, de Erausquin GA (2014) Transcranial magnetic stimulation and deep brain stimulation in the treatment of alcohol dependence. Addictive Disorders & their Treatment 13:159–169. doi:10.1097/ADT.0b013e31829cf047
Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A (2009) Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction 104:653–660. doi:10.1111/j.1360-0443.2008.02448.x
Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK (1999) Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry 156:1758–1764. doi:10.1176/ajp.156.11.1758
Attwood AS, Williams T, Adams S, McClernon FJ, Munafo MR (2014) Effects of varenicline and cognitive bias modification on neural response to smoking-related cues: study protocol for a randomized controlled study. Trials 15, Artn 391. doi:10.1186/1745-6215-15-391
Baker DA, McFarland K, Lake RW, Shen H, Toda S, Kalivas PW (2003) N-acetyl cysteine-induced blockade of cocaine-induced reinstatement. Ann N Y Acad Sci 1003:349–351. doi:10.1196/annals.1300.023
Baler RD, Volkow ND (2006) Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med 12:559–566. doi:10.1016/j.molmed.2006.10.005
Barros-Loscertales A, Bustamante JC, Ventura-Campos N, Llopis JJ, Parcet MA, Avila C (2011) Lower activation in the right frontoparietal network during a counting Stroop task in a cocaine-dependent group. Psychiatry Res-Neuroim 194:111–118. doi:10.1016/j.pscychresns.2011.05.001
Batista EK, Klauss J, Fregni F, Nitsche MA, Nakamura-Palacios EM (2015) A randomized placebo-controlled trial of targeted prefrontal cortex modulation with bilateral tDCS in patients with crack-cocaine dependence. Int J Neuropsychopharmacol/Off Sci J Coll Int Neuropsychopharmacologicum 18. doi:10.1093/ijnp/pyv066
Bechara A (2005) Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci 8:1458–1463. doi:10.1038/nn1584
Beck A et al (2012) Effect of brain structure brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry 69:842–853. doi:10.1001/archgenpsychiatry.2011.2026
Bellamoli E, Manganotti P, Schwartz RP, Rimondo C, Gomma M, Serpelloni G (2014) rTMS in the treatment of drug addiction: an update about human studies. Behav Neurol 2014:815215. doi:10.1155/2014/815215
Boggio PS et al (2008) Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend 92:55–60. doi:10.1016/j.drugalcdep.2007.06.011
Bohil CJ, Alicea B, Biocca FA (2011) Virtual reality in neuroscience research and therapy. Nat Rev Neurosci 12:752–762. doi:10.1038/nrn3122
Bradley BP, Field M, Healy H, Mogg K (2008) Do the affective properties of smoking-related cues influence attentional and approach biases in cigarette smokers? J Psychopharmacol 22:737–745. doi:10.1177/0269881107083844
Britton JC, Suway JG, Clementi MA, Fox NA, Pine DS, Bar-Haim Y (2015) Neural changes with attention bias modification for anxiety: a randomized trial. Soc Cogn Affect Neurosci 10:913–920. doi:10.1093/scan/nsu141
Brody AL et al (2004) Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: a preliminary study. Psychiatry Res 130:269–281. doi:10.1016/j.pscychresns.2003.12.006
Canterberry M, Hanlon CA, Hartwell KJ, Li X, Owens M, LeMatty T, Prisciandaro JJ, Borckardt J et al. (2013) Sustained reduction of nicotine craving with real-time neurofeedback: exploring the role of severity of dependence. Nicotine Tob Res 15:2120–2124. doi:10.1093/ntr/ntt122
Chang LD, Munsaka SM, Kraft-Terry S, Ernst T (2013) Magnetic resonance spectroscopy to assess NeuroInflammation and neuropathic pain. J Neuroimmune Pharmacol 8:576–593. doi:10.1007/s11481-013-9460-x
Childress AR et al (2008) Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS One 3, e1506. doi:10.1371/journal.pone.0001506
Chua HF, Ho SS, Jasinska AJ, Polk TA, Welsh RC, Liberzon I, Strecher VJ (2011) Self-related neural response to tailored smoking-cessation messages predicts quitting. Nat Neurosci 14:426–427. doi:10.1038/nn.2761
Chua HF, Liberzon I, Welsh RC, Strecher VJ (2009a) Neural correlates of message tailoring and self-relatedness in smoking cessation programming. Biol Psychiatry 65:165–168. doi:10.1016/j.biopsych.2008.08.030
Chua HF, Polk T, Welsh R, Liberzon I, Strecher V (2009b) Neural responses to elements of a web-based smoking cessation program. Studies Health Technol Inform 144:174–178
Conklin CA, Tiffany ST (2002) Applying extinction research and theory to cue-exposure addiction treatments. Addiction 97:155–167
Conti CL, Moscon JA, Fregni F, Nitsche MA, Nakamura-Palacios EM (2014) Cognitive related electrophysiological changes induced by non-invasive cortical electrical stimulation in crack-cocaine addiction. Int J Neuropsychopharmacol/Off Sci J Coll Int Neuropsychopharmacologicum 17:1465–1475. doi:10.1017/S1461145714000522
Conti CL, Nakamura-Palacios EM (2014) Bilateral transcranial direct current stimulation over dorsolateral prefrontal cortex changes the drug-cued reactivity in the anterior cingulate cortex of crack-cocaine addicts. Brain Stimulation 7:130–132. doi:10.1016/j.brs.2013.09.007
Costello MR et al (2010) Effects of treatment for tobacco dependence on resting cerebral glucose metabolism. Neuropsychopharmacology 35:605–612. doi:10.1038/npp.2009.165
Courtney KE, Schacht JP, Hutchison K, Roche DJ, Ray LA (2016) Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict Biol. doi:10.1111/adb.12314
Cousijn J, Goudriaan AE, Wiers RW (2011) Reaching out towards cannabis: approach-bias in heavy cannabis users predicts changes in cannabis use. Addiction 106:1667–1674. doi:10.1111/j.1360-0443.2011.03475.x
Cox WM, Fadardi JS, Pothos EM (2006) The addiction-stroop test: theoretical considerations and procedural recommendations. Psychol Bull 132:443–476. doi:10.1037/0033-2909.132.3.443
Culbertson CS et al (2011) Effect of bupropion treatment on brain activation induced by cigarette-related cues in smokers. Arch Gen Psychiatry 68:505–515. doi:10.1001/archgenpsychiatry.2010.193
da Silva MC et al (2013) Behavioral effects of transcranial direct current stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J Physiol Paris 107:493–502. doi:10.1016/j.jphysparis.2013.07.003
De Ridder D, Vanneste S, Kovacs S, Sunaert S, Dom G (2011) Transient alcohol craving suppression by rTMS of dorsal anterior cingulate: an fMRI and LORETA EEG study. Neurosci Lett 496:5–10. doi:10.1016/j.neulet.2011.03.074
den Uyl TE, Gladwin TE, Wiers RW (2015) Transcranial direct current stimulation, implicit alcohol associations and craving. Biol Psychol 105:37–42. doi:10.1016/j.biopsycho.2014.12.004
Denier N et al (2013) Association of frontal gray matter volume and cerebral perfusion in heroin addiction: a multimodal neuroimaging study. Front Psychiatry 4:135. doi:10.3389/fpsyt.2013.00135
DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Kober H, Potenza MN (2012) A preliminary study of the neural effects of behavioral therapy for substance use disorders. Drug Alcohol Depend 122:228–235. doi:10.1016/j.drugalcdep.2011.10.002
Drummond DC, Cooper T, Glautier SP (1990) Conditioned learning in alcohol dependence: implications for cue exposure treatment. Br J Addict 85:725–743
Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW (2008) A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry 165:179–187. doi:10.1176/appi.ajp.2007.06111851
Eberl C, Wiers RW, Pawelczack S, Rinck M, Becker ES, Lindenmeyer J (2013) Approach bias modification in alcohol dependence: do clinical effects replicate and for whom does it work best? Dev Cogn Neuros-Neth 4:38–51. doi:10.1016/j.dcn.2012.11.002
Fabiani M, Gratton G, Federmeier KD (2000) Event-related brain potentials: methods, theory, and applications handbook of psychophysiology, 3rd Edn:85–119 doi:10.1017/Cbo9780511546396.004
Fadardi JS, Cox WM (2009) Reversing the sequence: reducing alcohol consumption by overcoming alcohol attentional bias. Drug Alcohol Depend 101:137–145. doi:10.1016/j.drugalcdep.2008.11.015
Feldstein Ewing SW, Filbey FM, Sabbineni A, Chandler LD, Hutchison KE (2011) How psychosocial alcohol interventions work: a preliminary look at what fMRI can tell us. Alcohol-Clin Exp Res 35:643–651. doi:10.1111/j.1530-0277.2010.01382.x
Field M, Duka T, Tyler E, Schoenmakers T (2009) Attentional bias modification in tobacco smokers. Nicotine Tob Res 11:812–822. doi:10.1093/ntr/ntp067
Field M, Mogg K, Mann B, Bennett GA, Bradley BP (2013) Attentional biases in abstinent alcoholics and their association with craving. Psychol Addict Behav 27:71–80. doi:10.1037/a0029626
Foley KF, DeSanty KP, Kast RE (2006) Bupropion: pharmacology and therapeutic applications. Expert Rev Neurother 6:1249–1265. doi:10.1586/14737175.6.9.1249
Franklin T et al (2011) Effects of varenicline on smoking cue-triggered neural and craving responses. Arch Gen Psychiatry 68:516–526. doi:10.1001/archgenpsychiatry.2010.190
Galynker II et al (2007) Cerebral metabolism and mood in remitted opiate dependence. Drug Alcohol Depend 90:166–174. doi:10.1016/j.drugalcdep.2007.03.015
Ghahremani DG, Tabibnia G, Monterosso J, Hellemann G, Poldrack RA, London ED (2011) Effect of modafinil on learning and task-related brain activity in methamphetamine-dependent and healthy individuals. Neuropsychopharmacology 36:950–959. doi:10.1038/npp.2010.233
Goldstein RZ et al (2008) Compromised sensitivity to monetary reward in current cocaine users: an ERP study. Psychophysiology 45:705–713. doi:10.1111/j.1469-8986.2008.00670.x
Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159:1642–1652. doi:10.1176/appi.ajp.159.10.1642
Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669. doi:10.1038/nrn3119
Gonzales RA, Weiss F (1998) Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci 18:10663–10671
Gorelick DA, Zangen A, George MS (2014) Transcranial magnetic stimulation in the treatment of substance addiction. Ann N Y Acad Sci 1327:79–93. doi:10.1111/nyas.12479
Goudriaan AE, Veltman DJ, van den Brink W, Dom G, Schmaal L (2013) Neurophysiological effects of modafinil on cue-exposure in cocaine dependence: a randomized placebo-controlled cross-over study using pharmacological fMRI. Addict Behav 38:1509–1517. doi:10.1016/j.addbeh.2012.04.006
Grant KA (1995) The role of 5-Ht3 receptors in drug-dependence. Drug Alcohol Depend 38:155–171. doi:10.1016/0376-8716(95)01120-N
Greenwald MK, Woodcock EA, Khatib D, Stanley JA (2015) Methadone maintenance dose modulates anterior cingulate glutamate levels in heroin-dependent individuals: a preliminary in vivo (1)H MRS study. Psychiatry Res 233:218–224. doi:10.1016/j.pscychresns.2015.07.002
Han DH, Kim SM, Choi JE, Min KJ, Renshaw PF (2013) Adjunctive aripiprazole therapy with escitalopram in patients with co-morbid major depressive disorder and alcohol dependence: clinical and neuroimaging evidence. J Psychopharmacol 27:282–291. doi:10.1177/0269881112472563
Hanlon CA et al (2013) Reduction of cue-induced craving through realtime neurofeedback in nicotine users: the role of region of interest selection and multiple visits. Psychiatry Res 213:79–81. doi:10.1016/j.pscychresns.2013.03.003
Hartwell KJ et al (2016) Individualized real-time fMRI neurofeedback to attenuate craving in nicotine-dependent smokers. J Psychiatry Neurosci: JPN 41:48–55
Hayashi T, Ko JH, Strafella AP, Dagher A (2013) Dorsolateral prefrontal and orbitofrontal cortex interactions during self-control of cigarette craving. Proc Natl Acad Sci U S A 11:4422–4427. doi:10.1073/pnas.1212185110
Heinz A et al (2004) Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry 161:1783–1789. doi:10.1176/appi.ajp.161.10.1783
Hermann D et al (2006) Blockade of cue-induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcohol Clin Exp Res 30:1349–1354. doi:10.1111/j.1530-0277.2006.00174.x
Herremans SC, De Raedt R, Van Schuerbeek P, Marinazzo D, Matthys F, De Mey J, Baeken C (2016) Accelerated HF-rTMS protocol has a rate-dependent effect on dACC activation in alcohol-dependent patients: an open-label feasibility study alcoholism. Clin Exp Res 40:196–205. doi:10.1111/acer.12937
Horrell T, El-Baz A, Baruth J, Tasman A, Sokhadze G, Stewart C, Sokhadze E (2010) Neurofeedback effects on evoked and induced EEG gamma band reactivity to drug-related cues in cocaine addiction. J Neurother 14:195–216. doi:10.1080/10874208.2010.501498
Janes AC et al (2010) Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry 67:722–729. doi:10.1016/j.biopsych.2009.12.034
Jansen JM, Wingen G, Brink W, Goudriaan AE (2015) Resting state connectivity in alcohol dependent patients and the effect of repetitive transcranial magnetic stimulation. Eur Neuropsychopharmacol: J Eur Coll Neuropsychopharmacol 25:2230–2239. doi:10.1016/j.euroneuro.2015.09.019
Johnson BA, Ait-Daoud N, Prihoda TJ (2000) Combining ondansetron and naltrexone effectively treats biologically predisposed alcoholics: from hypotheses to preliminary clinical evidence. Alcohol Clin Exp Res 24:737–742
Karila L et al (2016) Dopamine transporter correlates and occupancy by modafinil in cocaine-dependent patients: a controlled study with high-resolution PET and [C]-PE2I. Neuropsychopharmacology. doi:10.1038/npp.2016.28
Karch S et al (2015) Modulation of craving related brain responses using real-time fMRI in patients with alcohol use disorder. PLoS One 10, e0133034. doi:10.1371/journal.pone.0133034
Kirsch M, Gruber I, Ruf M, Kiefer F, Kirsch P (2015) Real-time functional magnetic resonance imaging neurofeedback can reduce striatal cue-reactivity to alcohol stimuli. Addict Biol. doi:10.1111/adb.12278
Konova AB, Moeller SJ, Goldstein RZ (2013) Common and distinct neural targets of treatment: changing brain function in substance addiction. Neurosci Biobehav Rev 37:2806–2817. doi:10.1016/j.neubiorev.2013.10.002
Koob GF (1992) Neural mechanisms of drug reinforcement. Ann N Y Acad Sci 654:171–191
Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. doi:10.1038/npp.2009.110
Kravitz AV, Tomasi D, LeBlanc KH, Baler R, Volkow ND, Bonci A, Ferre S (2015) Cortico-striatal circuits: novel therapeutic targets for substance use disorders. Brain Res 1628:186–198. doi:10.1016/j.brainres.2015.03.048
Langleben DD et al (2009) Reduced prefrontal and temporal processing and recall of high “sensation value” ads. NeuroImage 46:219–225. doi:10.1016/j.neuroimage.2008.12.062
Langleben DD et al (2008) Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry 165:390–394. doi:10.1176/appi.ajp.2007.07010070
Langleben DD et al (2014) Extended-release naltrexone modulates brain response to drug cues in abstinent heroin-dependent patients. Addict Biol 19:262–271. doi:10.1111/j.1369-1600.2012.00462.x
Lee E et al (2013) Neural evidence for emotional involvement in pathological alcohol craving. Alcohol Alcohol 48:288–294. doi:10.1093/alcalc/ags130
Lee JH et al (2003) Experimental application of virtual reality for nicotine craving through cue exposure Cyberpsychology & behavior: the impact of the Internet. Multimed Virtual Real Behav Soc 6:275–280. doi:10.1089/109493103322011560
Li X et al (2013) Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: a preliminary real-time fMRI study. Addict Biol 18:739–748. doi:10.1111/j.1369-1600.2012.00449.x
Linden DE (2012) The challenges and promise of neuroimaging in psychiatry. Neuron 73:8–22. doi:10.1016/j.neuron.2011.12.014
Liu X et al (2011) Chronic cocaine exposure induces putamen glutamate and glutamine metabolite abnormalities in squirrel monkeys. Psychopharmacology (Berl) 217:367–375
Loeber S, Croissant B, Heinz A, Mann K, Flor H (2006) Cue exposure in the treatment of alcohol dependence: effects on drinking outcome, craving and self-efficacy. Br J Clin Psychol/The British Psychological Society 45:515–529. doi:10.1348/014466505X82586
Lu H, Stein EA (2014) Resting state functional connectivity: its physiological basis and application in neuropharmacology. Neuropharmacology 84:79–89. doi:10.1016/j.neuropharm.2013.08.023
Lubman DI, Yücel M, Pantelis C (2004) Addiction, a condition of compulsive behaviour? Neuroimaging and neuropsychological evidence of inhibitory dysregulation. Addiction 12:1491–1502
Lukas SE et al (2013) Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers: a bold FMRI study. NeuroImage 78:176–185. doi:10.1016/j.neuroimage.2013.03.055
Lyoo IK, Renshaw PF (2002) Magnetic resonance spectroscopy: current and future applications in psychiatric research. Biol Psychiatry 51:195–207
Ma N et al (2010) Addiction related alteration in resting-state brain connectivity. NeuroImage 49:738–744. doi:10.1016/j.neuroimage.2009.08.037
MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L (2002) Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. J Abnorm Psychol 111:107–123
Mann K et al (2014) Predicting naltrexone response in alcohol-dependent patients: the contribution of functional magnetic resonance imaging. Alcohol Clin Exp Res 38:2754–2762. doi:10.1111/acer.12546
Meunier D et al (2012) Brain functional connectivity in stimulant drug dependence and obsessive-compulsive disorder. NeuroImage 59:1461–1468. doi:10.1016/j.neuroimage.2011.08.003
Michaelides M, Thanos PK, Volkow ND, Wang GJ (2012) Translational neuroimaging in drug addiction and obesity ILAR journal/National Research Council. Inst Lab Anim Resour 53:59–68
Mishra BR, Nizamie SH, Das B, Praharaj SK (2010) Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction 105:49–55. doi:10.1111/j.1360-0443.2009.02777.x
Mogg K, Bradley BP, Field M, De Houwer J (2003) Eye movements to smoking-related pictures in smokers: relationship between attentional biases and implicit and explicit measures of stimulus valence. Addiction 98:825–836
Mogg K, Field M, Bradley BP (2005) Attentional and approach biases for smoking cues in smokers: an investigation of competing theoretical views of addiction. Psychopharmacology 180:333–341. doi:10.1007/s00213-005-2158-x
Moon J, Lee JH (2009) Cue exposure treatment in a virtual environment to reduce nicotine craving: a functional MRI study cyberpsychology & behavior: the impact of the Internet. Multimed Virtual Real Behav Soc 12:43–45. doi:10.1089/cpb.2008.0032
Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K (2008) Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry 65:466–475. doi:10.1001/archpsyc.65.4.466
Myrick H, Li X, Randall PK, Henderson S, Voronin K, Anton RF (2010) The effect of aripiprazole on cue-induced brain activation and drinking parameters in alcoholics. J Clin Psychopharmacol 30:365–372. doi:10.1097/JCP.0b013e3181e75cff
Nestler EJ (2001) Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2:119–128. doi:10.1038/35053570
Pariyadath V, Gowin JL, Stein EA (2016) Resting state functional connectivity analysis for addiction medicine: from individual loci to complex networks. Prog Brain Res 224:155–173. doi:10.1016/bs.pbr.2015.07.015
Parvaz MA, Alia-Klein N, Woicik PA, Volkow ND, Goldstein RZ (2011) Neuroimaging for drug addiction and related behaviors. Rev Neurosci 22:609–624. doi:10.1515/RNS.2011.055
Parvaz MA, Konova AB, Tomasi D, Volkow ND, Goldstein RZ (2012) Structural integrity of the prefrontal cortex modulates electrocortical sensitivity to reward. Journal of cognitive neuroscience 24:1560–1570. doi:10.1162/jocn_a_00166
Peniston EG, Kulkosky PJ (1989) Alpha-theta brainwave training and beta-endorphin levels in alcoholics. Alcohol Clin Exp Res 13:271–279
Pripfl J, Tomova L, Riecansky I, Lamm C (2014) Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimulation 7:226–233. doi:10.1016/j.brs.2013.11.003
Ray LA, Courtney KE, Ghahremani DG, Miotto K, Brody A, London ED (2015) Varenicline, naltrexone, and their combination for heavy-drinking smokers: preliminary neuroimaging findings. Am J Drug Alcohol Abuse 41:35–44. doi:10.3109/00952990.2014.927881
Reissner KJ, Kalivas PW (2010) Using glutamate homeostasis as a target for treating addictive disorders. Behav Pharmacol 21:514–522. doi:10.1097/FBP.0b013e32833d41b2
Schacht JP, Anton RF, Myrick H (2013) Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol 18:121–133. doi:10.1111/j.1369-1600.2012.00464.x
Schacht JP, Anton RF, Randall P, Li XB, Henderson S, Myrick H (2014) Varenicline effects on drinking, craving and neural reward processing among non-treatment-seeking alcohol-dependent individuals. Psychopharmacology 231:3799–3807. doi:10.1007/s00213-014-3518-1
Schmaal L, Goudriaan AE, Joos L, Dom G, Pattij T, van den Brink W, Veltman DJ (2014) Neural substrates of impulsive decision making modulated by modafinil in alcohol-dependent patients. Psychol Med 44:2787–2798. doi:10.1017/S0033291714000312
Schmaal L, Goudriaan AE, Joos L, Kruse AM, Dom G, van den Brink W, Veltman DJ (2013a) Modafinil modulates resting-state functional network connectivity and cognitive control in alcohol-dependent patients. Biol Psychiatry 73:789–795. doi:10.1016/j.biopsych.2012.12.025
Schmaal L, Joos L, Koeleman M, Veltman DJ, van den Brink W, Goudriaan AE (2013b) Effects of modafinil on neural correlates of response inhibition in alcohol-dependent patients. Biol Psychiatry 73:211–218. doi:10.1016/j.biopsych.2012.06.032
Schmaal L, Veltman DJ, Nederveen A, van den Brink W, Goudriaan AE (2012) N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacology 37:2143–2152. doi:10.1038/npp.2012.66
Schmidt A et al (2015a) Increased functional connectivity in the resting-state basal ganglia network after acute heroin substitution. Transl Psychiatry 5, e533. doi:10.1038/tp.2015.28
Schmidt A et al (2015b) Normalizing effect of heroin maintenance treatment on stress-induced brain connectivity. Brain: J Neurol 138:217–228. doi:10.1093/brain/awu326
Schoenmakers TM, de Bruin M, Lux IF, Goertz AG, Van Kerkhof DH, Wiers RW (2010) Clinical effectiveness of attentional bias modification training in abstinent alcoholic patients. Drug Alcohol Depend 109:30–36. doi:10.1016/j.drugalcdep.2009.11.022
Shi J et al (2008) PET imaging of dopamine transporter and drug craving during methadone maintenance treatment and after prolonged abstinence in heroin users. Eur J Pharmacol 579:160–166. doi:10.1016/j.ejphar.2007.09.042
Sinha R (2012) How does stress lead to risk of alcohol relapse? Alcohol Res-Curr Rev 34:432–440
Sokunbi MO, Linden DEJ, Habes I, Johnston S, Ihssen N (2014) Real-time fMRI brain-computer interface: development of a “motivational feedback” subsystem for the regulation of visual cue reactivity. Front Behav Neurosci 8, Artn 392. doi:10.3389/Fnbeh.2014.00392
Son JH et al (2015) Virtual reality therapy for the treatment of alcohol dependence: a preliminary investigation with positron emission tomography/computerized tomography. J Stud Alcohol Drugs 76:620–627
Stewart JL, May AC (2016) Electrophysiology for addiction medicine: from methodology to conceptualization of reward deficits. Prog Brain Res 224:67–84. doi:10.1016/bs.pbr.2015.07.013
Stotts AL, Potts GF, Ingersoll G, George MR, Martin LE (2006) Preliminary feasibility and efficacy of a brief motivational intervention with psychophysiological feedback for cocaine abuse. Subst Abus 27:9–20
Tabatabaei-Jafari H et al (2014) Patterns of brain activation during craving in heroin dependents successfully treated by methadone maintenance and abstinence-based treatments. J Addict Med 8:123–129. doi:10.1097/ADM.0000000000000022
Taylor CT, Aupperle RL, Flagan T, Simmons AN, Amir N, Stein MB, Paulus MP (2014) Neural correlates of a computerized attention modification program in anxious subjects. Soc Cogn Affect Neurosci 9:1379–1387. doi:10.1093/scan/nst128
Terraneo A, Leggio L, Saladini M, Ermani M, Bonci A, Gallimberti L (2016) Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: A pilot study. Eur Neuropsychopharmacol: J Eur Coll Neuropsychopharmacol 26:37–44. doi:10.1016/j.euroneuro.2015.11.011
Thanos PK, Wang GJ, Volkow ND (2008) Positron emission tomography as a tool for studying alcohol abuse. Alcohol Res Health 31:233–237
Tomasi D et al (2010) Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One 5, e10815. doi:10.1371/journal.pone.0010815
Trojak B et al (2015) Transcranial magnetic stimulation combined with nicotine replacement therapy for smoking cessation: a randomized controlled trial. Brain Stimulation 8:1168–1174. doi:10.1016/j.brs.2015.06.004
Volkow ND et al (2009) Effects of modafinil on dopamine and dopamine transporters in the male human brain clinical implications. Jama-J Am Med Assoc 301:1148–1154
Volkow ND, Fowler JS, Wang GJ (2003) The addicted human brain: insights from imaging studies. J Clin Invest 111:1444–1451. doi:10.1172/JCI18533
Volkow ND, Fowler JS, Wang GJ (2004) The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology 47:3–13. doi:10.1016/j.neuropharm.2004.07.019
Volkow ND et al (1991) Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry 148:621–626. doi:10.1176/ajp.148.5.621
Volkow ND et al (2011) Reduced metabolism in brain “control networks” following cocaine-cues exposure in female cocaine abusers. PLoS One 6, e16573. doi:10.1371/journal.pone.0016573
Volkow ND et al (2005) Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci 25:3932–3939. doi:10.1523/Jneurosci.0433-05.2005
Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L (1992) Long-term frontal brain metabolic changes in cocaine abusers. Synapse 11:184–190. doi:10.1002/syn.890110303
Volkow ND, Morales M (2015) The brain on drugs: from reward to addiction. Cell 162:712–725. doi:10.1016/j.cell.2015.07.046
Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K (1988) Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br J Psychiatry J Ment Sci 152:641–648
Vollstädt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Bühler M, von der Goltz C, Hermann D, Mann K, Kiefer F (2011) Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biol Psychiatry 69:1060–1066. doi:10.1016/j.biopsych.2010.12.016
Wang AL et al (2013) Content matters: neuroimaging investigation of brain and behavioral impact of televised anti-tobacco public service announcements. J Neurosci 33:7420–7427. doi:10.1523/JNEUROSCI.3840-12.2013
Wang W et al (2011) Brain fMRI and craving response to heroin-related cues in patients on methadone maintenance treatment. Am J Drug Alcohol Abuse 37:123–130. doi:10.3109/00952990.2010.543997
Wang Y et al (2014) Reduced responses to heroin-cue-induced craving in the dorsal striatum: effects of long-term methadone maintenance treatment. Neurosci Lett 581:120–124. doi:10.1016/j.neulet.2014.08.026
Watson P, de Wit S, Hommel B, Wiers RW (2013) Motivational mechanisms underlying the approach bias to cigarettes. J Exp Psychopathol 4:250–262
Weerts EM, Kim YK, Wand GS, Dannals RF, Lee JS, Frost JJ, McCaul ME (2008) Differences in delta- and mu-opioid receptor blockade measured by positron emission tomography in naltrexone-treated recently abstinent alcohol-dependent subjects. Neuropsychopharmacology 33:653–665. doi:10.1038/sj.npp.1301440
Wheelock MD, Reid MA, To H, White DM, Cropsey KL, Lahti AC (2014) Open label smoking cessation with varenicline is associated with decreased glutamate levels and functional changes in anterior cingulate cortex: preliminary findings. Front Pharmacol 5:158. doi:10.3389/fphar.2014.00158
Wiers CE et al (2013) Automatic approach bias towards smoking cues is present in smokers but not in ex-smokers. Psychopharmacology 229:187–197. doi:10.1007/s00213-013-3098-5
Wiers CE et al (2015a) Effects of cognitive bias modification training on neural signatures of alcohol approach tendencies in male alcohol-dependent patients. Addict Biol 20:990–999. doi:10.1111/adb.12221
Wiers CE et al (2015b) Effects of cognitive bias modification training on neural alcohol cue reactivity in alcohol dependence. Am J Psychiatry 172:335–343. doi:10.1176/appi.ajp.2014.13111495
Wiers CE et al (2014) Neural correlates of alcohol-approach bias in alcohol addiction: the spirit is willing but the flesh is weak for spirits. Neuropsychopharmacology 39:688–697. doi:10.1038/npp.2013.252
Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J (2011) Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychol Sci 22:490–497. doi:10.1177/0956797611400615
Wilson SJ, Sayette MA, Fiez JA (2013) Neural correlates of self-focused and other-focused strategies for coping with cigarette cue exposure. Psychol Addict Behav 27:466–476. doi:10.1037/a0027055
Yang S, Salmeron BJ, Ross TJ, Xi ZX, Stein EA, Yang Y (2009) Lower glutamate levels in rostral anterior cingulate of chronic cocaine users - A (1)H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy. Psychiatry Res 174:171–176
Young KA et al (2014) Nipping cue reactivity in the bud: Baclofen prevents limbic activation elicited by subliminal drug cues. J Neurosci 34:5038–5043. doi:10.1523/Jneurosci.4977-13.2014
Zhou Y, Li X, Zhang M, Zhang F, Zhu C, Shen M (2012) Behavioural approach tendencies to heroin-related stimuli in abstinent heroin abusers. Psychopharmacology 221:171–176. doi:10.1007/s00213-011-2557-0
Zilverstand A, Parvaz MA, Moeller SJ, Goldstein RZ (2016) Cognitive interventions for addiction medicine: understanding the underlying neurobiological mechanisms. Prog Brain Res 224:285–304. doi:10.1016/bs.pbr.2015.07.019
Acknowledgments
The authors thank Dr. Joanna Fowler for proofreading.
This work was supported by NIH/NIAAA intramural grant Y1AA-3009 to NDV.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cabrera, E.A., Wiers, C.E., Lindgren, E. et al. Neuroimaging the Effectiveness of Substance Use Disorder Treatments. J Neuroimmune Pharmacol 11, 408–433 (2016). https://doi.org/10.1007/s11481-016-9680-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-016-9680-y