Abstract
A tapered optical fiber fabricated by a simple chemical etching method and modified with Ag nanoparticles (AgNPs) by chemical deposition was evaluated for surface-enhanced Raman scattering (SERS). The fiber probe was used for SERS measurements in both direct and remote scattering modes, yielding desired performance in both scattering configurations. The state of the obtained AgNPs made a significant contribution to the high sensitivity of SERS to Rhodamine 6G (R6G) molecules (down to a concentration of 10−7 M), and the substrate had an analyst enhancement factor (AEF) on the order of ∼108. Meanwhile, the SERS intensity during the evaporation process was investigated, showing a good stability at the later stage of the evaporation process. The fiber SERS probes demonstrated good reproducibility with the average relative standard deviation (RSD) values being less than 0.2 for the major Raman peaks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to their great flexibility, optical fibers are widely used as either light carriers or analytical or sensing platforms in a wide range of application fields, such as telecommunications, analytical chemistry, and remote sensing [1, 2]. Applications of optical fiber coupled with surface plasmonic structures have also been reported for surface-enhanced Raman scattering (SERS) [3, 4]. The SERS effect was discovered 40 years ago [5, 6]. Compared to traditional Raman technology, SERS can provide significant signal amplification on the order of 106 to 108 for analyte molecules adsorbed on a SERS-active substrate [7–9]. Usually, the SERS signals are detected utilizing a SERS substrate with metal nanostructures placed on a planar platform. Compared with traditional SERS substrates, optical fibers provide an ideal platform for detecting SERS signal of analytes at remote locations, making in situ sensing possible. Generally, the metallic SERS-active nanostructures in the fiber probe are fabricated directly at the end of the excitation fiber. The metal structures/films/nanoparticles characterized by localized surface plasmon resonances are used to enhance the normally weak Raman scattering. However, we face many challenges when fabricating micro/nanoscale structures on the fiber, because the large aspect ratio and microscopic cross-section of the fiber are incompatible with many fabrication platforms [4]. There are several approaches to prepare fiber SERS probes [1, 10–13]. Some use optical fibers simply as light carriers, by etching the cladding of optical fibers and modifying them with metallic films or nanoparticles, in order to take advantage of the evanescent wave field [10–14]. Some modify the tip of the optical fiber with plasmonic nanostructures for SERS application, including photochemical reduction of Ag on the optical fiber tip [15], nanoimprinting using cicada wings as templates [16], nanolithography fabrication of Au nanostructures [17], sol-gel deposition of Ag nanoparticles (AgNPs) [18], Au films milling and deposition of Au nanoislands [11], chitosan-gold nanocomposites via electrodes plating [19], and Au nanoparticles via electro-static self-assembly technology [20]. Among these methods, the simplest approach to functionalize the fiber tip is decoration or self-assembly. This technique can produce stochastic assemblies of fractal objects with the benefit of low cost and ready access to the nano-regime, while it sacrifices the geometrical control offered by more precise lithographic techniques [4]. The choice of techniques is directly related to cost; low cost is a particular consideration in disposable devices.
Until now, fiber probes with different geometries have been explored in order to improve the SERS activity [21], such as flat facets, angled facets, and tapered fibers. Among them, the tapered fiber probe can increase both the number of internal reflections at the silica-metal interface and the SERS-active surface [22].
In this article, we used a chemical etching and deposition approach; Ag nanoparticles were directly synthesized on the tapered surface of the fiber. The geometric morphology of the fiber tip was characterized by optical microscope and scanning electron microscope (SEM). The performances of the devices as SERS substrates were evaluated both at direct scattering and remote scattering configurations, using Rhodamine 6G (R6G) as the target molecules. The results show that the analyst enhancement factor was up to ∼108 and its detection limit reached as low as 10−7 M.
Experimental Sections
Materials
Multimode fiber with a core/cladding diameter of 50/125 μm is chosen in the experiments. Ethanol, hydrofluoric (HF) acid, edible oil, and solid powder of NH4F and SnF2 are used without further purification.
Sample Preparation
Fabrication of the AgNPs-decorated optical fibers is shown in Fig. 1. There are two steps, firstly pointed cone fiber preparation with chemical etching [4], and secondly, fiber probe with AgNPs using chemical deposition.
Preparation of the Tapered Fiber
Chemical etching was adopted for the fabrication of a tapered-end on the optical fiber (Fig. 1). Firstly, the coating layer with a length of 20 mm is removed by mechanical stripping; the end face is cut to flat-end, and cleaned by ethyl alcohol. Then, the naked fiber is vertically immersed in the HF-edible oil immiscible solution, where the edible oil layer is essential to prevent corrosion of the part that is not immersed in the HF solution. After etching, the fiber is rinsed by ethanol and dried in vacuum.
Preparation of the Fiber SERS Probe
One of the key factors to be taken into consideration when designing a SERS fiber probe is the morphology and aggregation of metal nanoparticles used to induce the SERS effect. In this paper, chemical deposition was used to assemble Ag nanostructures onto the tapered-end of the sample fiber (Fig. 1), which is capable of maximizing the surface coverage of AgNPs. To achieve this aim, firstly, we use ethanol to clean the lubricants and dirt produced during the process of removing the coating layer. Secondly, the tapered fiber is dipped in a mixture solution (0.1 g NH4F, 1.75 mL HF solution, 3.25 mL distilled water) for 5 min for coarsening (to enhance the binding force of the fiber and Ag nanoparticles), then dipped in another mixture solution (0.1 g SnF2, 0.75 mL HF solution, and 49.25 mL distilled water) for 5 min for sensitization (to be beneficial to AgNPs deposition), and then washed in ethanol, and dried in vacuum. After that, it is immersed in another mixture solution (1 mL 0.1 mol/L AgNO3, 1 mL 10 g/L seignette salt solution, and appropriate amount of ammonia water) in 80 °C water bath for 65 s. Finally, it is cleaned by ethanol and dried in vacuum.
Experimental Configuration for SERS Measurement
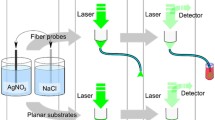
Experimental setup for SERS detection using the prepared tapered fiber probes is schematically shown in Fig. 2. The fiber SERS probes are coupled to a Raman spectrometer (Horiba Jobin Yvon LabRAM HR Evolution), with an excitation wavelength of 532 nm, power of 50 mW (available filter of 1 %, 5 %, 10 %, 25 %, 50 %), objective lens of ×10 (numerical aperture (NA) of 0.25, working distance (WD) of 10.6 mm), ×50 (NA = 0.75, WD = 0.37 mm), and ×100 (NA = 0.9, WD = 0.21 mm). All spectra are collected using an accumulation time of 2 s.
Schematic experiment setup of SERS measurement; the sample is coupled to a wavelength excitation (532 nm) Raman spectrometer, working at direct scattering configuration (10 % filter to the laser source, ×50 objective lens) and remote scattering one (25 % filter to the laser source, ×10 objective lens). The optical image shows the R6G solution at different concentrations
In order to compare direct and remote detection activity of our fiber probe, there are two working configurations, direct scattering configuration and remote scattering one. For direct scattering configuration, the SERS-active end of the fiber is fixed perpendicularly to the objective lens by a fiber holder, by which the position of the fiber probe can be adjusted. While for remote scattering configuration, flat-end of the fiber is coupled to the optics system, the same fiber carries the excitation light to the fiber tip and provides a return light path for the Raman-scattered light to the collecting optics system and the Raman spectrometer. There are two points worth mentioning: (1) the thickness of the decorated metal on the fiber tip should allow optical transmission to the analyte and its return to the fiber core; (2) the length of the fiber is limited by Raman contribution of the fiber itself [4, 23].
Considering the difference of light transmission route and loss, the Raman spectrometer setup parameter is different for two different scattering configurations, 10 % filter and ×50 objective lens for direct configuration, and 25 % filter to the laser source, ×10 objective lens for remote configuration.
Prior to detection, different amounts of the R6G molecule are dissolved in diluted water to make solutions with different concentrations ranging from 1 × 10−7 to 1 × 10−4 M. Droplets of 10 μL are dropped on the tip of the fiber substrate and naturally dried in air before SERS characterization (except part 3.4).
Experimental Results
Sample Characterization
The Bare Fiber Taper
Optical microscope photograph of the fiber sample is presented in Fig. 3a, when the etching time was about 28 min. It can be seen that the tip constructed by dynamic chemical etching formed a tapered region, where the fiber taper was about 28°, while the taper length was measured at around 240–260 μm.
During the etching process, two different etching processes start simultaneously [24–26], one is bulk etching, occurring in the bulk HF fluid, and the other is meniscus etching, taking place at the HF-edible oil-fiber interface. The two processes proceed on different time scales and give rise to two different tip shapes. The former process with a higher etching rate gradient (because of the larger silica surface exposed to the HF) is faster than the latter (driven by HF-edible oil) [24]. The dynamic process of tip formation is as follows: HF attacks the fiber, reducing its radius; the oil layer covers the other part and prevents it from further corrosion; and the etching process terminates when the fiber core is totally etched and the conical tip is completely shaped, shown in Fig. 4a (the first row). The fiber tip is related with process parameters, including the contact angle, the Euler constant, the surface tension, the density of the liquid, and the gravity acceleration constant [25].
a The fiber is immersed in the mixture, then the etching process begins, finally the etching process terminates when the fiber core is totally etched and the final tip is formed. The second row shows the images of the tip of the fiber from the beginning of etching to 28 min, at a 5-min interval. b Plot of the fiber diameter vs. the etching time. The red lines are linearly fit for two different stages, and the pink line indicates the core diameter value (horizontal)
In order to investigate the etching process, we considered the diameter change with the etching time. We etched a piece of fiber, 20 mm long, retracting it every 5 min. The optical images of the final conical tip at different etching times are given in Fig. 4a (the second row). The diameter as a function of the etching time is presented in Fig. 4b (black square), and the diameter decreases with the etching time. When the etching time is less than 25 min, the diameter fits in the following relation: d(t) = d 0 − αt, where d 0 is the cladding diameter of 125 μm, and α is the etching rate of 2.75 according to the experimental data. As the etching time becomes longer than 25 min, the diameter reduces sharply.
During the etching process, the Si–O bond is broken and exchanged with a Si–F one. Therefore, the concentrations of all the H+, HF, and HF2 − ions are important [24]. The presence of an oil layer can slow down the meniscus rise time [27]. A surface roughness induced by the chemical attack can lead to some meniscus pinning effects [28]. Moreover, according to the effects of the HF-oil interface and HF concentration, all these effects and processes result in a variation of the final tip angle.
When the tapered fiber probe is used for remote-excitation SERS, the small cone angle can increase the SERS-active area and numbers of internal reflections, thus allowing more excitation laser to interact with Ag nanoparticles and target molecule. Although smaller core angle could be better for Raman signal enhancement, the tapered SERS probe with an even smaller cone angle is more fragile and difficult to prepare with our current method. The fact is that the tapered fiber requires removing the protective coating, etching of the fiber to decrease the cladding thickness, which makes the tapered portion more vulnerable and fragile [21]. Therefore, in this paper, through lots of sample preparation experiments, the optimized cone angle for tapered fiber probes used for SERS activity experiments is 28° under our current experimental conditions.
The Fiber SERS Probe
The tapered fiber SERS probe was prepared by direct composition of AgNPs on the fiber taper surface through the chemical deposition method. Figure 3b gives an overall SEM image of the tapered fiber probe we prepared, and reveals that it still retains the tapered structure after the chemical deposition process, and the fiber taper surface is covered by a layer of silver. In enlarged SEM image (Fig. 3c), the AgNPs are uniformly distributed on the probe surface, with a diameter of less than 100 nm, the gap of neighboring AgNPs of 1∼10 nm. Figure 3d shows the size distribution histograms of AgNPs, revealing that the diameter of AgNPs is concentrated at the range of 40 to 80 nm, with an average diameter of 57.7 nm. In order to keep Ag nanoparticles coating on the surface of the fiber, there are several important process parameters to be taken into consideration, such as the concentration of AgNO3, the solution PH value, the deposition temperature, time, etc. After many experiments of tuning parameters, we chose parameters with AgNO3 concentration of 0.1 mol/L, PH of 8, deposition temperature of 80 °C, and deposition time of 65 s.
Effect of Different R6G Concentrations
The SERS performances of the tapered fiber probes were investigated by using R6G as a target molecule. Figure 5 illustrates the SERS response of our fiber probes, with R6G solution of different concentrations (10−4, 10−5, 10−6, and 10−7 M), while Fig. 5a for direct scattering configuration and Fig. 5b for remote scattering one, respectively. The characteristic peaks of R6G molecules are presented at 614, 773, 1187, 1312, 1365, 1509, 1575, and 1652 cm−1, and the observed Raman peaks can be attributed to the C-C-C ring in-plane, out-of-plane, and C-H in-plane bending vibrations, and to symmetric modes of in-plane C-C stretching vibrations [29].
With baseline correction obtained by subtracting the background signals introduced by the fiber itself, Fig. 6 shows the corresponding SERS intensities of R6G. The Raman peaks observed in the SERS spectra are consistent with previous report [8, 9]. For remote configuration (Fig. 6b), the spectrum peaks are still clearly visible at the concentration of 10−6 M, demonstrating high sensitivity of the tapered fiber probe based on silver nanoparticles. In both working configurations, the Ag nanoparticles in AgNPs-fiber composites could serve as hot spots with large electromagnetic field contributing to the generation of high SERS enhancement. The capability of detecting molecules at low concentrations makes our fiber SERS probe promising for future applications.
Some typical Raman peak intensities at different concentrations are shown in Table 1 (direct scattering) and Table 2 (remote scattering).
Enhancement Factor Calculation
The enhancement factor (EF) strongly depends on the exact SERS conditions, such as substrate, analyte, excitation wavelength, etc. There is a variety of major definitions to EF [30]: (a) single molecule enhancement factor (SMEF) which is suitable for theoretical estimations of the EF, rather than experimental measurements; (b) average SERS EF, which is used to compare the average SERS enhancements across different substrates; and (c) the analytical enhancement factor (AEF), which is also directly perceivable and particularly relevant for analytical chemistry applications, and suitable for SERS in active liquids. The AEF can be defined as AEF = (I SERS /c SERS )/(I RS /c RS ), where I RS is a Raman signal under non-SERS conditions with an analyte solution concentration c RS, I SERS is a Raman signal under SERS conditions with an analyte solution concentration c SERS . The AEF is a simple figure for the EF and is easily reproducible. In this paper, we calculate the AEF, with our fiber probe working both at direct and remote configurations. Figure 7 gives the SERS signal of probe fiber and fluorescence signal of the tapered fiber (non-SERS condition), for 10−4 M R6G concentration, while working at direct configuration (Fig. 7a) and remote configurations (Fig. 7b). The AEF calculated is given in Table 3.
For the values of AEF, we intend to discuss the following aspects:
-
(1)
In fact, small variations in AEFs could be partly attributed to electromagnetic effects. The SERS effect greatly depends on the dimension, shape, size, composition, and agglomeration of the metallic nanostructures [21, 31]. The observed SERS signals originate from a small congregation of molecules located at hot spots exhibiting higher enhancement. If we could position all or most of the analyst molecules at these hot spots, much larger enhancement could be obtained. Besides, the random distribution of Ag nanoparticles would influence the stability of the final SERS results. Different Ag size leads to different maximal absorption wavelength. The non-uniformity of the AgNPs in the sample we prepared could lead to different resonance absorption wavelength, which results in the different enhancement activity, thus affects the AEFs.
-
(2)
The changes in relative peak intensities from non-SERS to SERS are directly reflected in the variations of the AEFs at different peaks. As demonstrated in Table 3, comparing AEF at 614 cm−1 with that at other energy modes, there is a trend of a slight decrease of the AEFs. The AEF is a factor of ∼2.8 and 2.7 smaller for modes around 1652 cm−1 as compared to the low-energy mode of 614 cm−1, when working at direct and remote configurations, respectively. This could be explained by the fact that the electromagnetic EFs in SERS have two multiplicative contributions from the underlying plasmon resonance, one at the incident laser wavelength and one at the Stokes wavelength [12]. Because of the finite spectral width of the plasmon resonance, it is not possible to simultaneously fulfill resonance conditions for large Stokes shifts.
-
(3)
For I SERS , one can tune the localized surface plasmon resonance (LSPR) frequency of the substrate to match the incident laser frequency, and the effective coupling between metal nanoparticles can be induced to maximize the enhancement [32]. So, for prepared samples, the excitation wavelength would influence I SERS , and thus affect the AEF. Meanwhile, I SERS is a function of number of analyte adsorbed, so the higher molecule concentration, the higher Raman intensity. In order to obtain optimal enhancement, excitation laser wavelength would be tuned to different SERS substrates, while taking consideration of the LSPR frequency, metal materials, and size. In this paper, 532-nm laser is used as incident laser, and the same molecule concentration is used to calculate AEF.
-
(4)
In addition, the effect of the chemisorption of molecules onto the Ag nanoparticles could be a related factor. The detection of different molecules could lead to different results, which is affected by two factors [3]: (a) the exact number of molecules adsorbed on the surface of the fiber probe, (b) the Raman emission efficiency of molecules adsorbed at different positions of the fiber probe.
Time-Course SERS Measurement
In this section, we mainly focus on the SERS performance during the evaporation process. Time-course SERS measurement is performed to monitor the evaporation process on the fiber probe, working at remote configuration (Fig. 8). Before detection, the probe fiber is dipped in the R6G solution (10−5 M concentration) for 1 min, then taken to detection without other drying process.
During the first 2 min, the fluorescent signal is obvious, which could be attributed to the fluorescent enlargement of the fiber to the solvent liquid. Nevertheless, the fluorescent signals gradually reduce and the fingerprints of R6G enhance and become stable after 2 min. During the evaporation process, the R6G solution on the surface of the fiber probe becomes nearly stable. The number of molecules excited by surface plasmon of Ag nanoparticles remains unchanged in the later stage of the evaporation process. When the R6G solution has dried completely, the interface effect between the Ag nanoparticles and molecules remains stable, which leads to stable SERS signals. By reduction of the background noise and baseline correction, the SERS signals as a function of evaporation time are shown in Fig. 8b. Some obvious Raman peak intensities are given in Fig. 8c.
Reproducibility of Different SERS Probes
To test whether the as-prepared fiber SERS probes with the same preparation process are able to give reproducible SERS signals, we collected SERS spectra of R6G molecules with a concentration of 10−4 M from 8 probes, shown in Fig. 9. Qualitatively, the Raman signals of R6G maintained their fine features and intensities. The relative standard deviation (RSD) is used to estimate the reproducibility of SERS probes. The RSD values corresponding to the major Raman peaks are shown in Table 4, revealing a good reproducibility. The difference among the probes could be due to the random distribution of Ag nanoparticles.
Conclusions
In summary, the SERS probes based on combination of optical fiber and Ag nanoparticles are presented, by a simple and low-cost etching and deposition method. The probes show effective SERS activity, working at direct and remote scattering configurations. The sensitivity could reach 10−7 M and AEFs of on the order of ∼108 with R6G as target molecules, which shows potential application in sensing and SERS detection. In order to improve the SERS activities, efforts could be made in the following aspects: (1) optimization of the angle of the fiber, (2) uniformity of nanoparticles decorated on the tapered fiber tip, and (3) an attempt of on-line detection.
References
Andrade GFS, Hayashi JG, Rahman MM, Salcedo WJ, Cordeiro CMB, Brolo AG (2013) Surface-enhanced resonance Raman scattering (SERRS) using Au nanohole arrays on optical fiber tips. Plasmonics 8(2):113–1121
Wolfbeis OS (2006) Fiber-optic chemical sensors and biosensors. Anal Chem 78:3859–3873
Liu T, Zhou L, Zhang Z, Xiao X, Zhou M, Yang C (2014) Combined taper-and-cylinder optical fiber probes for highly sensitive surface-enhanced Raman scattering. Appl Phys B 116(4):799–803
Kostovski G, Stoddart PR, Mitchell A (2014) The optical fiber tip: an inherently light-coupled microscopic platform for micro- and nanotechnologies. Adv Mater 26:3798–3820
Fleischmann M, Hendra PJ, McQuillan AJ (1974) Raman spectra of pyridine adsorbed at a silver electrode. Chem Phys Lett 26:163–166
Jeanmaire DL, Van Duyne RP (1977) Surface Raman spectroelectrochemistry part i. heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J Electroanal Chem 84:1–20
Zhang J, Zhang X, Lai C, Zhou H, Zhu Y (2014) Silver-decorated aligned CNT arrays as SERS substrates by high temperature annealing. Opt Express 22(18):21157–21166
Zhang J, Fan T, Zhang X, Lai C, Zhu Y (2014) Three-dimensional multi-walled carbon nanotube arrays coated by gold-sol as a surface-enhanced Raman scattering substrate. Appl Opt 53(6):1159–1165
Gong T, Zhu Y, Zhang J, Ren W, Quan J, Wang N (2015) Study on surface-enhanced Raman scattering substrates structured with hybrid Ag nanoparticles and few-layer graphene. Carbon 87:385–394
Stoddart PR, White DJ (2009) Optical fiber SERS sensor. Anal Bioanal Chem 394:1761–1774
Homola J, Yee SS, Gauglitz G (1999) Surface plasmon resonance sensors: review. Sensors Actuators B 54:3–15
Sharma AK, Jha R, Gupta BD (2007) Fiber-optic sensors based on surface plasmon resonance: a comprehensive review. IEEE Sensors J 7:1118–1129
Guieu V, Lagugne-labarthet F, Talaga D, Servant L, Sojic N (2008) Ultrasharp optical-fiber nanoprobe array for Raman direct-enhancement imaging. Small 4(1):96–99
Brolo AG, Arctander E, Gordon R, Leathem B, Kavanah KL (2004) Nanohole-enhanced Raman scattering. Nano Lett 4(10):2015–2018
Zheng XL, Guo DW, Shao YL, Jia SJ, Xu SP, Zhao B, Xu WQ, Corredor C, Lombardi JR (2008) Photochemical modification of an optical fiber tip with a silver nanoparticle film: a SERS chemical sensor. Langmuir 24(8):4394–4398
Kostovski G, White DJ, Mitchell A, Austin MW, Stoddart PR (2009) Nanoimprinted optical fibers: biotemplated nanostructures for SERS sensing. Biosens Bioelectron 24:1531–1535
Smythe EJ, Dickey MD, Bao JM, Whitesides GM, Capasso F (2009) Optical antenna arrays on a fiber facet for in situ surface-enhanced Raman scattering detection. Nano Lett 9(3):1132–1138
Andrade GFS, Fan MK, Brolo AG (2010) Multilayer silver nanoparticles-modified optical fiber tip for high performance SERS remote sensing. Biosens Bioelectron 25(10):2270–2275
Lim JW, Kang IJ (2014) Fabrication of chitosan-gold nanocomposites combined with optical fiber as SERS substrates to detect dopamine molecules. Bull Kor Chem Soc 35(1):25–29
Li L, Liu S, Chen Z, Dai Z, Chen N, Pang F, Shang Y, Lu B, Wang T (2014) Remote detection of the surface-enhanced Raman spectrum with the optical fiber nanoprobe. Opt Spectrosc 116(4):575–578
Cao J, Wang J (2015) Development of Ag nanopolyhedra based fiber-optic probes for high performance SERS detection. New J Chem 39:2421–2424
Viets C, Hill W (2001) Fiber-optic SERS sensors with conically etched tips. J Mol Struct 563–564:163–166
Jayawardhana S, Kostovski G, Mazzolini AP, Stoddart PR (2011) Optical fiber sensor based on oblique angle deposition. Appl Opt 50(2):155–162
Foti A, D’Andrea C, Bonaccorso F, Lanza M, Calogero G, Messina E, Marago OM, Fazio B, Gucciardi PG (2013) A shape-engineered surface-enhanced Raman scattering optical fiber sensor working from the visible to the near-infrared. Plasmonics 8(1):13–23
Puygranier BAF, Dawson P (2000) Chemical etching of optical fibre tips—experiment and model. Ultramicroscopy 85(4):235–248
Puygranier BAF, Montgomery S, Ashe J, Turner RJ, Dawson P (2001) Imaging tip formation in single-mode optical fibres. Ultramicroscopy 86(1–2):233–239
Clanet C, Quere D (2002) Onset of menisci. J Fluid Mech 460:131–149
Schaffer E, Wong PZ (1998) Dynamics of contact line pinning in capillary rise and fall. Phys Rev Lett 80(14):3069–3072
Tang H, Meng G, Huang Q, Zhang Z, Huang Z, Zhu C (2012) Arrays of cone-shaped ZnO nanorods decorated with Ag nanoparticles as 3D surface-enhanced Raman scattering substrates for rapid detection of trace. Polychlorinated biphenyls. Adv Funct Mater 22(10):218–224
Ru ECL, Blackie E, Meyer M, Etchegoin PG (2007) Surface enhanced Raman scattering enhancement factors: a comprehensive study. J Phys Chem C 111(37):13794–13803
Ahn H, Thiyagarajan P, Jia L, Kim S, Yoon J, Thomas EL, Jang J (2013) An optimal substrate design for SERS: dual-scale diamond-shaped gold nano-structures fabricated via interference lithography. Nanoscale 5(5):1836–1842
Lin X, Cui Y, Xu Y, Ren B, Tian T (2009) Surface-enhanced Raman spectroscopy: substrate-related issues. Anal Bioanal Chem 394:1729–1745
Acknowledgments
We would like to thank Mr. Xueqiang Qi from the College of Chemistry and Chemical Engineering in Chongqing University for SEM and Raman spectrometer help. This research is funded by National Natural Science Foundation of China (No. 61376121), National High Technology Research and Development Program of China (863 Program, No. 2015AA034801), and the Fundamental research Funds for the central Universities (106112013CDJZR 125502, 20003, 20008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Chen, S., Gong, T. et al. Tapered Fiber Probe Modified by Ag Nanoparticles for SERS Detection. Plasmonics 11, 743–751 (2016). https://doi.org/10.1007/s11468-015-0105-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11468-015-0105-1