Abstract
A combined taper-and-cylinder optical fiber fabricated by simple tube-etching and modified with silver nanoparticles is developed for surface-enhanced Raman scattering (SERS) sensing. It has the advantages of high light transmission efficiency and large interaction areas for light and silver nanoparticles. The detection of rhodamine 6G in remote mode indicates that the sensitivity could realize 10−13 M. The fiber SERS probe with high flexibility and sensitivity shows great potential for molecule detection in various sensing applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Surface-enhanced Raman scattering (SERS) is an important tool for molecular characterization due to its capability of providing “fingerprints” of molecules in low concentrations. Since its discovery in 1974 [1], it has attracted extensive attentions and been widely used in various fields, such as biology, chemistry, environment and military. Compared with traditional SERS substrates fixing metal nanostructures on glass [2–4] or quartz [5, 6] plates, fiber SERS probes are produced by directly preparing metal nanostructures on optical fiber, which are more simple and effective. Moreover, they can realize remote sensing and in situ detection due to the flexibility of optical fiber.
Since the original single multimode fiber SERS probe was first demonstrated by Mullen and Carron in 1991 [7], many improvements have been made to realize higher sensitivity and better reproducibility of fiber SERS detections. These improvements are generally made with the optimization of fiber sensing tips and the preparation of SERS substrates. Several fiber tips have been made for SERS detections recently. Among them, end facet-based fiber probes [8–11] are the most commonly used because of their easier preparation. These probes are made by directly fixing metal nanostructures on the end facet of the fiber. But limited by the laser light propagating through the core region of the fiber, only the metal nanostructures around the core area can be excited for SERS, which decreases the detection sensitivity of the fiber SERS sensor. To address the restriction, tapered fiber tips [12–14] and double-tapered fiber tips [15, 16] are proposed. They are fabricated by modifying metal nanoparticles on the tapered surface of the fiber, which increases the interaction area between the laser light and the metal nanoparticles. However, the continuous taper leading to the mode mismatch still causes a large loss of signal or laser light. Furthermore, their tips are so fragile that they need special protections. In recent years, photonic crystal fiber (PCF)-based fiber probes [17–19] have been developed for SERS detections. The pore structures of PCF provide spaces for the interaction between the light and the metal nanoparticles. However, the high cost and difficult integration with other equipments restrict their use in applications.
An alternative method is to use combined taper-and-cylinder optical fiber (CTOF) consisting of a tapered and a cylindrical portion [20] as SERS probe. The tapered portion serving as the mode converter can reduce the light transmission loss between the non-sensing portion and the sensing portion, which increases the utilization of excitation laser and collection of SERS signal. The cylindrical portion for sensing can provide large interaction area for excitation laser and the metal nanoparticles so that the sensitivity could be improved. Moreover, the CTOF could realize evanescent wave excitation to reduce the metal nanoparticle damage caused by the high-power laser. Additionally, silver colloid is an effective SERS substrate with high-enhancement factor. Here, the CTOF fabricated by simple tube-etching and modified with silver nanoparticles (AgNPs) through silver colloid is used for SERS sensing. The detection of rhodamine 6G (R6G) shows that the sensitivity of the CTOF probe can realize 10−13 M. The improvement of sensitivity and flexibility shows potential applications in various fields.
2 Experiment
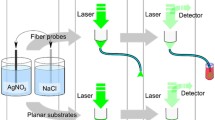
The principle schematic of the CTOF probe for SERS sensing is shown in Fig. 1a. The CTOF is composed of a tapered portion and a cylindrical portion, which are both modified with AgNPs. The tapered portion functions as a waveguide mode converter so that the light can leakage-freely transmit between the non-sensing portion and the sensing portion if the parameters of the tapered portion are properly designed. Since the cladding is replaced by the aqueous phase, the mode capacity would be different between the non-sensing portion and the sensing portion. The mismatch in mode capacity can be calculated by comparing the V-number
which is a dimensionless number that indicates how many modes the fiber waveguide can support. To satisfy V-number matching, the diameter of the taper end d match should be lower than that of the clad fiber and is determined by the following equation:
where d cl is the diameter of the clad fiber; n co is the refractive index of the fiber core; n cl is the refractive index of the fiber cladding; and n aq is the refractive index of the aqueous solution. When transmitted from the taper end to the cylindrical portion, the laser light exists in the form of evanescent wave, which could excite the metal nanoparticles generating surface plasmon resonance (SPR) effect and causing the enhancement of the localized fields. Once the sample molecules are located in the enhanced localized fields, their Raman signal could be greatly enhanced. Meanwhile, the SERS light radiates isotropically, and some of the light is coupled back into the probe and detected as signal.
The fiber used in the experiment is the common multimode quartz optical fiber (NA = 0.22) with a core diameter of 105 μm and a cladding diameter of 125 μm. In view of the parameter values that n co = 1.456, n cl = 1.444 and n aq = 1.333, the diameter of the taper end should be d match = 40 μm. Considering that the maximum incident angle of the fiber is 8.5°, the taper length should be larger than 243 μm to meet the total internal reflection condition. The CTOF satisfying the above conditions is fabricated by simple tube-etching method [21] using 40 % hydrofluoric acid (HF) as the etchant. Prior to etching, the fiber coatings from 2.5 cm along the fiber distal ends are removed by the fiber buffer stripper. The coating-removed fibers are then fixed on a homemade holder which can hold 10 fibers at a time. After that, these fibers with a length of 3 cm along the fiber distal ends are immersed into HF. The CTOFs are formed under the effect of the static tube-etching. The parameters of the CTOFs can be controlled by etching time and temperature. The microscope photograph of the prepared CTOF is shown in Fig. 1b. The lengths of the tapered portion and the cylindrical portion are, respectively, about 0.03 cm and 2.5 cm, and the taper end diameter is appropriately 40 μm, which conform to the previous model. To remove the residual HF, the probe is cleaned with a piranha solution (2:1 mixture of concentrated sulfuric acid and 30 % hydrogen peroxide) and washed with deionized water. Due to the affinity of metal nanoparticles toward amine group [22], the probe is further functionalized with (3-aminopropyl) triethoxysilane (APTES) to introduce amine terminal groups. Then, the silanized surface is soaked in colloidal silver solution to allow the modification of the AgNPs, which are synthesized using the Lee and Meisel protocol [23]. Basically, silver nitrate is used as the metal precursor and sodium citrate as the reducing agent. The morphology of the immobilized AgNPs is observed by the scanning electron microscopy (SEM, Opton). The SPR spectrum of the AgNPs is measured by the UV–Vis spectrophotometer (PerkinElmer LAMBDA 950).
Rhodamine 6G (R6G) is taken as the sample molecules to test the SERS activity of the CTOF probe. To avoid exciting the additional background fluorescence of R6G in the sample solution, the detections are performed in water. Prior to detection, the fiber probe is cut into a segment of 15 cm length and the modified end of the probe is immersed into the sample solution for 3 min and then removed. The detection is made in remote mode employing the typical optrode configuration in which the excitation laser and back-scattering SERS signal are, respectively, coupled and collected from the modified end of the probe. All the detections are carried out by a microscopic confocal Raman spectrometer (Renishaw System RM2000), which operates with the excitation laser of 514 nm, 4.7 mW. A 5 × objective lens is used to focus the excitation light into the fiber core and to collect the scattered Raman signal. All the SERS spectra are obtained with the scanning time of 10 s and the accumulation time of one.
The performance of the CTOF probe is evaluated in two stages. Firstly, the silanized CTOFs without modification of AgNPs are used for measurement to determine the enhancement factor of the AgNPs. The measurement solutions are R6G, the mixture of R6G and AgNPs with the volume ratio of 1:1, the mixture of R6G, AgNPs and sodium chloride (NaCl) with the volume ratio of 5:5:1, respectively. The concentrations of R6G and NaCl are, respectively, 10−9 M and 10 mM. Then, the AgNPs-modified fiber probes are directly used to detect R6G aqueous solutions with serial concentrations (10−10, 10−11, 10−12, 10−13 M) to evaluate the sensitivity.
3 Results and discussion
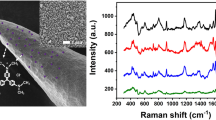
Figure 2a shows the SEM image of the AgNPs modified to the fiber probe. It can be seen that they are relatively evenly distributed on the surface of the probe with sizes ranging from 20 to 30 nm. The UV–Vis spectrum of the silver colloid aqueous is given in Fig. 2b, which indicates that the SPR peak wavelength of the AgNPs is around 410 nm. The concentration of AgNPs is estimated to be 3 × 10−11 M.
The SERS enhancement factor of AgNPs is estimated by the measurement using the silanized CTOFs without modification of AgNPs as demonstrated in Fig. 3. Curve A is the background spectrum of the CTOF. As seen, it has no effect on the Raman or SERS spectra of sample molecules. Curve B is the Raman spectrum of R6G, which indicates that no regular Raman signal is observed except for the fluorescence background as the resonant Raman scattering cross sections (~10−22 cm2) are much smaller than that of fluorescence (~10−16 cm2) [24]. However, when the sample solution is the mixture of R6G and AgNPs, the fluorescence is quenched and the resonant Raman intensity is dramatically enhanced as shown in curve C. Generally, the ratio between the SERS intensity per molecule and the regular Raman intensity per molecule at the same excitation light is used to account for the SERS enhancement factor. Since the regular Raman signal can be hardly detected, we estimate the enhancement factor in the comparison of Raman scattering cross section and fluorescence cross section. Both of the SERS and fluorescence spectra are taken at the same excitation and detection conditions. By data processing according to Ref. [25], the integrated intensities of SERS and fluorescence are respective ~6,230 and ~216,200 counts s−1. So the intensity of fluorescence signal is 34 times stronger than that of SERS. Considering that the number of R6G molecules in the mixture of R6G and AgNPs is halved than that in R6G solution and the total fluorescence cross section is ~10−16 cm2, the total Raman cross section of R6G in AgNPs is ~5.9 × 10−18 cm2. As the total resonance Raman cross section is ~2.6 × 10−22 cm2 [24], the surface effects account for ~2.3 × 104 enhancement in the experiment. When the solution is further added with NaCl, there is another more ninefold enhancement of the SERS intensity as shown in curve D. It is mainly because that NaCl can induce aggregation of AgNPs in the solution to provide more “hot spots” for sample molecules [26].
The spectra measured by the silanized CTOF. Curve A the background of the CTOF; Curve B R6G; Curve C the mixture of R6G and AgNPs with the volume ratio of 1:1; Curve D the mixture of R6G, AgNPs and NaCl with the volume ratio of 5:5:1. The concentrations of R6G and NaCl are, respectively, 10−9 M and 10 mM
The sensitivity of the CTOF probe for SERS sensing is demonstrated by obtaining the spectrum curves of R6G with serial low concentrations (10−10, 10−11, 10−12, 10−13 M). The detection limit is considered as the minimum concentration of R6G that could be detected. The SERS signals against the R6G concentrations are shown in Fig. 4. Although the detections are made in remote mode, the spectrum peaks are still clearly visible at the low concentrations of R6G. The sensitivity of the R6G detection could realize 10−13 M. To our knowledge, the sensitivity of the CTOF probe for SERS sensing is much better than that of fiber probes with other sensing tips in the optrode detection configuration. By comparison, R6G in colloidal suspensions [27] or on surfaces [28] both could realize single-molecule detection. However, both of them are based on local signal measurements. For R6G in colloidal suspensions, the statistical analysis is needed as the colloid system is highly heterogeneous. For R6G on surfaces, the special measuring sites need to be found as some of the metal nanoparticles are inactive. Conversely, fiber probe measures the sum of the SERS signals of R6G adsorbed on the probe surface, which may lead to better repeatable signals. The premise is that the sample molecules adsorb to the surface of the probe. Furthermore, the relationship between the integrated intensity at 1,362 cm−1 and the concentration is shown in Fig. 5. The SERS intensity versus the concentration plot shows a log–log linear dependence with a linear regression slope (α = log I/log C) of 0.20 and a correlation coefficient (R) of 0.96. The relationship can be used for the concentration measurement of R6G. The detection of different molecules may lead to different results. They could be affected by many factors, such as the number of molecules adsorbed on the probe, the Raman emission efficiency of molecules at different positions of the probe, the preferred chemisorption of molecules onto the silver colloid or the aminosilane and so on. The related mechanism needs further research.
4 Conclusions
In summary, highly sensitive SERS probes based on combination of effective CTOFs and high-enhancement factor AgNPs are presented. The CTOFs are fabricated by a simple and low-cost tube-etching method. The enhancement factor of AgNPs could achieve four orders of magnitude and would be even larger when adding with NaCl. The SERS activity of the CTOF probe is demonstrated in remote detection mode employing R6G as sample molecules. The sensitivity could realize 10−13 M, which is superior to fiber probes with other sensing tips. The CTOF probe with such high sensitivity and flexibility shows the potential applications in SERS detections.
References
M. Fleischmann, P. Hendra, A. McQuillan, Chem. Phys. Lett. 26, 163 (1974)
E.J. Bjerneld, F. Svedberg, M. Käll, Nano Lett. 3, 593 (2003)
L.O. Brown, S.K. Doorn, Langmuir 24, 2178 (2008)
L. Xia, Z. Yang, S. Yin, W. Guo, S. Li, W. Xie, D. Huang, Q. Deng, H. Shi, H. Cui, Opt. Express 21, 11349 (2013)
R. Ossig, Y.-H. Kwon, F. Hubenthal, H.-D. Kronfeldt, Appl. Phys. B 106, 835 (2012)
Y. Yang, Z.-Y. Li, K. Yamaguchi, M. Tanemura, Z. Huang, D. Jiang, Y. Chen, F. Zhou, M. Nogami, Nanoscale 4, 2663 (2012)
K.I. Mullen, K.T. Carron, Anal. Chem. 63, 2196 (1991)
C. Viets, W. Hill, Sens. Actuators B. Chem. 51, 92 (1998)
E.J. Smythe, M.D. Dickey, J. Bao, G.M. Whitesides, F. Capasso, Nano Lett. 9, 1132 (2009)
T. Liu, X. Xiao, C. Yang, Langmuir 27, 4623 (2011)
X. Yang, N. Ileri, C.C. Larson, T.C. Carlson, J.A. Britten, A.S. Chang, C. Gu, T.C. Bond, Opt. Express 20, 24819 (2012)
C. Viets, W. Hill, J. Mol. Struct. 563, 163 (2001)
Z. Xie, J. Tao, Y. Lu, K. Lin, J. Yan, P. Wang, H. Ming, Opt. Commun. 282, 439 (2009)
Q. Fan, J. Cao, Y. Liu, B. Yao, Q. Mao, Appl. Opt. 52, 6163 (2013)
A. Lucotti, G. Zerbi, Sens. Actuators B. Chem. 121, 356 (2007)
A. Pesapane, A. Lucotti, G. Zerbi, J. Raman Spectrosc. 41, 256 (2010)
H. Yan, C. Gu, C.X. Yang, J. Liu, G.F. Jin, J.T. Zhang, L.T. Hou, Y. Yao, Appl. Phys. Lett. 89, 204101 (2006)
A. Amezcua-Correa, J. Yang, C.E. Finlayson, A.C. Peacock, J.R. Hayes, P.J.A. Sazio, J.J. Baumberg, S.M. Howdle, Adv. Funct. Mater. 17, 2024 (2007)
Y. Zhang, C. Shi, C. Gu, L. Seballos, J.Z. Zhang, Appl. Phys. Lett. 90, 193504 (2007)
F. Long, M. He, H.C. Shi, A.N. Zhu, Biosens. Bioelectron. 23, 952 (2008)
R. Stockle, C. Fokas, V. Deckert, R. Zenobi, B. Sick, B. Hecht, U.P. Wild, Appl. Phys. Lett. 75, 160 (1999)
K. Aslan, P. Holley, C.D. Geddes, J. Mater. Chem. 16, 2846 (2006)
P. Lee, D. Meisel, J. Phys. Chem. 86, 3391 (1982)
S. Shim, C.M. Stuart, R.A. Mathies, Chem. Phys. Chem. 9, 697 (2008)
P. Etchegoin, E. Le Ru, Phys. Chem. Chem. Phys. 10, 6079 (2008)
A.M. Michaels, J. Jiang, L. Brus, J. Phys. Chem. B 104, 11965 (2000)
K. Kneipp, Y. Wang, H. Kneipp, L.T. Perelman, I. Itzkan, R.R. Dasari, M.S. Feld, Phys. Rev. Lett. 78, 1667 (1997)
S. Nie, S.R. Emory, Science 275, 1102 (1997)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, T., Zhou, L., Zhang, Z. et al. Combined taper-and-cylinder optical fiber probes for highly sensitive surface-enhanced Raman scattering. Appl. Phys. B 116, 799–803 (2014). https://doi.org/10.1007/s00340-014-5764-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-014-5764-7