Abstract
Ground granulated blast-furnace slag (GGBS), activated with olivine (Mg2SiO4) and sodium hydroxide (NaOH), was used to stabilise a clayey soil. Mechanical and microstructural properties of the stabilised soil were assessed through uniaxial compression strength (UCS) tests, X-ray diffraction, scanning electron microscopy and energy-dispersive X-ray spectroscopy (EDS), after curing periods of 7, 18 and 90 days. The UCS of the GGBS-treated soil (without activation with NaOH), even at the highest slag dosage (G20S), after 90 days, showed only a slight increase (142 kPa) relatively to the original soil. When olivine was added to the GGBS-treated mixture (O20G20S), the UCS increased to 444 kPa, after 90 days. However, when NaOH was used as an activator, the UCS of the olivine–GGBS-treated soil (NO20G20S) increased to more than 6000 kPa, after 90 days. This significant strength increase was attributed to the higher reaction degree provided by the NaOH, which enabled a more effective exploitation (dissolution) of the Ca and Mg present in the slag and olivine, respectively, forming a mixture of C–S–H and M–S–H gels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Among the several ground improvement techniques now available, soil stabilisation with cement and lime is mostly and extensively used in road and railways, airport pavements, shallow foundations, embankments and deep soil stabilisation [3, 14, 15, 24, 25]. Although such traditional binders can improve many engineering properties of the original soils, they also possess several shortcomings, especially when viewed from an environmental perspective. In the case of Portland cement (OPC), its production requires high energy inputs and generates around 7% of anthropogenic CO2 emissions [11]. It is estimated that every ton of cement produces nearly an equivalent amount of CO2, a greenhouse gas that plays a major role in global warming [18, 20]. In addition to the CO2 emissions, another by-product of cement production is NOx. Indeed, a very significant volume of nitrogen oxides are produced in cement kilns, which can also contribute to the greenhouse effect and acid rain [28].
To reduce the environmental impacts associated with soil stabilisation, efforts are often focused on the development of new soil stabilisation methods that reduce the need for lime and, especially, cement. An interesting alternative are microbial biopolymers (i.e. excretions) capable of significant soil strengthening with as low as 10% of the equivalent cement content [6], or the better-known microbially induced carbonate precipitation technique, used to bind soil particles either for strength increase or pore filling [33]. This technique is already moving to the next evolution stage, as solutions for application of a single all-in-one shot are being successfully tested [7, 30]. Another popular route for developing new and environmentally friendly binders is based on industrial by-products and wastes, preferably those which are mostly constituted by amorphous aluminosilicates and exhibit pozzolanic properties. A wide variety of by-products was already successfully tested, including ground granulated blast-furnace slag (GGBS), which proved to be a promising option for the replacement of traditional binders in soil stabilisation [8, 21]. Apart from the strong environmental benefit of reusing GGBS for soil stabilisation applications, there are also technical and economic reasons and advantages [17, 37].
According to the study conducted by Fasihnikoutalab et al. [29], a layer of Si–Al–O forms on the GGBS particle surfaces, when in contact with water. This layer can absorb H+ ions, resulting in an increase of OH− ions as well as on the pH of the solution. However, this can be insufficient to efficiently break the Si–O and Al–O bonds, thus limiting the formation of calcium silicate hydrate (CSH) and calcium aluminate hydrate (CAH) compounds. Therefore, the hydration of GGBS can be enhanced via chemical activators. Most common activators used for this purpose are lime (calcium oxide, CaO) and calcium hydroxide (Ca(OH)2) [21]. Previous applications of lime–GGBS mixtures in ground improvement included the treatment of sulphate-bearing soils [13, 31, 32] and flooded low-capacity soils [22, 23].
Recent evidence suggests reactive magnesia (MgO) can also act as a sustainable GGBS activator in ground improvement applications. Yi et al. [38] investigated the use of reactive magnesia (MgO) and carbide slag (CS) as sustainable activators for GGBS in clayey soil stabilisation, concluding that the MgO–GGBS stabilised marine clay developed a substantially higher 90-day compressive strength than the corresponding CS–GGBS stabilised marine clay. Also, the 90-day UCS of MgO–GGBS stabilised soil doubled the strength of the same soil stabilised with cement. In a different study, Yi et al. [35] compared the activating efficiency of a MgO–GGBS paste with a GGBS-hydrated lime paste and concluded that reactive MgO could act as an effective alkali activator of GGBS, achieving higher 28-day strength than the corresponding GGBS-hydrated lime system.
Despite these findings, an important obstacle in the widespread application of MgO–GGBS in soil stabilisation is related to environmental and economic issues. Given the fact that global production of MgO is around 20 million tonnes per year, the price of the MgO that is suitable for GGBS activation varies between 180$ and 350$ per ton [1]. Moreover, MgO is usually produced by heating magnesium carbonate, which releases CO2 into the atmosphere [9]. A possible solution is the substitution of the MgO by olivine (Mg2SiO4), a magnesium silicate mineral containing 45–49% of magnesium oxide (MgO) and 40% of silicon dioxide (SiO2), which can be considered a valid alternative source of MgO, to be used in soil improvement [3, 10, 16, 25].
This study investigates the effectiveness of olivine (i.e. individually and in the presence of NaOH) for GGBS activation and for soil stabilisation applications. To achieve this, the UCS test was used as a practical indicator of strength development. The influence of GGBS and olivine contents, as well as curing age, on the mechanical performance of stabilised soil samples is discussed. These outcomes were further supported with microstructural analysis to identify the mechanism responsible for strength development.
2 Experimental work
2.1 Materials
The geotechnical properties and chemical composition of the clayey soil used in this experiment are listed in Tables 1 and 2, respectively. The soil was classified, according to the Unified Soil Classification System [2], as a ‘high-plasticity clay’ (CH).
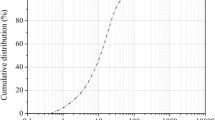
The chemical composition of the olivine mineral, obtained from Maha Chemicals Asia, is also listed in Table 2, showing MgO and SiO2 contents of 48% and 40%, respectively. In its original state, olivine had a significant volume of larger particles, thus requiring ball milling, for 24 h at 60 rpm (around 65% of the critical speed), to decrease and homogenise the particle size distribution, both presented in Fig. 1. This approach was in line with the pre-treatment process reported in earlier studies [3, 25] to increase the specific surface area and, consequently, the reactivity of the olivine.
The GGBS, whose chemical composition is also listed in Table 2, was obtained from the company YTL Cement. Sodium hydroxide (NaOH), supplied in pellets, was employed as an alkali activator after dissolution in distilled water, to a pre-designed concentration of 10 M.
2.2 Specimen preparation and testing
Table 3 presents the composition of the mixtures submitted to the UCS tests. Six distinct groups were defined, each composed by different combinations, namely:
Soil (S).
Sodium hydroxide and soil (NS).
GGBS and soil (GS).
Olivine, GGBS and soil (OGS).
Sodium hydroxide, GGBS and soil (NGS).
Sodium hydroxide, olivine, GGBS and soil (NOGS).
The dry soil was initially mixed with the GGBS and, whenever necessary, with the olivine. For the NGS and NOGS groups, the NaOH solution was added to the solids and thoroughly mixed until a uniform blend was achieved. During this stage, additional water was added to the mixture to meet the optimum moisture content of the stabilised samples.
Standard Proctor compaction tests were conducted for each mixture to obtain the moisture–density relationship of the mixtures [4]. The maximum dry density (MDD) and optimum water content (OWC) of each mixture are presented in Table 3.
Once mixing was completed, the specimens were manually compacted in cylindrical moulds of 50 mm in diameter and 100 mm in height, using a 45 mm diameter steel rod to apply a static load, in three layers. After compaction, the specimens were extruded and immediately wrapped in plastic film and polythene covers to prevent moisture loss. The curing occurred at room temperature (24°) for 7, 28 and 90 days. In order to achieve a state of near saturation, thus avoiding any suction effects, the specimens were unwrapped and submerged in water for the 24 h prior to the UCS test. The exception to this saturation procedure were the S and GS groups, due to the loss of structural integrity of these samples when submerged.
The UCS test was conducted in accordance with Ball et al. [5]. An Instron 3366 universal testing machine, fitted with a 100 kN load cell, was used for the test, which was carried out under monotonic displacement control, at a rate of 0.2 mm/min. The entire stress–strain curve was obtained for each test. Three different specimens were used for each data point.
The effect of the different activators and mix designs on sample development were further investigated via energy-dispersive X-ray spectroscopy (EDS), scanning electron microscopy (SEM) and X-ray diffraction (XRD). Suitable samples for these analyses were extracted from the UCS specimens, after testing. Specimens for SEM/EDS analysis were prepared by crushing the treated soil specimens and then mounting them on Al stubs with double-sided carbon tapes prior to sputter coating with a thin layer of platinum. Analysis was performed on a field emission scanning electron microscope (JSM 5700) coupled with an energy-dispersive X-ray spectrometer. XRD was performed on a Bruker D8 ADVANCE X-ray diffractometer, with CuKα radiation, at 40 kV and 40 mA emission current.
3 Results
3.1 Mechanical performance
The stress–strain behaviour of the olivine–GGBS-treated soil, containing different percentages of olivine and GGBS (OGS group), at curing periods of 7, 28 and 90 days, is shown in Fig. 2. The stress–strain behaviour of the natural soil (S) and the GGBS-treated soil (GS group) is also presented in these figures, for comparison purposes. The 7-day UCS values of the GS group specimens improved slightly with the increase in GGBS content, which is most likely related to a higher volume of calcium silicate hydrate (C–S–H) gel, resulting from the soluble calcium present in the GGBS. The UCS of the mixtures that included olivine in its composition (OGS group) achieved higher values than the corresponding mixtures without olivine (GS group). The presence of olivine creates a source of partially dissolved MgO, allowing the formation of a magnesium silicate hydrate (M–S–H) gel that coexists with the main C–S–H gel.
Regarding the UCS evolution with curing time, as presented in Fig. 3, the data indicate that an increase in GGBS content enhances the influence of curing time on compressive strength, even if the short-term improvement is very similar for all three contents. This effect was also observed for the O15GS and O20GS groups, although only for the 90-day curing period, since the differences after 7 and 38 curing were practically neglectable. The 90-day UCS of the GS and OGS groups was approximately 2× and 11× times higher than the UCS of the natural soil (S), respectively. In short, these results indicate that, for longer curing periods (28 days and, especially, 90 days), the MgO potentiates the GGBS performance.
Figure 4 shows a comparative analysis of the stress–strain behaviour of the NaOH–GGBS–olivine-treated soil (NOGS group), after 7-, 28- and 90-day curing. The stress–strain curves of the natural soil (S), of the soil activated with NaOH (NS) and of the NaOH–GGBS-treated soil (NGS group) were also included in these figures. The sodium hydroxide, as expected, did not produce any effect on the original compressive strength of the soil, showing a very similar stress–strain path during the test, which did not evolve with curing time. After 7-day curing, the UCS of the NGS mixtures slightly increased with higher GGBS contents, suggesting that the presence of GGBS in the NaOH solution formed an aluminium-substituted calcium silicate hydrate gel, commonly known as C–A–S–H gel. The existence of Al ions resulted in a higher degree of polymerisation and, also, on more efficient crosslinking between the C–S–H chains. This finding is consistent with the work of Fasihnikoutalab et al. [27], who found that the availability of Al ions results in the formation of stronger C–S–H chains. Further strength development was achieved by the addition of olivine to the mixture (NOGS group), reaching a maximum value of 6.1 MPa for the highest GGBS and olivine contents. The different UCS obtained by the NGS and NOGS groups was probably due to the higher amount of MgO dissolved by the NaOH.
The influence of the curing period on these pastes activated with sodium hydroxide is clearly lower than that shown for the no-NaOH pastes (Fig. 5), even though the 90-day curing represented the highest UCS values, with the exception of the NO15G20S paste. Nevertheless, the curing period has to be considered a significant variable in the UCS of these pastes, since an increase between 20 and 100% was obtained when the curing period was extended between 7 and 90 days.
3.2 Microstructural analysis
SEM images of the olivine–GGBS-treated soil (O20G20S and NO20G20S mixtures), after 90-day curing, are presented in Fig. 6. The microstructure reveals the formation of a binding gel, resulting from the reactions between the olivine and GGBS precursors and the water or alkaline activator, connecting the unreacted olivine and GGBS particles and the clay particles. However, the use of water alone showed less dense formations (Fig. 6a) than those obtained with an alkaline activator (Fig. 6b), suggesting that the resulting gel and the subsequent crystallisation, produced by the latter, were more effective at occupying the initial voids of the soil, generating a more compact microstructure. This is probably a consequence of a higher dissolution rate of the amorphous species present in the olivine and GGBS [34]. This also explains the higher UCS values obtained by the mixture NO20G20S, as shown in Fig. 6.
The EDX data obtained from mixtures O20G20S and NO20G20S, also shown in Fig. 6 (only two points per image, out of six, are presented) allowed the comparison between the composition of the gels developed with and without NaOH. Ideally, this elemental analysis would have been made using back-scattering, guaranteeing enhanced reliability and precision. Since such option was not available, the spectra obtained can still be used to detect gel areas, by comparison. This semi-quantitative elemental analysis (Na, Si, Al, Ca and Mg) was used in the calculation of the Na/Al, Si/Al, Ca/Al, Mg/Si and Ca/Si atomic ratios, as presented in Table 4.
Differences in the nature of the gel are easily identifiable between the mixture fabricated with a highly alkaline activator and the mixture fabricated with water. With the addition of NaOH, the Si/Al ratio increased, as a result of a more effective capacity, shown by the NaOH-based mixture, to dissolve the Si present in the olivine and GGBS. (Both precursors had originally a significantly lower content in Al than Si.) However, and according to Provis [26], the soil particles could also have reacted with the alkaline solution, thus contributing to the Si released into the ion ‘soup’ that later resulted in the binding gel. The Mg/Si and Ca/Si ratios also increased with the inclusion of sodium hydroxide in the mixture (from 0.031 to 0.063 and 0.124 to 0.133, respectively), suggesting that the Ca from the GGBS and the Mg from the olivine were also more effectively dissolved with the NaOH, favouring the development of a combination of C–S–H and M–S–H gels. The idea that the dissolution of Al was hindered by the presence of NaOH, comparing with the remaining species, is reinforced by the fact that the increase in the Mg/Al and Ca/Al ratios, from OGS to NOGS mixtures (from 0.046 to 0.162 and 0.182 to 0.291, respectively), was significantly higher than the corresponding Mg/Si and Ca/Si increases.
The crystalline phases formed in mixtures O20G20S and NO20G20S, as determined by XRD analysis, are shown in Fig. 7. The main phases observed in the O20G20S sample were quartz, kaolinite, magnesium and magnesium oxide, while calcium oxide and calcium silicate hydrate were also detected. All these are common phases in olivine–GGBS stabilised clayey soils, with intensities varying only with the type of clay mineral. The same main phases were observed in the NO20G20S mixture, although part of the kaolinite phase appears to have been dissolved in the reactions promoted by the alkaline activator. The XRD data supported the presence of gel-like or reticular C–S–H fume in sample O20G20S, as a result of the hydration process, which is in line with the findings reported by Porbaha [9], Riemer [9] and Higgins et al. [19]. The intensity of the magnesium-based peaks is lower in the NO20G20S mixture, revealing that the olivine is more effectively incorporated with NaOH than water. Haha et al. [12] demonstrated that increasing the MgO content in MgO–GGBS mixtures resulted in a higher volume of hydration products and higher strength development in slag pastes activated by NaOH. Therefore, these findings could explain the high strength developed in OGS and NOGS groups presented in Figs. 2, 3, 4, 5, 6 and 7.
4 Discussion
The UCS as a function of the stabiliser/solids ratio, after 7, 28 and 90 days, is presented in Fig. 8. The terms ‘stabiliser’ and ‘solids’ were defined as the sum of components of the stabiliser, in dry form (GGBS + Olivine), and as the sum of these components with the soil (Soil + GGBS + Olivine), respectively.
Both the OGS group (without NaOH) and the NOGS group (with NaOH) are represented. Two observations can easily be drawn: an increase in curing time (up to 90 days) yielded higher compressive strength; and an increase in the stabiliser content was also highly beneficial for strength development. This second observation was particularly valid for the mixtures activated with sodium hydroxide, which showed R-squared values not lower than 95%. The strength gain rate of these mixtures was also superior to that of the OGS mixtures, further highlighting the role of the alkaline activator. The R-squared value for the 90-day curing of the mixtures without NaOH was relatively low (64%), mostly due to the UCS values registered by the mixtures prepared with a stabiliser/solids ratio of 0.35, which are clearly lower than the 0.30 and 0.40 UCS values. This is a possible consequence of the fact that such mixtures were prepared with the lowest olivine/GGBS ratio (0.75) of the whole experimental campaign.
The highest UCS values obtained by the 15% olivine mixtures (either in the OGS and NOGS groups), after 90 days, were inferior to the lowest UCS obtained by the 20% olivine mixtures. However, the latter group also had a higher stabiliser/solids content than the former. In order to better assess the effect of the olivine on the quality of the mixtures, the UCS values obtained with mixtures with the same stabiliser/solids content (0.20 or 0.25) are compared in Fig. 9. The positive influence of the MgO is especially clear with the increase from 0 to 15%, and especially when sodium hydroxide was used. Note that this increase in olivine represented a decrease in the GGBS content, from 20 to 5%, suggesting that the MgO plays a more relevant role than the Ca from the slag. The reason behind the favourable effect of the olivine in the overall mechanical strength of the mixtures is probably related to the capacity of the MgO to reduce porosity [38]. Nonetheless, the increase in olivine from 15 to 20% did not produce such a positive effect, indicating there is an optimum ratio olivine/GGBS.
Other authors, studying the stabilisation of a marine soft clay with GGBS activated by carbide slag [37], found a maximum UCS value of 3.8 MPa (after 28 days) for a carbide/GGBS ratio of 0.15, after which the UCS steadily decreased, reaching a value of 2.5 MPa for a 0.40 ratio. These values were obtained for a GGBS/soil ratio of 0.30. When the GGBS/soil ratio decreased to 0.20, the peak UCS, after 28 days, decreased also to 2.8 MPa, obtained with a carbide/GGBS ratio of 0.25. During the present research, similar GGBS/soil ratios were used (values of 0.053, 0.111 and 0.250), although with very different activator/GGBS ratios (the activator, in this case, was olivine), ranging from 0.75 to 4.0. Nonetheless, the results are comparable and consistent with those presented by other authors, since the UCS, after 28 days, starts at approximately 0.4 MPa and 0.5 MPa, for olivine/GGBS ratios of 0.75 and 1.0, respectively. These values are in line with the findings of the research mentioned above, assuming that the UCS values would continue to decrease with the increase in the activator/GGBS ratio.
A similar study, from the same authors, tested the effectiveness of lime to act as the GGBS activator [39]. The results showed a similar trend, i.e. the existence of an optimum activator/GGBS dry weight ratio, although, in this case, lower UCS values were obtained: approximately 1.8 MPa and 1.6 MPa, for quicklime and hydrated lime, respectively, both with an activator/GGBS ratio of 0.20 and a GGBS/soil ratio of 0.20.
Yi et al. [36] also studied the effect of binders made from GGBS activated either with lime or MgO on the stabilisation of two soils. The results are in accordance with those presented above. The MgO-based UCS results were, once again, far superior to the lime-based results, and the activator (MgO or lime)/GGBS ratio proved also to have an optimum value which, in this case, was again 0.20. Further increase in this ratio was detrimental to the UCS development, even if the binder contents tested are significantly lower (only up to 0.10) than the ones used in the current study.
Based on the results and subsequent discussion and comparison with similar studies, it is possible to assume that the increase in stabiliser content improves the mechanical behaviour of the soil and that the inclusion of olivine has a positive effect on the formation of hydration gel, but also that such olivine content has an optimum value to potentiate the quality of the binding gel formed.
5 Conclusions
The present study focused on the use of olivine, as a reliable and sustainable source of MgO, to enhance the effectiveness of alkali-activated ground granulated blast-furnace slag. The resulting binder was applied to the stabilisation of a clayey soil, which was then assessed through uniaxial compression strength tests, X-ray diffraction, scanning electron microscopy and energy-dispersive X-ray spectroscopy, after curing periods of 7, 18 and 90 days. The following conclusions were drawn:
The high alkalinity of the NaOH promoted a more effective dissolution of the olivine and GGBS precursors, leading also to higher strength development of the stabilised soil mixtures, compared with the water-based mixtures.
UCS results demonstrated that the addition of olivine to the GGBS–soil combinations improved strength development, as demonstrated by the UCS values obtained with 15% and 20% olivine.
The olivine/GGBS ratio should be optimised, as an increase in such ratio produces a strength decrease, for all curing periods, but only up to a certain level.
There was a clear strength increase with curing time, at least until 90 days, regardless of the composition considered.
The UCS clearly increased with the stabiliser/solids wt. ratio. Since this ratio increase represented also a decrease in the olivine/GGBS wt. ratio, it was necessary to establish which of these two factors was responsible for the strength increase.
A combination of C–S–H gel and M–S–H gel was observed in the SEM/EDS analysis, as a result of the addition of olivine (MgO) to the GGBS (CaO) precursor.
References
Al-Tabbaa A, Liu C, Gao L et al (2015) Incorporation of reactive magnesia and quicklime in sustainable binders for soil stabilisation. Eng Geol 195:53–62. https://doi.org/10.1016/j.enggeo.2015.05.025
ASTM D2487-11 (2011) Standard practice for classification of soils for engineering purposes (Unified Soil Classification System). Am Soc Test Mater
Ball RJ, Pourakbar S, Huat BK et al (2016) Utilisation of carbonating olivine for sustainable soil stabilisation. Environ Geotech 4:184–198. https://doi.org/10.1680/jenge.15.00018
BSi 1377–4 (1990) BS 1377-4: 1990 - Methods of test for soils for civil engineering purposes, Part 4: Compaction-Related Tests. Br Stand Institution, London, p 4
BSi 1377–7 (1990) BS 1377-7: 1990 - Methods of test for soils for civil engineering purposes, Part 7: Shear Strength Tests (Total Stress). Br Stand Institution, London, p 7
Chang I, Cho GC (2019) Shear strength behavior and parameters of microbial gellan gum-treated soils: from sand to clay. Acta Geotech 14:361–375
Cheng L, Shahin MA, Chu J (2019) Soil bio-cementation using a new one-phase low-pH injection method. Acta Geotech 14:615–626
Du YJ, Wu J, Bo YL, Jiang NJ (2019) Effects of acid rain on physical, mechanical and chemical properties of GGBS–MgO-solidified/stabilized Pb-contaminated clayey soil. Acta Geotech. https://doi.org/10.1007/s11440-019-00793-y
Fasihnikoutalab MH, Asadi A, Kim Huat B et al (2016) Laboratory-scale model of carbon dioxide deposition for soil stabilisation. J Rock Mech Geotech Eng 8:178–186. https://doi.org/10.1016/j.jrmge.2015.11.001
Fasihnikoutalab MH, Pourakbar S, Ball RJ, Huat BK (2017) The effect of olivine content and curing time on the strength of treated soil in presence of potassium hydroxide. Int J Geosynth Gr Eng 3:12. https://doi.org/10.1007/s40891-017-0089-3
Gartner E (2004) Industrially interesting approaches to “low-CO2” cements. Cem Concr Res 34:1489–1498. https://doi.org/10.1016/j.cemconres.2004.01.021
Haha MB, Lothenbach B, Le Saout G, Winnefeld F (2011) Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part I: effect of MgO. Cem Concr Res 41:955–963. https://doi.org/10.1016/J.CEMCONRES.2011.05.002
Higgins DD, Kinuthia JM, Wild S (1998) Soil stabilization using lime-activated ground granulated blast furnace slag. Spec Publ 178:1057–1074. https://doi.org/10.14359/6023
Horpibulsuk S, Bergado DT, Lorenzo GA (2004) Compressibility of cement-admixed clays at high water content. Géotechnique 54:151–154. https://doi.org/10.1680/geot.54.2.151.36341
Horpibulsuk S, Miura N, Nagaraj TS (2004) Assessment of strength development in cement-admixed high water content clays with Abrams’ law as a basis. Géotechnique 53:439–444. https://doi.org/10.1680/geot.53.4.439.37319
Huat BK, Ball RJ, Asadi A et al (2017) Utilization of alkali-activated olivine in soil stabilization and the effect of carbonation on unconfined compressive strength and microstructure. J Mater Civ Eng 29:06017002. https://doi.org/10.1061/(asce)mt.1943-5533.0001833
Jegandan S, Liska M, Osman AA-M, Al-Tabbaa A (2010) Sustainable binders for soil stabilisation. Proc Inst Civ Eng—Gr Improv 163:53–61. https://doi.org/10.1680/grim.2010.163.1.53
Kim Y, Worrell E (2002) CO2 emission trends in the cement industry: an international comparison. Mitig Adapt Strateg Glob Change 7:115–133. https://doi.org/10.1023/A:1022857829028
Konsta-Gdoutos MS, Shah SP (2003) Hydration and properties of novel blended cements based on cement kiln dust and blast furnace slag. Cem Concr Res 33:1269–1276. https://doi.org/10.1016/S0008-8846(03)00061-9
Lothenbach B, Scrivener K, Hooton RD (2011) Supplementary cementitious materials. Cem Concr Res 41:1244–1256. https://doi.org/10.1016/j.cemconres.2010.12.001
Nidzam RM, Kinuthia JM (2010) Sustainable soil stabilisation with blastfurnace slag—a review. Proc Inst Civ Eng—Constr Mater 163:157–165. https://doi.org/10.1680/coma.2010.163.3.157
Obuzor GN, Kinuthia JM, Robinson RB (2011) Enhancing the durability of flooded low-capacity soils by utilizing lime-activated ground granulated blastfurnace slag (GGBS). Eng Geol 123:179–186. https://doi.org/10.1016/j.enggeo.2011.07.009
Obuzor GN, Kinuthia JM, Robinson RB (2012) Soil stabilisation with lime-activated-GGBS-A mitigation to flooding effects on road structural layers/embankments constructed on floodplains. Eng Geol 151:112–119. https://doi.org/10.1016/j.enggeo.2012.09.010
Porbaha A (1998) State of the art in deep mixing technology: part I. Basic concepts and overview. Proc Inst Civ Eng—Gr Improv 2:81–92. https://doi.org/10.1680/gi.1998.020204
Pourakbar S, Asadi A, Huat BBK, Fasihnikoutalab MH (2015) Stabilization of clayey soil using ultrafine palm oil fuel ash (POFA) and cement. Transp Geotech 3:24–35. https://doi.org/10.1016/j.trgeo.2015.01.002
Provis JL (2014) Geopolymers and other alkali activated materials: why, how, and what? Mater Struct Constr 47:11–25
Richardson IG, Brough AR, Groves GW, Dobson CM (1994) The characterization of hardened alkali-activated blast-furnace slag pastes and the nature of the calcium silicate hydrate (C-S-H) phase. Cem Concr Res 24:813–829. https://doi.org/10.1016/0008-8846(94)90002-7
Riemer P, Hendriks C, Ozawa Meida L et al (2007) Emission reduction of greenhouse gases from the cement industry. Greenhouse Gas Control Technol 4:939–944
Shi C, Day RL (1993) Chemical activation of blended cements made with lime and natural pozzolans. Cem Concr Res 23:1389–1396. https://doi.org/10.1016/0008-8846(93)90076-L
Wang X, Tao J (2019) Polymer-modified microbially induced carbonate precipitation for one-shot targeted and localized soil improvement. Acta Geotech 14:657–671
Wild S, Kinuthia JM, Jones GI, Higgins DD (1998) Effects of partial substitution of lime with ground granulated blast furnace slag (GGBS) on the strength properties of lime-stabilised sulphate-bearing clay soils. Eng Geol 51:37–53. https://doi.org/10.1016/S0013-7952(98)00039-8
Wild S, Kinuthia JM, Robinson RB, Humphreys I (2006) Effects of ground granulated blast furnace slag (GGBS) on the strength and swelling properties of lime-stabilized kaolinite in the presence of sulphates. Clay Miner 31:423–433. https://doi.org/10.1180/claymin.1996.031.3.12
Wu C, Chu J, Wu S et al (2019) Microbially induced calcite precipitation along a circular flow channel under a constant flow condition. Acta Geotech 14:673–683
Yi Y, Liska M, Al-Tabbaa A (2013) Properties and microstructure of GGBS–magnesia pastes. Adv Cem Res 26:114–122. https://doi.org/10.1680/adcr.13.00005
Yi Y, Li C, Liu S, Al-Tabbaa a A (2014) Resistance of MgO–GGBS and CS–GGBS stabilised marine soft clays to sodium sulfate attack. Géotechnique 64:673–679. https://doi.org/10.1680/geot.14.t.012
Yi Y, Liska M, Al-Tabbaa A (2014) Properties of two model soils stabilized with different blends and contents of GGBS, MgO, lime, and PC. J Mater Civ Eng 26:267–274
Yi Y, Gu L, Liu S, Puppala AJ (2015) Carbide slag–activated ground granulated blastfurnace slag for soft clay stabilization. Can Geotech J 52:656–663
Yi Y, Zheng X, Liu S, Al-Tabbaa A (2015) Comparison of reactive magnesia- and carbide slag-activated ground granulated blastfurnace slag and Portland cement for stabilisation of a natural soil. Appl Clay Sci 111:21–26. https://doi.org/10.1016/j.clay.2015.03.023
Yi Y, Gu L, Liu S (2015) Microstructural and mechanical properties of marine soft clay stabilized by lime-activated ground granulated blastfurnace slag. Appl Clay Sci 103:71–76
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fasihnikoutalab, M.H., Pourakbar, S., Ball, R.J. et al. Sustainable soil stabilisation with ground granulated blast-furnace slag activated by olivine and sodium hydroxide. Acta Geotech. 15, 1981–1991 (2020). https://doi.org/10.1007/s11440-019-00884-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11440-019-00884-w