Abstract
Previously, we reported that cyclolinopeptides (CLs) extracted from flaxseed inhibited receptor activator of nuclear factor κ-B ligand (RANKL)-induced osteoclastogenesis from mouse bone marrow cells in vitro. However, mode of action involved in CLs-inhibited osteoclastogenesis has been yet unknown. Therefore, in this study, we investigated the details of inhibitory activity of cyclolinopeptide-F (CL-F) in osteoclastogenesis, as a representative of CLs. CL-F dose-dependently inhibited RANKL-induced osteoclastogenesis (IC50 0.58 µM) without cytotoxic effects. The inhibition by CL-F was mainly observed in macrophage colony-stimulating factor (M-CSF)-induced proliferation/differentiation phase from M-CSF responsive immature myeloid cells to monocyte/macrophage (M/Mϕ) lineage. Additionally, CL-F also slightly inhibited RANKL-induced differentiation phase from M/Mϕ to mature osteoclasts. Expression of RANKL receptor, RANK, in M-CSF-induced M/Mϕ, i.e. osteoclast progenitor cells, was decreased by CL-F treatment. Furthermore, RT-PCR analysis revealed that CL-F inhibited c-fos gene expression, which is reported to be crucial for RANK expression in osteoclast progenitor cells induced with M-CSF from myeloid lineage cells. These results suggested that CL-F inhibits osteoclastogenesis via down regulation of c-fos expression, which leads to the down-regulation of RANK expression in M-CSF-induced osteoclast progenitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flax (Linum usitatissimum L.) belongs to the Linaceae family. Flaxseeds contain α-linolenic acid, omega-3 fatty acid, and lignans, which are used for natural health products [1,2,3,4]. Flaxseeds also contain cyclic peptides known as cyclolinopeptides (CLs). Cyclolinopeptide A (CL-A) has been isolated from flaxseed oil and reported to have various biological activity, especially immunosuppressive activity [5, 6].
Previously, we reported that most of CLs inhibited receptor activator of nuclear factor κ-B ligand (RANKL)-induced osteoclast formation. However, the inhibitory mechanism has not been known yet [7].
Bone tissue is continuously remodeled by osteoblasts and osteoclasts, which is in charge of bone formation and bone resorption respectively. The balance maintained by these cells is important for bone homeostasis. Most adult skeletal diseases, such as osteoporosis are due to disruption of bone remodeling balance by the dominant activity of bone resorption. Therefore, appropriate regulation of osteoclast differentiation and activation is important for prevention of these bone diseases [8]. Osteoclasts are giant multinucleated cells existing along the bone surface, which originate from myeloid cells derived from hematopoietic stem cells [9]. M-CSF-responsive myeloid cells (colony-forming unit macrophage; CFU-M) proliferate and differentiate into monocyte/macrophage lineage cells (M/Mϕ), and then into monocytes and resident macrophages. In the process of the generation of M/Mϕ lineage cells, appropriate stimulation by RANKL, expressed on osteoblasts, induce differentiation from M/Mϕ to mononuclear osteoclasts, which fuse to each other and mature into multinuclear osteoclasts, which then acquire bone resorption function [10]. Osteoclastogenesis, differentiation from myeloid lineage cells to mature osteoclast, is mainly regulated by osteoblasts. M-CSF and RANKL are expressed in osteoblasts, and both are essential cytokines for osteoclastogenesis [11,12,13].
M-CSF is necessary to CFU-M for survival, proliferation, and differentiation. In osteoclastogenesis, M-CSF stimulation leads to differentiation from myeloid cells into M/Mϕ lineage precursors [14]. Under M-CSF stimulation, M/Mϕ lineage precursors express c-Fos, a component of activator protein 1 (AP-1), and promotes RANK expression, a receptor to RANKL [15]. RANKL-induced expression of nuclear factor of activated T cells c1 (NFATc1), a master regulator of osteoclast differentiation, is also regulated by c-Fos [16]. Therefore, c-Fos is thought to play an essential role in M-CSF and RANKL signaling for osteoclastogenesis.
In this study, we investigated the mechanism of osteoclastogenesis inhibitory activity of CL-F in detail. Estimated from its oxidation states, cyclolinopeptide F (CL-F) is a stable peptide and abundantly contained in flaxseeds compared with other CLs exhibiting inhibitory activity. Furthermore, CL-F potently inhibited RANKL-induced osteoclast differentiation without cytotoxicity [7]. Therefore, CL-F is suitable to be developed as health supplement for adult skeletal diseases since flaxseed is already used as health assistant food around the world [1,2,3].
Experimental procedure
Isolation and purification of CL-F
CL-F was isolated according to the methods described previously [17]. In brief, flaxseed pomace was extracted with methanol and the extract was fractionated by HP-20. The fraction eluted with 100% methanol was then fractionated by silica gel column and ODS HPLC. Purification of CL-F was done by recrystallization and the purity was examined using HPLC, which is over 97%.
Preparation of bone marrow cells, bone marrow macrophages (BMMs), and induction of differentiation into osteoclasts
Bone marrow cells were prepared from femur and tibia of 4-8 weeks old male ICR mice (SLC Inc. Shizuoka, Japan). After hemolysis, bone marrow cells were seeded to petri-dish and cultivated in α-minimum essential medium (α-MEM: Wako Pure Chemical Industries, Ltd., Osaka, Japan) supplemented with 10% FBS (Equitec-Bio, Inc., TX, USA) and 100 ng/mL of M-CSF (leukoprol; Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan).
After 72 h cultivation with M-CSF, non-adherent cells were washed out with PBS and adherent M/Mϕ were harvested as BMMs by pipetting. After re-seeding of BMMs with 20 ng/mL of M-CSF, osteoclast differentiation was induced by the addition of soluble RANKL (sRANKL, 40 ng/mL, Wako Pure Chemical Industries, Ltd.) for 4 days [18, 19]. All experimental procedures were approved by the Hoshi University Animal Care and Use Committee.
Evaluation of CL-F activity on osteoclastogenesis by tartrate-resistant acid phosphatase (TRAP) assay
Bone marrow cells were seeded 1.5 × 105 cells/well in a 96-wells microtiter plate and cultivated in α-MEM supplement with 10% FBS and 20 ng/mL of M-CSF. After priming M/Mϕ for 24 h, sRANKL was added to a final concentration of 40 ng/mL. Various concentration of CL-F was simultaneously added to M/Mϕ with sRANKL. The cells were then incubated for 72 h before subjected to TRAP assay. The TRAP assay was based on the method described by Janckila, et al. [20]. Briefly, cells cultivated in a 96 well plate were lysed with 0.5% Triton-X 100 diluted in 50 mM sodium-tartrate dihydrate in 0.1 M acetate buffer (pH 5.4). After that, naphthol-AS-BI phosphate solution (Wako Pure Chemical Industries, Ltd.) was added to each well to a final concentration of 2.5 mM. Then, 96 well plate was incubated at 37 °C for 60 min, fluorescence signal at EX405/EM495–505 was measured. Cyclosporine A (Tokyo chemical industry Co., LTD, Tokyo, Japan), used for positive control, is known to suppress osteoclast differentiation via inhibition of calcineurin signal and the following NFATc1 translocation.
Staining of osteoclasts for TRAP activity
The solution that mixed naphtol AS-BI dissolved in N, N-dimethylformamide (Wako Pure Chemical Industries, Ltd.) and FAST red ITR salt (Sigma-Aldrich Co. LLC., USA) dissolved in TRAP buffer [50 mM sodium tartrate dihydrate, 0.1 M acetate buffer (pH 5.7)], were used as TRAP staining buffer. Cells including osteoclasts were fixed by 10% formalin, then washed 3 times by purified water. After addition of TRAP staining solution and incubation at 37 °C for 20 min, TRAP-positive cells were stained as red for its enzymatic activity.
Evaluation of CL-F activity on M-CSF-induced cell proliferation by MTT assay
The influence of CL-F on cell proliferation was evaluated by the MTT assay, which is based on mitochondrial succinate dehydrogenase activity. Bone marrow cells were incubated with samples and M-CSF (20 ng/mL) for 4 days. At the end of the incubation period, 15 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 5 mg/mL, Sigma) were added to each of the wells. The formed formazan crystal after another 3 h incubation was dissolved with 150 µL of dimethyl sulfoxide (Wako Pure Chemical Industries, Ltd.) and its optical density was measured using a microplate reader (Bio-Rad, Hercules, CA, USA) at 550 nm, with a reference wavelength at 700 nm.
Detection of NO production in LPS-stimulated BMMs
NO production was determined by the Griess assay. Supernatant of the cultured medium (100 µL) of LPS-stimulated BMMs for 24 h was transferred to a 96-well microtiter plate, and then 100 µL of Griess reagent (1% sulfanilamide, 0.1% N-1-naphthylethylenediamine dihydrochloride in 2.5% H3PO4) was added. After incubation at room temperature for 15 min, the absorbances at 540 nm and 620 nm were measured with a microplate reader.
Evaluation of BMMs with flow cytometry
BMMs were harvested using flow cytometry buffer [10 mM Hepes (pH 7.4), 137 mM NaCl, 1 mg/mL glucose, 2 mM EDTA, 0.1% NaN3, 0.5% BSA] and incubated with anti-CD16/32 antibody (Bio Legend, San Diego, CA, USA) on ice, for the prevention of non-specific binding, for 15 min. Each antibody was then added to the cells, and the mixture was incubated on ice for another 30 min. Before flow cytometric analysis, cells were washed and resuspended in flow cytometry buffer and analyzed by FACSVerse (BD biosciences, Franklin Lakes, NJ, USA). APC-labeled anti-mouse CD115, FITC-labeled anti-F4/80, FITC-labeled anti-mouse CD68, PE-labeled mouse anti-TLR4, and PE-labeled mouse anti-CD14 antibodies were purchased from Bio Legend (San Diego, CA, USA).
Isolation of CD115 positive cells
BMMs induced by M-CSF were harvested by flow cytometry buffer, and incubated with biotinylated anti-CD115 antibody (Bio Legend) on ice for 30 min. Streptavidin Particles Plus-DM (BD biosciences) was added to cells and then incubated on ice for 10 min. CD115 positive cells were isolated using BD IMag™ (BD Biosciences) at room temperature for 10 min, and then washed by flow cytometry buffer. After 3 times washing, isolated CD115 positive cells were verified using flow cytometer or analyzed by RT-PCR and WB.
Reverse transcription-PCR (RT-PCR)
Total RNA was prepared from BMMs induced from bone marrow cells at various times of harvest using TRIzol (Ambion, Austin, TX, USA). Reverse transcriptions were performed using the ReverTra Ace kit (Toyobo, Osaka, Japan). Polymerase chain reaction (PCR) were carried out using the PCR kit from Qiagen (Hilden, Germany). The synthesized forward and reverse primer sequences are as follows: β-actin (forward: 5′-TCACCCACACTGTGCCCATCTAC-3′, reverse: 5′-GAGTAC TTGCGCTCAGGAGGAGC-3′), c-fos (forward: 5′-GAGAAGGGGCAGGGTGAAGG-3′, reverse: 5′-CAATATAAGGGCTGTAAAAGCCT-3′), fosl-1 (forward: 5′-AACCTTGCTCCTCCGCTCACC-3′, reverse: 5′-GCTGCTGGCTGTTGATGCTGT-3′), rank (forward: 5′-CTCTGCGTGCTGCTCGTTCC-3′, reverse: 5′-TTGTCCCCTGGTGTGCTTCT-3′), hur (forward: 5′-TCCTCCGAGCCCATCACAGT-3′, reverse: 5′-CAAAGGGGCCAAACATCTGC-3′), ttp (forward: 5′-CCACCATGGATCTCTCTGCC -3′, reverse: 5′-GGCGAAGTAGGTGAGGGTGA-3′), c-fms (forward: 5′-TCGAAACGTGCTGTTGACCA-3′, reverse: 5′-TTCTTCGGTGCAAATACAGG-3′), PU.1 (forward: 5′-TGTGCTTCCCTTATCAAACC-3′, reverse: 5′-TTCTTCACCTCGCCTGTCTT-3′).
All PCR products were separated by 1.5% agarose gel electrophoresis and visualized by ethidium bromide staining.
Western blotting (WB)
Total cellular lysate was prepared from BMMs subjected to various treatments with laemmli buffer after isolation with CD115 antibody. The total protein concentrations were quantified using the BCA protein assay kit (ThermoScientific, Rockford, IL, USA). Twenty micrograms of total protein were loaded into each well of an SDS–polyacrylamide gel. Separated proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Amersham GE Healthcare, Buckinghamshire, UK). Primary antibody was prepared by diluting with BlockAce (DS Pharma Biomedical Co., Ltd. Osaka, Japan). The primary antibodies used in this study are anti-Akt, anti-p-Akt Ser473, anti-p-Akt Thr308, and RANK (Cell Signaling Technology, Beverly, California, USA). Horseradish peroxidase-linked secondary antibody was used in this study (Amersham, GE Healthcare). Fluorescence generated by the addition of ImmunoStar LD (Wako Pure Chemical Industries, Ltd.) was detected with BIAquire 285II Imaging System.
Results
CL-F inhibited osteoclasts formation, but not influenced on M-CSF-induced cell proliferation in bone marrow cells
TRAP is known as a marker enzyme for osteoclast differentiation. Therefore, its gene expression, protein expression, and enzyme activity are utilized for index of osteoclastogenesis and identification of osteoclast lineage cells. CL-F reduced TRAP activity in a dose dependent manner from 0.31 to 10 µM (IC50 0.58 µM). CL-F (1.25 µM) showed inhibitory activity equal to or higher than that of cyclosporine A (1 µM) used as positive control (Fig. 1a). High dose CL-F (5–10 µM) potently inhibited generation of TRAP-positive mononuclear osteoclasts by microscopic observation (Figs. 1b4). Interestingly, low dose CL-F (0.07 µM) did not inhibit differentiation from M/Mϕ cells to TRAP-positive mononuclear osteoclasts (Fig. 1a), but inhibited formation of multinuclear osteoclasts (Figs. 1b3). Effect of CL-F on M-CSF-induced cell proliferation was examined using MTT assay and via microscope observation. Although M-CSF-induced cell proliferation with cyclosporine A (1 µM) resulted in 75% viability compared with vehicle control (Fig. 2), cyclosporine A above 1 µM showed obvious cytotoxicity under microscopic observation (data not shown). On the other hand, CL-F did not show definite influences on M-CSF-induced cell proliferation at 20 µM, approximately 35 times of the IC50 evaluated in TRAP assay (Figs. 1a, 2).
Inhibitory activity of CL-F on osteoclastogenesis. Inhibitory activity of CL-F on osteoclastoganesis was evaluated using TRAP assay and TRAP staining. a Bone marrow cells (1.5 × 105 cells/well) were cultured in a 96-well plate with M-CSF (20 ng/mL). After 24 h, sRANKL (40 ng/mL) was added for induction of osteoclast differentiation with various concentration of CL-F. TRAP activity was measured 3 days after sRANKL addition. Cyclosporine A (Cyc A; 1 µM) used as a positive control. All experimental groups were done with n = 4. b Bone marrow cell were cultured in 24-well plate (1 × 106 cells/well) with M-CSF (20 ng/mL). After 24 h, bone marrow cells were treated RANKL with/without CL-F. After cultivation for 3 days, osteoclasts were stained based on TRAP activity. b1; M-CSF + RANKL, b2; M-CSF, b3; M-CSF + RANKL + 0.07 µM CL-F, b4; M-CSF + RANKL + 5 µM CL-F
Influence of CL-F on M-CSF-induced cell proliferation. Effects of CL-F on cell proliferation were evaluated using MTT assay. Bone marrow cells (1.5 × 105 cells/well) were cultured in a 96-well plate and treated M-CSF (20 ng/mL) with various concentration of CL-F. After 3 days cultivation, MTT solution was added to cells and incubated for 4 h. CL-F (
 ), cyclosporine A (
), cyclosporine A (
 1 µM) used as a positive control. All experimental groups were done with n = 4
1 µM) used as a positive control. All experimental groups were done with n = 4
CL-F-affected phase in the process of osteoclastogenesis. To determine at what stage of osteoclast differentiation CL-F inhibits osteoclastogenesis, BMMs with/without CL-F (20 µM) for 3 days of the culture in the presence of M-CSF (20 ng/mL) was prepared. Isolated BMMs and F-BMMs were reseeded and induced differentiation to osteoclasts lineage with M-CSF and sRANKL (40 ng/mL) for 4 days. Generated osteoclasts in each treatment were measured by TRAP assay (n = 4). a Scheme of the culture protocol of this experiment. b TRAP activity after respective treatments according to a. Column 1–4; BMMs and F-BMMs induced by M-CSF with/without CL-F were respectively reseeded and stimulated only M-CSF (20 ng/mL). Column 1′–4′; BMMs and F-BMMs induced by M-CSF with/without CL-F were stimulated to differentiation by M-CSF (20 ng/mL) + RANKL (40 ng/mL) after reseeding. column 1 and 1′; DMSO → DMSO, column 2 and 2′; DMSO → CL-F, column 3 and 3′; CL-F → DMSO, column 4 and 4′; CL-F → CL-F. *; p < 0.05 by Tukey’s multiple comparison test
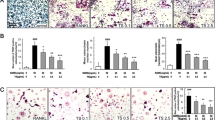
Morphological difference between BMMs and F-BMMs and respective expression of macrophagic surface antigens. a–f Bone marrow cells were treated M-CSF (20 ng/mL) with/without CL-F (20 µM) for 2 days. a CD115 expression of proliferated BMMs and F-BMM were detected. b BMMs and c F-BMM were gated with CD115, and evaluated by FSC/SSC. d F4/80, e CD14 and f CD68 expression in CD115-gated BMMs and F-BMM were detected with specific antibodies. a, d–f Black line indicates BMMs and dashed line indicates F-BMMs respectively
CL-F affected mainly the priming of M/Mϕ toward differentiation into osteoclasts
To determine at what stage of osteoclast differentiation CL-F inhibits osteoclastogenesis, we prepared M/Mϕ lineage cells as bone marrow macrophages (BMMs) with/without CL-F for 3 days of the culture in the presence of M-CSF. Isolated BMMs and F-BMMs (F-BMMs; BMMs proliferated in the presence of CL-F) were respectively reseeded, and induced differentiation to osteoclasts lineage with M-CSF and sRANKL with/without CL-F for 4 days (Fig. 3a). When the BMMs as osteoclast progenitors were exposed to CL-F for the entire 7 days, TRAP activity was decreased to 20% compared with that in the cultures without CL-F (Fig. 3b; column 1′ vs 4′). The CL-F-exposure for only 0–3 days resulted in 30% TRAP activity compared with that in the cultures without CL-F for the entire 7 days (Fig. 3b; column 1′ vs 3′). However, when the cells were treated only for the last 4 days with CL-F, the TRAP activity was approximately 50% compared to the control group (Fig. 3b; column 1′ vs 2′), suggesting that CL-F acts on M-CSF-dependent osteoclast progenitors at the early stage in the process of osteoclast differentiation.
CL-F modulated M-CSF-induced differentiation toward M/Mϕ lineage as osteoclast progenitor and generated cells with different morphology
We found that M-CSF-induced BMMs treated with CL-F (F-BMMs) had different morphology compared with that of BMMs without CL-F (BMMs) under microscopic observation (data not shown). F-BMMs showed a slightly larger spherical morphology than BMMs and showed weak adhesion. Therefore, flowcytometric analysis was employed to investigate the details of morphological difference between BMMs and F-BMMs.
CD115 is a M-CSF receptor expressed in cells from M-CSF-responsive immature myeloid to M/Mϕ lineage, of which expression increase as proliferation and differentiation progressed after M-CSF stimulation. CD115 expression in F-BMMs was slightly higher than that in BMMs (Fig. 4a).
In analysis of CD115-gated cells indicated in Fig. 4a, F-BMM showed higher values of forward scattered light (FSC) and side scattered light (SSC) than that in BMMs, indicating F-BMMs have larger cell size and higher internal complexity (Fig. 4b, c). Next, the expression of F4/80, CD14 and CD68 in CD115-positive BMMs and F-BMMs, which are known as surface markers of M/Mϕ lineage cells, were investigated. All of macrophagic surface marker, F4/80, CD14, and CD68, were expressed higher in F-BMMs than in BMMs, speculating CL-F enhanced differentiation toward the cells with features of mature macrophages (Fig. 4d–f).
Then, LPS-stimulated NO production was investigated for finding functional difference between BMMs and F-BMMs. However, NO production stimulated by LPS was not influenced by CL-F treatment (Fig. 5). Furthermore, expression of TLR4 (CD165), a LPS receptor, was also not influenced in F-BMMs as shown by flow cytometric analysis (data not shown).
LPS-induced NO production in BMMs and F-BMMs. Bone marrow cells were cultured in a 96-well plate and stimulated with M-CSF (20 ng/mL) with/without CL-F (none; open column, 5 microM; hatched column, 10 microM; filled column). After 2 days cultivation, BMMs were stimulated by LPS (100 ng/mL) for 24 h, and NO amount produced in BMMs and F-BMMs were respectively measured using Griess assay
CL-F down-regulates c-fos expression and RANK expression in CD115 positive cells
For further investigation of CL-F activity, CD115 positive cells were isolated from other cells mixed in the culture by using biotinylated anti-CD115 antibody and avidin-conjugated magnetic particles. Indicated in appendix figure, the purity of CD115-positive cells was above 95% after the isolation.
The expressions of c-fos and fosl1, which belongs to the c-Fos family, are known as crucial transcription factors in the process of osteoclast differentiation, which also regulates RANK expression, receptor for RANKL, in osteoclast progenitor, i.e., BMMs. The expressions of c-fos and fosl1 in isolated CD115-positive cells after 48 h treatment with/without CL-F were investigated using RT-PCR, and were revealed to be decreased in F-BMMs compared with BMMs. Furthermore, rank expression regulated by the c-Fos family was also decreased in F-BMMs (Fig. 6a). On the other hand, gene expression of M-CSF receptor, c-fms, or PU.1 was not altered. The attenuation of RANK protein expression in F-BMMs was also confirmed by western blotting using RANK specific antibody (Fig. 6b). In the same samples, expression and phosphorylation of AKT, a downstream factor of M-CSF signaling, were not influenced by CL-F (Fig. 6b). The stability of c-fos mRNA is regulated by HuR and TTP. Since the expression level of c-fos mRNA in F-BMM was decreased, the expression of hur and ttp were investigated by RT-PCR. The differences in the expression levels of hur and ttp were not found between F-BMM and BMM (Fig. 6a).
Downregulation of c-fos and RANK expression in CD115 positive F-BMMs. a Bone marrow cells were stimulated by M-CSF (20 ng/mL) with/without CL-F (20 µM) for 2 days. Proliferated BMMs and F-BMMs were isolated using anti-CD115 antibody. Total mRNA in BMMs and F-BMMs was respectively extracted and cDNA was prepared by RT-reaction. PCR was performed using cDNAs, and specific primers of c-fos, fosl1-1, c-fms, PU.1, rank, hur, ttp, and β-actin, and the expression level of mRNA was semi-quantitatively examined. The numbers in parentheses indicate the number of PCR cycles. b Total protein was prepared from BMMs and F-BMMs isolated with anti-CD115 antibody as same procedure as a. WB was performed using specific antibodies to RANK, AKT, p-AKT (ser473) and p-AKT (thr308). The experiments of a and b were performed three times, and the reproducibility was confirmed
CL-F also down-regulates RANKL-induced c-fos expression in osteoclast differentiation processs
During RANKL-induced osteoclast differentiation process, sustaining c-Fos expression in progenitor cells is essential for NFATc1 expression, master regulator for osteoclastic differentiation. In Fig. 6a, the down-regulation of c-fos by CL-F in F-BMMs was revealed. However, as shown in Fig. 3, when the cells were treated only for the last 4 days with CL-F, the TRAP activity was also decreased to approximately 50%. Therefore, the effect of CL-F on c-fos expression in RANKL-RANK signaling, differentiation signaling toward osteoclasts, was investigated. Total mRNA was harvested at 1, 3 and 24 h after sRANKL stimulation with/without CL-F in BMMs, and c-fos mRNA was detected using RT-PCR. As a result, c-fos mRNA expression 24 h after RANKL addition was obviously decreased in BMMs treated with CL-F, but but no obvious change was found from 1 to 3 h after RANKL stimulation (Fig. 7).
Downregulation of c-fos mRNA expression by CL-F in RANK signaling. Bone marrow cells harvested were treated 100 ng/mL M-CSF for 2 days. Proliferated BMMs were reseeded and stimulated by RANKL (40 ng/mL) with/without CL-F (20 µM). Total mRNA was extracted at 1, 3 and 24 h after RANKL stimulation and cDNA was prepared by RT-reaction. PCR was performed using cDNAs, and specific primers of c-fos and β-actin, and the expression level of mRNA was semi-quantitatively examined. The numbers in parentheses indicate the number of PCR cycles
Discussions
We previously reported the osteoclastogenesis inhibitory activity of cyclolinopeptides (CLs) isolated from Flax [7]. In this study, we investigated the inhibitory mechanism of CL-F on osteoclastogenesis.
CL-F inhibited osteoclastogenesis in a dose dependent manner and its IC50 was determined to be 0.58 µM (Fig. 1a). Furthermore, CL-F inhibited the formation of multinuclear osteoclasts even at 70 nM (Fig. 1b), suggesting it is effective in the process of RANKL-induced osteoclast differentiation and maturation. Moreover, CL-F did not show obvious effects on M-CSF-induced cell proliferation and cell viability (Fig. 2). Cyclosporine A, a positive control, is a cyclic peptide similar to CL-F which showed osteoclastogenesis inhibitory activity at 1 µM. However, it showed cell toxicity at 1 µM or higher concentration. It is known that cyclosporine A inhibits phosphatase activity of calcineurin whose activity is required for NFAT activation. Considering it together with our result, CL-F was suggested to have different inhibitory mechanism from that of cyclosporine A in osteoclastogenesis (Fig. 1a, b).
During the whole process of osteoclast differentiation, CL-F showed more effective inhibition in the M-CSF-induced proliferation/differentiation phase than in the RANKL-induced differentiation phase. Moreover, M/Mϕ cells pretreated with CL-F during M-CSF-induced differentiation phase were hard to differentiate into osteoclasts compared to that treated with CL-F under RANKL stimulation (Fig. 3b). However, CL-F also showed inhibitory activity during RANKL-stimulated differentiation phase and maturation into multinuclear osteoclasts. These data suggested that CL-F interferes with common factor in both M-CSF and RANKL signaling leading to osteoclastogenesis.
In the process of these studies, we found that M-CSF induced BMMs treated with CL-F (F-BMMs) had different morphology compared to M-CSF induced BMMs without CL-F (BMMs) under microscopic observation (data not shown). Furthermore, F-BMM indicated the feature hard to differentiate into osteoclasts by RANKL stimulation. From these results, the difference between F-BMMs and BMMs was investigated for characterization of F-BMM and clarification of CL-F activity. Flow cytometry analysis revealed that, F-BMM showed larger FSC and SSC values than BMMs, indicating F-BMMs have larger cell size and higher internal complexity (Fig. 4a, b).
Signaling of M-CSF via its receptor, CD115, is essential for both M/Mϕ lineage progenitor generation and RANKL-induced osteoclast differentiation. CD115 expression is increased on the cells in proliferation and differentiation process stimulated by M-CSF, i.e. from M-CSF-responsive immature myeloid to M/Mϕ lineage cells [21]. Indicated in Fig. 4a, although CD115 expression in F-BMMs was slightly higher than that in BMMs, significant difference of CD115 expression between BMMs and F-BMMs was not observed.
Next, proliferated BMMs and F-BMM were gated with CD115, and evaluated by the expressions of F4/80, CD14 and CD68 as surface markers of M/Mϕ lineage cells. As a result, F4/80, CD14, and CD68 expression were found to be increased in F-BMMs, suggesting CL-F enhanced differentiation toward cells that have features of mature macrophages (Fig. 4d–f).
Integrins are crucial adherence molecules expressed on cell surface of macrophages and osteoclasts. In particular, αvβ3 is prominently expressed on osteoclasts, and is known as an essential factor for differentiation and function of osteoclasts [21]. However, significant differences were not found in expression of integrin αv, α5, β1, β3 (data not shown). For finding functional differences between F-BMMs and BMMs, the LPS-stimulated NO production was determined by Griess method, but it was not influenced by CL-F treatment (Fig. 5). Expression of TLR4 (CD165), a LPS receptor in macrophages, was also not influenced in F-BMMs, as shown by flow cytometric analysis (data not shown).
Bone marrow contains various hematopoietic cells. CD115 (CSF-1R, c-Fms) is a receptor of M-CSF and CD115 positive cells were known as osteoclast precursors [22]. Since the ratio of CD115 positive cells, CFU-M, in freshly prepared bone marrow cells was extremely low [23], CD115 positive cells proliferation was stimulated by M-CSF to become the dominant population in the culture system employed in this study. However, CD115 positive cells had to be isolated from other lineage cells since other mixed cells interfere in further investigation (Fig. 6). In analysis using isolated CD115-positive cells, expression of c-fos and fosl-1 were found to be lower in F-BMMs compared to BMMs. Furthermore, both rank mRNA and RANK protein expression regulated by c-Fos family were also decreased in F-BMMs. In RANKL-promoted differentiation phase, expression of c-fos was decreased in CL-F-treated BMMs 24 h after RANKL stimulation although initial expression of that from 1 to 3 h was not altered (Fig. 7).
It is known that c-Fos is involved in cell proliferation, differentiation and apoptosis. Since c-fos−/− mice were observed to have osteopetrosis, which is caused by the absence of osteoclasts, c-Fos is thought to be an essential factor in osteoclastogenesis [24, 25]. In osteoclastogenesis, c-Fos expression is continuously elevated by RANKL stimulation [26, 27]. Fra-1 (fosl-1) known as c-Fos family is a transcriptional target of AP-1 including c-Fos. Overexpression of Fra-1 by viral gene transfer in vitro or in transgenic mice was able to rescue c-Fos-dependent osteoclast differentiation [28]. Recently, it is reported that c-Fos expression in M-CSF signaling leads to up-regulation of RANK expression in BMMs [15]. Considering these reports and our results, F-BMM is suggested to be unable to differentiate into osteoclast lineage because of the lack of RANKL-RANK signaling caused by c-Fos down-regulation. F4/80 up-regulation in F-BMM was observed in Fig. 5c. F4/80 is known as a marker of mouse mature macrophage, and its expression in BMMs is induced by M-CSF [29]. The number of F4/80 positive macrophage was reported to increase in c-Fos lacking mice [26]. From this observation, it is suggested that F4/80 up-regulation in F-BMM was caused by c-fos reduction by CL-F. The stability of c-fos mRNA is regulated by HuR and TTP, both of which are mRNA stabilizing factor. HuR stabilizes mRNA by binding on AU-rich element (ARE) on 3′-UTR and TTP leads to degradation by binding ARE as well [30, 31]. The expression level of hur and ttp in F-BMM and BMM were found to be similar (Fig. 6). These data suggested that reduction of c-fos mRNA expression in F-BMM was caused by transcriptional regulation independent of mRNA stability.
Osteoporosis is a disease characterized by low bone mass and microarchitectural deterioration of bone tissue, leading to enhanced bone fragility and a consequent increase in fracture risk. At the moment, most of natural products thought to be effective on osteoporosis are isoflavones, lignans, and coumestan known as phytoestrogen. Actually, ipriflavone, a derivative of isoflavone, has been clinically used on osteoporosis [32, 33].
Our study suggests that CL-F inhibits c-fos expression in M-CSF and RANKL signaling, which are common factor leading to osteoclastogenesis. Especially, CL-F leads to the down-regulation of RANK expression and reduction of RANKL-RANK signaling via inhibition of c-fos expression. Additionally, CL-F is expected not to have health risks because cytotoxic effects were not observed. CL-F has new inhibitory mechanism for inactivating osteoclastogenesis, and is expected to become an effective and safe health supplement for adult skeletal diseases.
References
Shim YY, Gui B, Arnison PG, Wang Y, Reaney MJT (2014) Flaxseed (Linum usitatissimum L.) compositions and processing: a review. Trends Food Sci Technol 38:5–20
Aladedunye F, Sosinska E, Przybylski R (2013) Flaxseed cyclolinopeptides: analysis and storage stability. J Am Oil Chem Soc 90:419–428
Schmidt TJ, Klaes M, Sendker J (2012) Lignans in seeds of Linum species. J Phytochem 82:89–99
Wang YF, Xu ZK, Yang DH, Yao HY, Ku BS, Ma XQ, Wang CZ, Liu SL, Cai SQ (2013) The antidepressant effect of secoisolariciresinol, a lignan-type phytoestrogen constituent of flaxseed, on ovariectomized mice. J Nat Med 67:222–227
Kaufmann HP, Tobschirbel A (1959) An oligopeptide from linseed. Chem Ber 92:2805–2809
Wieczorek Z, Bengtsson B, Trojnar J, Siemion IZ (1991) Immunosuppressive activity of cyclolinopeptide A. Pept Res 4:275–783
Kaneda T, Yoshida H, Nakajima Y, Toishi M, Nugroho Alfarius Eko, Morita H (2016) Cyclolinopeptides, cyclic peptides from flaxseed with osteoclast differentiation inhibitory activity. Bioorg Med Chem Lett 26:1760–1761
Rodan GA, Martin TJ (2000) Therapeutic approaches to bone diseases. Science 289:1508–1514
Loutit JF, Nisbet NW (1982) The origin of osteoclasts. Immunobiology 161:193–203
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323
Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW Jr, Ahmed-Ansari A, Sell KW, Pollard JW, Stanley ER (1990) Total absence of colonystimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci 87:4828–4832
Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD, Nishikawa S (1990) The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345:442–444
Takayanagi H (2007) Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 7:292–304
Arai A, Mizoguchi T, Harada S, Kobayashi Y, Nakamichi Y, Yasuda H, Penninger JM, Yamada K, Udagawa N, Takahashi N (2012) Fos plays an essential role in the upregulation of RANK expression in osteoclastprecursors within the bone microenvironment. J Cell Sci 125:2910–2917
Kim JH, Kim N (2014) Regulation of NFATc1 in osteoclast differentiation. J Bone Metab 21:233–241
Matsumoto T, Shishido A, Morita H, Itokawa H, Takeya K (2001) Cyclolinopeptides F-I, cyclic peptides from linseed. Phytochemistry 57:251–260
Kaneda T, Nojima T, Nakagawa M, Ogasawara A, Kaneko H, Sato T, Mano H, Kumegawa M, Hakeda Y (2000) Endogenous production of TGF-beta is essential for osteoclastogenesis induced by a combination of receptor activator of NF-kappa B ligand and macrophage-colony-stimulating factor. J Immunol 165:4254–4263
Hayashi T, Kaneda T, Toyama Y, Kumegawa M, Hakeda Y (2002) Regulation of receptor activator of NF-kappa B ligand-induced osteoclastogenesis by endogenous interferon-beta (INF-beta) and suppressors of cytokine signaling (SOCS). The possible counteracting role of SOCSs-in IFN-beta-inhibited osteoclast formation. J Biol Chem 277:27880–27886
Janckila AJ, Takahashi K, Sun SZ, Yam LT (2001) Naphthol-ASBI phosphate as a preferred substrate for tartrate-resistant acid phosphatase isoform 5b. J Bone Miner Res 16:788–793
Feng X, Teitelbaum SL (2013) Osteoclasts: new Insights. Bone Res 1:11–26
Sherr CJ (1990) Colony-stimulating factor-1 receptor. Blood 75:1–12
Sato N, Sawada K, Kannonji M, Tarumi T, Sakai N, Ieko M, Sakurama S, Nakagawa S, Yasukouchi T, Krantz SB (1991) Purification of human marrow progenitor cells and demonstration of the direct action of macrophage colony-stimulating factor on colony-forming unit-macrophage. Blood 78:967–974
Wang ZQ, Ovitt C, Grigoriadis AE, Möhle-Steinlein U, Rüther U, Wagner EF (1992) Bone and haematopoietic defects in mice lacking c-fos. Nature 360:741–745
Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF (1994) c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266:443–448
Matsuo K, Owens JM, Tonko M, Elliott C, Chambers TJ, Wagner EF (2000) Fosl1 is a transcriptional target of c-Fos during osteoclast differentiation. Nat Genet 24:184–187
Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, Yokochi T, Oda H, Nakamura K, Ida N, Wagner EF, Taniguchi T (2002) RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature 416:744–749
Fleischmann A, Hafezi F, Elliott C, Remé CE, Rüther U, Wagner EF (2000) Fra-1 replaces c-Fos-dependent functions in mice. Genes Dev 14:2695–2700
Hirsch S, Austyn JM, Gordon S (1981) Expression of the macrophage-specific antigen F4/80 during differentiation of mouse bone marrow cells in culture. J Exp Med 154:713–725
Ma WJ, Cheng S, Campbell C, Wright A, Furneaux H (1996) Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J Biol Chem 271:8144–8151
Raghavan A, Robison RL, McNabb J, Miller CR, Williams DA, Bohjanen PR (2001) HuA and tristetraprolin are induced following T cell activation and display distinct but overlapping RNA binding specificities. J Biol Chem 276:47958–47965
Glazier MG, Bowman MA (2001) A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Arch Intern Med 161:1161–1172
Rietjens IMCM, Louisse J, Beekmann K (2017) The potential health effects of dietary phytoestrogens. Br J Pharmacol 174:1263–1280
Acknowledgement
This work was partly supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaneda, T., Nakajima, Y., Koshikawa, S. et al. Cyclolinopeptide F, a cyclic peptide from flaxseed inhibited RANKL-induced osteoclastogenesis via downergulation of RANK expression. J Nat Med 73, 504–512 (2019). https://doi.org/10.1007/s11418-019-01292-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-019-01292-w