Abstract
Introduction
Throughout the field of orthopedic surgery, there has been a trend toward using smaller incisions and implants that preserve as much normal anatomy as possible. The use of bone sparing technology, such as partial and full surface replacements of the humeral head, while attractive in younger patients, does not allow the best exposure for proper glenoid replacement. Additionally, there are other situations when the use of surface replacements is contraindicated. There are also patients with an existing total elbow replacement or a humeral malunion or deformity in which a traditional long-stem component would not fit. For these reasons, a mini-stem humeral component for total shoulder arthroplasty was developed.
In this study, we hypothesized that total shoulder replacement using the mini-stem humeral component could provide low complication rates and good to excellent results, as measured by postoperative Constant–Murley and UCLA shoulder scores at minimum 2 years postoperatively.
Materials and Methods
This was a retrospective review of the first 49 mini-stem shoulder replacements (47 patients) for primary osteoarthritis. There were 26 male and 23 female patients. UCLA Shoulder Score and Constant Murley Scores were obtained on all patients at a minimum of 2 years postoperatively (average 29 months; range 24–43 months). Radiographs were interpreted by a musculoskeletal radiologist. Intraoperative blood loss was documented as was postoperative pain using a visual analog pain scale.
Results
Patients experienced over 90% good to excellent results at minimum 2 year follow up. ROM improved significantly in all parameters. Postoperative UCLA scores at final follow up averaged 27.5 while Constant–Murley scores averaged 91. Small lucent lines (<1 mm) were noted in 11 patients. Five of 49 stems were placed in varus but the postoperative result was not affected in any of these patients. One patient suffered an acute subscapularis rupture that required repair.
Conclusions
This is the first report to document the efficacy of mini-stemmed humeral components used during total shoulder arthroplasty. Our study group showed good to excellent results as well as improvement in range of motion at minimum 2-year follow-up. The results presented in this study are comparable to previous outcomes achieved with conventional length humeral components, and suggest that mini-stem humeral components are an effective option for total shoulder arthroplasty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Results of traditional shoulder arthroplasty (TSA) for primary osteoarthritis have been predictable and durable, with most series reporting over 90% good to excellent results in long term follow-up [3, 8, 12]. Throughout the field of orthopedic surgery, there has been a trend toward less invasive surgery and implants that preserve as much normal anatomy as possible. Several authors have reported favorable results treating humeral-sided arthritis with humeral surface replacements [7, 11]. The use of bone sparing technology, such as partial and full surface replacements of the humeral head, while attractive in younger patients, does not allow the best exposure for proper glenoid replacement. This is particularly true using a pegged or hybrid type glenoid component. Several authors have showed that when compared to hemiarthroplasty in same patient cohort group, TSA results have consistently trended better with regards to pain relief and functional outcome [4, 6, 10]. Additionally, there are other situations such as extensive humeral head loss (>40%) and soft humeral heads with large bone cysts (as may be seen in rheumatoid arthritis) when the use of surface replacements is contraindicated.
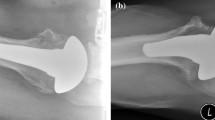
For all of the above reasons, we have utilized a mini-stem humeral component in total shoulder arthroplasty for primary osteoarthritis since 2004 (Fig. 1). This technology seems to have a number of advantages, which include proper glenoid exposure, less intramedullary reaming, and easier stem revision if necessary. It is also useful in proximal diaphyseal shaft malunions (Fig. 2) and in patients with long-stemmed total elbow replacements that make insertion of standard length stems impossible. To evaluate the efficacy of this new implant, we present the initial series of patients treated with this new technology.
In this study, we hypothesized that total shoulder replacement using the mini-stem humeral component could provide low complication rates, improvement in range of motion, and good to excellent results, as measured by postoperative Constant–Murley and UCLA shoulder scores at minimum 2 years postoperatively.
Materials and Methods
This study retrospectively reviewed the first 49 mini-stem shoulder replacements (47 patients) for primary osteoarthritis. There were 26 female and 23 male patients. The average age was 67 years (range 46–83 years). All patients were evaluated at a minimum 2-year follow-up. Visual analog scores were recorded at the final preoperative visit or in the preoperative holding area before surgery and again each day during their hospitalization. Constant–Murley scores and UCLA shoulder scores were recorded at final follow-up, as was postoperative range of motion. Radiographs were taken to document implant position and any abnormalities. The follow-up was a minimum of 2 years and averaged 29 months (range 24–43 months).
All patients were evaluated preoperatively with a physical examination by one of the two senior surgeons (DMD, DWA) and radiographs. Hematocrits within 1 week prior to surgery were documented, and patients documented their preoperative pain using a visual analog pain scale.
Each patient underwent TSR with a pegged or hybrid-pegged glenoid component. The mini humeral stems measured 52–66 mm in length, depending upon stem diameter. They are plasma sprayed proximally for press-fit porous in-growth fixation (Fig. 1). Each humeral mini-stem was press-fit. The head-shaft angle was 55° in the first 40 consecutive shoulders (Biomodular Shoulder, Biomet Inc., Warsaw, Ind.), while in the next 15 shoulder arthroplasties, a 45° head-shaft angle was used. This change in head-shaft angle was introduced in efforts to make the system more anatomic. (Comprehensive Shoulder System, Biomet Inc., Warsaw, Ind.). Operative records were reviewed to document any intraoperative complications.
Hematocrits were recorded on postoperative day 1. Each patient used a visual analog pain scale to document their pain each day during their hospital stay. At a time at least 2 years postoperatively, all patients were assessed with the Constant–Murley Score and The UCLA Shoulder Score. Postoperative X-rays were taken immediately following surgery, at the initial postoperative visit, at least once annually at follow-up visits, and at the most recent follow-up during which data were collected. Images were evaluated independently by an orthopedic radiologist.
Surgical Technique
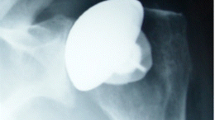
As with all shoulder arthroplasties, use of a mini-stem humeral component requires appropriate pre-operative planning to assess proximal bone stock and to adequately template the humeral shaft diameter to avoid under-reaming and subsequent varus positioning (Fig. 3). Successful placement requires implantation in neutral position to gain appropriate fixation in the proximal diaphyseal shaft.
We utilize a regional intrascalene block with general anesthesia for adequate relaxation and postoperative pain relief. The patient is placed in the beach chair position at 45° with the scapula stabilized and lateralized in order to allow full extension, adduction, and rotation of the freely draped arm.
A deltopectoral approach is carried out; after division of the subscapularis tendon, biceps tenotomy, and soft tissue tenodesis, the humerus is dislocated. An extramedullary cutting guide is placed approximately 4 mm below the native articular margin and the proximal humeral osteotomy is made. This cut must be made precisely to prevent varus or valgus positioning and allow for appropriate version. Next, based upon the pre-op templating, the humeral canal is sequentially reamed starting at the anatomic center of the humeral shaft, which lies about 5–7 mm medial and posterior to bicipital groove. Mini-reamers are utilized to limit diaphyseal cancellous bone destruction. Reaming continues until “chatter” at the final sized reamer.
At this point, the intramedullary cutting guide is assembled on the reamer shaft, and the forearm is externally rotated to the degree of retroversion (usually 30–40°). A reciprocating saw is used to remove the head fragment.
The canal is then rasped to the appropriate size and version. The final rasp is utilized as a dummy component and can be left in place or removed while appropriate glenoid replacement is carried out. The glenoid is replaced in standard fashion.
Once the glenoid component has been fixed appropriately, the humeral head component is selected based on pre-operative templating and direct measurement of the resected humeral head. A provisional trial head component of varying offset options is chosen and placed on the final mini-stem reamer which had been left in the canal as a dummy humeral component. A trial reduction is carried out. We like to have about 50% override of humeral head component on glenoid anteriorly, posteriorly, and inferiorly to allow for appropriate translation.
Once the final component sizes are chosen, the actual mini-stem humeral component is impacted into place, and the selected humeral head component is then impacted on to the stem at the proper degree of offset. Soft tissue balancing and subscapularis tendon repair are carried out, and closure is completed.
All patients were placed on a patient controlled anesthetic pump for pain control for the day of surgery and postoperative day 1. Postoperative rehabilitation is critical for success and begins on the first post-op day with protected passive ROM. Progressive ROM followed by gradual strengthening are carried out based on individual patient’s needs and surgeon’s preference.
Results
Postoperative UCLA scores averaged 27.5, while Constant–Murley Scores averaged 91. The final visual analog pain scale was recorded on postoperative day 2 and had improved, on average, from 1.3 to 4.5 on a five-point scale.
Pre-operative range of motion averaged 88° of forward elevation, 69° of abduction, 23° of ER and IR to L5. Average forward elevation at the time of final follow up improved to 142°, abduction improved to 136.2°, external rotation improved to 63.3°, and average internal rotation improved to L1. Pre-operative hematacrit averaged 40.6; the average postoperative day 1 hematocrit was 36.0, with four patients requiring transfusions (as determined by their internist based on hematocrit and symptoms including chest pain, tachycardia, shortness of breath).
There were two postoperative complications. One patient had an acute postoperative subscapularis tendon rupture and required revision surgery with marked improvement after revision, with range of motion and pain relief similar to the other patients in the study. A second patient had a nonfatal pulmonary embolism from which they recovered completely. There were no intra- or postoperative periprosthetic fractures, nor were there any other intraoperative complications. Small (<1 mm) lucent lines were note in 11 patients at 1 year postoperatively or greater (Fig. 4); however, there was no change in position or subsidence of any implant on serial X-rays. Five of the 49 components were placed in varus (Fig. 5), but each of these patients had good short-term results and no apparent complications at the time of final follow-up.
Discussion
This is the first report to document the efficacy of small-stemmed humeral components for the use in shoulder arthroplasty. The success of total shoulder arthroplasty for primary osteoarthritis is well documented and has been shown to give more predictable pain relief and functional outcomes than hemiarthroplasty and partial or full surface replacement with or without biologic glenoid arthroplasty. In this study, the use of mini-stem humeral components has provided good to excellent results and improved range of motion after minimum 2-year follow-up. While our current study design cannot provide a direct comparison to conventional total shoulder arthroplasty, we found that postoperative Constant–Murley and UCLA scores, active elevation and external rotation, as well as gains in active elevation and gains in external rotation were comparable to previously published studies [1, 2, 5, 9, 12, 13]. Eleven of our patients developed radiolucent lines of 1 mm or less postoperatively. None of these were progressive at later follow-up, and none of the patients were symptomatic. Other series of standard total shoulder arthroplasty have reported small numbers of patients with asymptomatic radiolucent lines [11]. Further follow-up will be required to determine if any of these will progress or become symptomatic in the long term.
We reported greater than 5% incidence of varus stem placement in this series. All procedures were performed by experienced shoulder surgeons; particular attention must be paid to proper stem insertion during surgery. As mentioned above, preoperative templating is critical, as is an appropriate proximal humeral osteotomy. In this series, up to 4 years postoperatively, outcomes were not affected by the varus positioning. Continued follow-up is clearly warranted to determine if late sequelae will be seen. Also, although not seen in this initial series, extreme varus malposition can create a loss of humeral offset and resultant tuberosity overhang, again illustrating the necessity of avoiding implant malposition.
The use of mini-stem technology has potential advantages. There is less reaming and broaching of the humeral canal which can theoretically decrease the incidence of cortical stress risers and potential perioperative periprosthetic fractures. Less reaming and broaching allows for less removal of bone stock, potentially allowing for less complicated revision surgery. With less distal in-growth, stem removal, may be less likely to cause humeral shaft fracture or require osteotomy. Further, if periprosthetic osteolysis were to occur, the effective joint space (which includes the entire area of the implant from the back side of the glenoid pegs to the tip of the humeral stem) and extent of potential bone resorption would be minimized. Shorter stems also are advantageous in cases of patients with diaphyseal humeral shaft malunions who require shoulder arthroplasty (Fig. 2). Similarly, those patients with previous long stemmed total elbow replacements might be treated more easily with these short stems.
This study has several limitations. As this is a descriptive report with no control group, no direct comparison can be made to conventional total shoulder arthroplasty, nor can we control for patient related variables. Also, with only 2-year follow-up, no information can be provided on the long-term outcome of mini-stem total shoulder arthroplasty. Longer-term follow-up and prospective studies are necessary to elucidate whether or not the theoretical benefits of such an implant translate to actual benefits. Similarly, longer-term data will be required to determine if varus positioning or the lucent lines seen in several patients will be associated with complications or early need for revision.
Conclusion
This is the first report to document the efficacy of mini-stemmed humeral components used during total shoulder arthroplasty. Our study group showed good to excellent results as well as improvement in range of motion at minimum 2-year follow-up. The results presented in this study are comparable to previous outcomes achieved with conventional length humeral components, and suggest that mini-stem humeral components are an effective option for total shoulder arthroplasty.
References
Boileau P, Walch G, Noel E, Liotard JP. Neer’s Shoulder Prosthesis: results according to etiology. Rev Rheum Ed Fr. 61:607–618, 1994.
Cofield RH. Total Shoulder Arthroplasty with the Neer Prosthesis. J Bone Joint Surg. 66A:899–906, 1984.
Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total Shoulder Arthroplasty: Long-term Survivorship, functional outcome and quality of life. J Shoulder Elbow Surg. 14(5):471–79, 2005.
Edwards TB, Kadakia NR, Boulahia A, et al. A Comparison of Hemiarthroplasty and Total Shoulder Arthroplasty in the Treatment of Primary Glenohumeral Osteoarthritis: Results of a Multicenter Study. J Shoulder Elbow Surg. 12(3): 207–213.
Gartsman GM, Russel JA, Gaenslen E. Modular Shoulder Arthroplasty. J Shoulder Elbow Surg. 6:333–339, 1997.
Gartsman GM, Roddey TS, Hammerman SM. Shoulder Arthroplasty with or without Resurfacing of the Glenoid in Patients who have osteoarthritis. J Bone Joint Surg. 82A(1):26–34, 2000.
Levy O, Copeland S. Cementless Surface Replacement Arthroplasty (Copeland CSRA) for Osteoarthritis of the Shoulder. J Shoulder Elbow Surg. 13(3):266–271, 2004.
Mansat P, Mansat M, Bellumore Y, et al. Mid-Term results of Shoulder Arthroplasty for Primary Osteoarthritis. Rev Chir Orthop reparatrice Appar Mot. 88(6):544–552, 2002.
Neer CS II, Watson KC, Stanton FJ. Recent Experiences in Total Shoulder Replacement. J Bone Joint Surg. 64A:319–337, 1982.
Orfaly RM, Rockwood CA Jr, Esenyel C, Wirth MA. A Prospective Functional Outcome Study of Shoulder Arthroplasty for Osteoarthritis with an intact Rotator Cuff. J Shoulder Elbow Surg. 12(3):214–221. 2003.
Thomas SR, Wilson AJ, Chambler A, et al. Outcome of Copeland Surface Replacement Shoulder Arthroplasty. J Shoulder Elbow Surg. 14(5):485–491, 2005.
Torchia ME, Cofield RH, Settergren CR. Total Shoulder Arthroplasty with the Neer Prosthesis: Long-term results. J Shoulder Elbow Surg. 6(6):495–505, 1997.
Walch G, Boileau P. Prosthetic Adaptability : A new concept in shoulder arthroplasty. J Shoulder Elbow Surg. 8:443–451, 1999.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she has no commercial associations (e.g., consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of this case, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Rights and permissions
About this article
Cite this article
Jost, P.W., Dines, J.S., Griffith, M.H. et al. Total Shoulder Arthroplasty Utilizing Mini-Stem Humeral Components: Technique and Short-Term Results. HSS Jrnl 7, 213–217 (2011). https://doi.org/10.1007/s11420-011-9221-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11420-011-9221-4