Abstract
Fourteen cyclolinopeptides (CLPs) from flaxseed oil and meal were separated, identified and quantified by HPLC coupled to an Orbitrap MS. The oxidative stability of the cyclolinopeptides was assessed during storage of flaxseed oil and meal. A significant decrease in the amounts of the methionine containing CLPs, namely CLP-B, CLP-J and CLP-M, and a concurrent increase in the amounts of methionine sulfoxide containing CLPs, such as CLP-C, CLP-E and CLP-G were observed. The cyclolinopeptides with two methionine units, CLP-L and CLP-M, exhibited the greatest decrease, followed by CLP-J, the major flaxseed oil bitter taste precursor, and CLP-B, a biologically active cyclolinopeptide. At the end of the storage period, the amount of the bitter CLP-E increased fourfold in the oil while the immunosuppressive cyclolinopeptides A remained unchanged. No significant changes in the amount of each of the CLPs were observed in the stored flaxseed meals. A fast and reliable procedure has been developed for quantitative analysis of cyclolinopeptides. Due to the high predisposition of methionine containing cyclolinopeptides to oxidation and the easiness of CLPs’ quantification with the proposed method, it is possible to reliably assess the extent of flaxseed meal and oil oxidation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The health benefits of flaxseed oil and meal, especially with regard to the prevention of cardiovascular disease are well established [1]. Most of the biological activity of flaxseed oil is attributed to its exceptionally high amount of α-linolenic acid, an essential omega-3 fatty acid [2, 3]. Flaxseed also contains bioactive compounds such as lignans and an abundance of hydrophobic cyclolinopeptides (CLPs) which have been shown to exhibit diverse biological activities in several laboratory studies [4–6]. For instance, cyclolinopeptide A (CLP-A), the most studied flaxseed cyclolinopeptide, with a primary sequence of cyclo(Pro–Pro–Phe–Phe–Leu–Ile–Ile–Leu–Val), exhibited immunosuppressive activity similar to that of cyclosporine [6, 7]; inhibitory activity towards calcium dependent activation of T-lymphocyte cell division [8]; and antimalarial activity [9]. Cyclopeptides B and E have also exhibited immunosuppressive activities on human and mouse peripheral blood lymphocytes [10].
Oxidative deterioration in flaxseed oil, and the resulting rancid off-flavor, is generally attributed to linolenic acid oxidation, because of its high content and relatively high susceptibility to oxidation [11–13]. However, it is also known that in addition to the rancid off-flavor, an unpleasant bitter off-taste begins to develop in flaxseed oil as early as after 1 day of storage [14, 15]. Recently, Brühl et al. [14] identified CLP-E as the component responsible for the development of the bitter off-taste in stored flaxseed oil. While the amount of CLP-E is usually small in the freshly pressed flaxseed oil, its content gradually increases during storage as effect of CLP-J oxidation, its precursor containing methionine, often represented as CLP-E′ [14–16]. Thus, oxidation of cyclolinopeptides can profoundly affect both functional and nutritional quality of the flaxseed oil.
Results from previous studies indicated that a number of other CLPs could also contribute to flaxseed oil bitter off-taste [14, 15]. Therefore, a study on the cyclolinopeptides profiles of flaxseed oil and their oxidative stabilities under storage condition is warranted. So far, the only studies on the storage stability of cyclolinopeptides were those by Brühl et al. [14, 15], with reference to only CLP-E.
Due to the complexity of flax CLPs mixture and the similarity in chemical structures, their separation on HPLC columns has been somewhat difficult. Recently, Bagoluri et al. [17] reported a rapid HPLC method for the analysis of flaxseed cyclolinopeptides; the method, however, was restricted to only five CLPs, namely, CLP-A, CLP-C, CLP-D, CLP-E, and CLP-G. In typical currently used analytical procedure, the cyclopeptide mixtures were first oxidized with hydrogen peroxide to convert methionine containing CLPs to the corresponding methionine sulfoxide moieties, thereby reducing the number of CLPs to be separated, this approach is limiting the versatility of the method. A recently published method by the same research group separated and identified six CLPs from difference flaxseed cultivars [18].
In the present study, fourteen CLPs were effectively separated by an optimized HPLC method using PDA detection and a high resolution Orbitrap mass spectrometer to verify the chemical identity of the compounds. The flax CLPs were further evaluated for oxidative stability during extended storage of the oil and meal.
Materials and Methods
Flaxseed Oil and Meals
Cold pressed flaxseed oil and meal were donated by Shape Foods Inc. (Brandon, MB, Canada). The commercial flaxseed oil was packaged under an argon atmosphere in a 250-mL dark bottle with minimal headspace. The meals were stored in a warehouse at ambient temperatures for different periods of time.
Chemicals
Acetonitrile and isopropanol used in the study were of MS grade and obtained from Fisher Scientific Co. (Toronto, ON, Canada). Ultrapure water was purified by a Nanopure Diamond laboratory water system (Barnstead, Dubuque, IA, USA). All other solvents and chemicals were of analytical grade and were purchased from Sigma-Aldrich (St. Louis, MO, USA). Standards of fatty acid methyl esters were purchased from Nu-Check-Prep (Elysian, MN, USA).
Storage Stability Test
Flaxseed oil in a commercially sealed dark bottle was stored in darkness at 65 ± 1 °C for up to 30 days. Samples were collected every other day and analyzed.
The meals were stored under warehouse conditions mostly at ambient temperatures for up to 48 months as follows:
Sample | Oil content | Storage condition |
|---|---|---|
Meal 1 | 17.8 ± 2.1 | 1 month at 27 °C; 47 months at 20 °C |
Meal 2 | 16.4 ± 1.5 | 1 month at 27 °C; 15 months at 20 °C |
Meal 3 | 16.6 ± 1.4 | 1 month at 27 °C; 14 months at 20 °C |
Meal 4 | 16.1 ± 1.3 | 1 month at 27 °C; 13 months at 20 °C |
Meal 5 | 15.4 ± 1.2 | 1 month at 27 °C; 12 months at 20 °C |
Meal 6 | 16.7 ± 1.7 | 1 month at 27 °C; 8 months at 20 °C |
Meal 7 | 17.2 ± 2.0 | 1 month at 27 °C; 7 months at 20 °C |
Meal 8 | 16.3 ± 1.8 | 1 month at 27 °C; 6 months at 20 °C |
Meal 9 | 17.6 ± 2.2 | 1 month at 27 °C; 5 months at 20 °C |
Meal 10 | 18.7 ± 1.8 | Fresh |
Extraction of Cyclolinopeptides
Cyclolinopeptides were isolated from the flaxseed oil and meal utilizing modified extraction procedure where sonication was applied [17]. Briefly, flaxseed oil or meal (1 g) was weighed into a threaded tube, then 10 mL of methanol added and the mixture vortexed for 1 min, followed by sonication at 50 °C for 1 h. The content was centrifuged at 4000×g for 30 min and the supernatant collected and subsequently analyzed by HPLC–MS.
Isolation of CLP Standards
Flaxseed meal (50 g) was extracted with methanol (100 mL) by sonication at 50 °C for 1 h. Thereafter, the mixture was filtered, and the filtrate evaporated at 40 °C under vacuum using a rotary evaporator (BÜCHI Labortechnik AG, Flawil, Switzerland) forming a reddish-brown oil (2.9 g). The dissolved oil (2.9 g) in methanol/diisopropyl ether (1/1, v/v; 5 mL) was applied to a glass column packed with a slurry of silica gel 60 Å (70–230 mesh; 35 g) deactivated with water (5 % w/w) and conditioned with diisopropyl ether/hexane (1/4, v/v). Separation was performed by successive elution with diisopropyl ether/hexane (1/4, v/v; 200 mL), diisopropyl ether/hexane (1/1, v/v; 200 mL), 100 % diisopropyl ether (200 mL), 100 % acetone (200 mL), acetone/methanol (1/4, v/v; 400 mL). Collected fractions were analyzed by HPLC for CLPs and fractions eluted with 100 % acetone contained 85 % of the flax CLPs, these fractions were combined and solvent evaporated under reduced pressure at 35 °C to form a light yellow powder (47 mg). The CLPs mixture was separated by HPLC using a Kinetex column and a gradient of a mixture of acetonitrile, water and isopropanol as described below, and fractions containing the cyclolinopeptides, A, B, C, E, and N were collected using a Gilson FC 203B fraction collector (Gilson Inc., Middleton, WI, USA). Fractions were concentrated under vacuum using a rotary evaporator at 35 °C to remove the acetonitrile. The aqueous portion containing the CLP standard was then extracted three times with dichloromethane (each time 2 × the volume of aqueous portion), and the combined dichloromethane extract concentrated under vacuum using a rotary evaporator at 35 °C, the residual solvent was removed by evaporation with a gentle stream of nitrogen.
Fatty Acid Analysis
Fatty acids were methylated following AOCS Official method Ce 1–62 [19]. The fatty acid methyl esters (FAME) were analyzed on a Trace GC Ultra gas chromatograph using a Trace TR-FAME fused silica capillary column (100 m × 0.25 mm × 0.25 μm). Hydrogen was used as the carrier gas with a flow rate of 1.5 mL/min. The column temperature was programmed from 70 to 160 °C at 25 °C/min and held for 30 min, and further programmed to 210 °C at 3 °C/min. Initial and final temperatures were held for 5 and 30 min, respectively. Splitless injection was made using a PTV injector. Detector temperature was set at 250 °C. FAME samples, 1 μL were injected with an AS 3000 autosampler. Fatty acids were identified by comparison of retention data with authentic standards.
Peroxide Values (PV)
Peroxide values (PV) were determined according to ISO Method 27107 using the potentiometric end point [20].
Anisidine Value
Anisidine values, a measure of secondary oxidation products, were obtained according to ISO Method 6885:2004 [21].
HPLC–MS
High performance liquid chromatography was carried out using an Accela HPLC system equipped with an Accela 1250 pump and autosampler (Thermo Fischer Scientific, West Palm Beach, FL). The sample was separated on a Kinetex C18 column (2.6 μm; 150 × 3 mm; Phenomenex, MA) at 25 °C using a mobile phase consisting of acetonitrile, water and isopropanol with the following gradient:
Time (min) | Acetonitrile | Water | Isopropanol | Flow rate (mL/min) |
|---|---|---|---|---|
0 | 40 | 60 | 0 | 0.30 |
20 | 60 | 35 | 5 | 0.30 |
25 | 70 | 25 | 5 | 0.30 |
27 | 94 | 0 | 6 | 0.60 |
37 | 94 | 0 | 6 | 0.60 |
38 | 40 | 60 | 0 | 0.30 |
45 | 40 | 60 | 0 | 0.30 |
The injection volume was 10 μL and the UV detector set at 210 nm (Accela PDA). For comparison, the HPLC conditions were also optimized for the following columns: Novapak (4 μm; 300 × 3.9 mm; Waters, MA) and Hypersil Gold (1.9 μm; 50 × 2.1 mm; Thermo Fisher Scientific, West Palm Beach, FL).
For subsequent identification of the cyclolinopeptides (CLPs), the column effluents from the HPLC were delivered to an online Exactive Orbitrap MS ion source (Thermo Fischer Scientific, West Palm Beach, FL) using a PEEK tubing (0.0005-in ID × 1/16-in OD). The mass spectrometer was equipped with an APCI ion source, operated in the positive mode. Xcalibur software was used for data acquisition and analysis. The mass spectrometer conditions were optimized for CLPs as follows: the APCI source temperature was set at 425 °C; ion transfer tube (capillary) temperature at 250 °C; sheath gas flow rate at 60, auxiliary gas flow at 5, corona discharge current at 5 μA; tube lens, capillary, and skimmer voltages at 100, 60 and 20 eV, respectively. Confirmation of amino acid sequence of the CLPs was achieved by fragmentation at 45 eV using high energy collision induced dissociation (HCD). The spectra were collected in a range of 55–1100 m/z at a scan rate of 1 scan/s. Quantification of CLPs A, B, C, E, and N was achieved by external calibration using the respective CLP standards, while the rest of the CLPs were quantified by external calibration with CLP-A.
Method Validation
Efficiency of CLPs Extraction
To evaluate the effectiveness of the CLP isolation using sonic assisted methanol extraction (SAME), CLP standards in minimal amount of methanol were added to 1 g flaxseed oil to achieve the following concentrations: 709, 177 and 1.5 μg/g of CLP-A; 557, 139 and 1.5 μg/g CLP-B; 195, 49 and 1.5 μg/g CLP-C; 281, 70 and 1.5 μg/g CLP-E; and 252, 63 and 1.5 μg/g of CLP-N. After mixing, the solvent was evaporated under a stream of nitrogen, the added CLPs were extracted using SAME, and the amounts analyzed by HPLC. The recovery experiments were performed in triplicate, and the recovery expressed as a percentage of the amount added to the sample.
To ascertain that the SAME conditions did not result in CLP’s oxidation, a standard solution of CLP-B in methanol was isolated under the same isolation conditions and changes in the amount of CLP-B or emergence of its oxidation product, CLP-C, evaluated.
Limit of Quantification (LOQ)
Standard solutions of CLP A, B, C, E, and N were diluted in methanol and analyzed by HPLC until the peak was observed at the signal-to-noise ratio of 10:1.
The linearity of calibration was assessed by using the standard solutions at ten different concentration levels within the range of 1.5–1,000 μg/mL. A calibration curve was generated for each CLP to confirm the linearity of the signal. The slope, intercept and correlation coefficient (r) were calculated.
Stability Assay
The stability of the CLPs under normal analytical conditions was evaluated by storing the flax CLP extracts in the HPLC autosampler for 24 h and after 1 month storage at 4 °C in a refrigerator. The parent and oxidized forms of CLPs were assessed.
Statistical Analysis
All storage experiments and analyses were performed in duplicate and the data are presented as means ± SD. Data were analyzed by single factor analysis of variance (ANOVA) and regression analysis using Minitab 2000 statistical software (Minitab Inc. PA, ver. 15). Statistical significant differences between means were determined by Duncan’s multiple range tests for P ≤ 0.05.
Results and Discussion
Efficiency of CLPs Extraction
As shown in Table 1, the SAME method used for the CLPs isolation was the most effective with recovery above 90 %. Methanol extraction with sonication offered more efficient extraction of CLPs than other two methods tested in this study (Table 1) [17, 18]. Marr et al. [22] reported a fivefold increase in the amounts of CLPs recovered using methanol extraction, compared to elution from a silica packed column. Further, the small coefficient of variation (<1.7 %) between CLP-B contents before and after processing under SAME conditions indicated that method did not induce any oxidative degradation of the cyclolinopeptides.
As shown in Table 2, the SAME method showed good accuracy and reproducibility with recoveries that ranged between 90 and 102 %, and coefficient of variation below 10 %.
HPLC–PDA–MS Separation of Cyclolinopeptides
The best separation of flaxseed CLPs was achieved using the Kinetex C18 column, where CLPs A and J, as well as CLPs N and D were well separated, whereas other tested columns were not able to separate these CLPs (Fig 1).
Fourteen CLPs were separated using a Kinetex column and were quantified in the present study (Fig 1). The identification of the CLPs was based on the determination of molecular mass of all compounds by mass spectrometer, which agreed with the calculated masses within 2 ppm. Identity of CLPs was also verified by amino acid sequencing using the HCD for collisional dissociation. As an example, the fragmentation pattern of CLP-A in Fig. 2 is presented. The fragmentation produces a series of ions with m/z at 941, 828, 715, 602, 489, 342, 245, and 70, which corresponded to the loss of valine, leucine, isoleucine, isoleucine, leucine, phenylalanine, proline, and phenylalanine. The cyclopeptide ring is opening at the amide nitrogen of proline and removal of amino acids from chain begins [16].Currently there is not unified nomenclature for cyclolinopeptides; Stefanowicz assigned connotation CLP K for a cyclolinopeptide with the amino acids sequence cyclo(Pro–Phe–Phe–Trp–Ile–Met–Leu–Leu), whereas Jadhav et al. [16, 23] used the same abbreviation for cyclolinopeptide with the following amino acid sequence cyclo(Met(O)–Leu–Ile–Pro–Pro–Phe–Phe–Val–Ile). In the present study we followed the configuration and abbreviations proposed by Stefanowicz [16].
Cyclolinopeptide A standard and its amino acid sequence using the MS high energy collision induced dissociation (HCD) fragmentation. Cyclopeptide fragments labelled according to a nomenclature system by Ngoka and Gross [30]. As suggested by Stefanowicz [16], fragment b2PP possibly formed by a loss of proline after intramolecular rearrangement of acylium ion HP–P–F+ to acylium ion HP–F–P+ similar to that described by Ngoka and Gross [31]
In the development of the HPLC method, it was observed that the use of acetonitrile as an organic modifier resulted in a better resolution of cyclolinopeptides compared to methanol. Furthermore, the use of methanol resulted in undesirable baseline drifts in the gradient profile [24]. Previous HPLC separation by Brühl et al. [15] using methanol/water was only able to separate five CLPs.
Furthermore, the method demonstrated high sensitivity for the CLPs examined in the range of 0.15 μg/mL and the calibration curves for all the CLP standards were linear over a wide range (1.5–1,000 μg/g) with correlation coefficient (r) >0.9995.
Storage Stability of Cyclolinopeptides
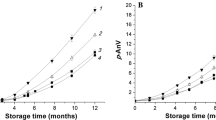
The evaluated CLPs can be broadly clustered into three groups based on their properties and changes in the amount during storage: (1) CLPs B, J, L, M, and K usually decreased; (2) CLPs C, D, E, F, and G increased; (3) CLPs A, H, I, and N that remained unchanged. Generally, the susceptibility of the CLPs to oxidative degradation is dependent on the presence and the number of methionine residues in the cyclopeptide structure. Thus, CLPs L and M, with two methionine molecules oxidized at the highest rate with the respective amounts in the oil decreasing by 72 and 73 % at the 2nd day of storage, thereafter plateaued (Fig 3). Similarly, CLPs B and J showed a twofold decrease of the amount in the oil at the 2nd day of storage; however, unlike CLPs L and M, the decrease continued up to the 12th day of storage, presumably due to the relatively higher starting concentrations of CLPs B and J in the oil. At the end of storage, 78 and 85 % of these CLPs disappeared (Fig 4). The same concentration effect may also be responsible for the relatively slower oxidative degradation of CLP K, showing 40 % decrease at the end of the storage period.
The oxidation of CLPs B, K and J resulted in the formation of the methionine sulfoxide containing cyclolinopeptides C, D and E, respectively [16]. Thus, a corresponding increase in the amounts of these CLPs was observed at the end of the storage period. As shown in Fig. 4, the amounts of CLPs C, D and E increased by 70, 27 and 65 %, respectively, at the end of the storage period. As shown in Fig. 5, CLPs containing two methionine residues may produce three oxidized forms depending on the extent of oxidation [10]. We observed relatively stable amounts of CLPs H and N throughout all storage time which may indicate faster oxidation from CLP-M into these CLPs than their further oxidation into CLP-G in which both methionine residues are oxidized (data not included).
Although the flaxseed oil was stored under an argon atmosphere, the oxidative degradation of methionine containing cyclolinopeptides may be related to the initial amounts of dissolved oxygen in the oil. According to Przybylski and Eskin [25], the usual content of dissolved oxygen in edible oil is sufficient to provide a peroxide value of 10 meq/kg. Interestingly, no significant decrease in the amounts of easily oxidizable linolenic acid was apparent in the flaxseed oil throughout the entire storage periods (results not shown). Furthermore, under the conditions used in the present study we observed only a very small increase in PV and AV, probably related to the applied analytical procedure and its error of estimation (Fig 6). Thus, the present study underscores the remarkably high predisposition of flaxseed cyclolinopeptides to oxidative deterioration even in the presence of limited amounts of oxygen, and the assessment of CLP prone to oxidation or their oxidation products can be used as flaxseed oil quality evaluation factor(s).
In contrast to the results obtained for flaxseed oil, the amounts and composition of CLPs in the meal samples remained practically unchanged throughout the entire time of warehouse storage (Table 3). The relatively higher stability of CLPs in the meal when compared to the bulk oil could be attributed to the antioxidative system present in flaxseed meal. Previous studies by Malcolmson et al. [26] and Przybylski and Daun [27] also reported an unusually high storage stability of flaxseed meal as measured by changes in fatty acid composition, conjugated diene and triene, peroxide values, and off-flavor components. Nevertheless, the presence of significant amounts of oxidized CLPs such as CLPs C, E, F, and G in the meal suggested an oxidation happened during oil cold pressing or in seeds during harvesting and storage. Gui et al. [18] also reported the presence of CLPs C, F and G in five flaxseed cultivars and suggested that methionine oxidation is happening prior to removal of oil from the seed. According to Vogt [28], methionine could be oxidized to the sulfoxide by the superoxide anions produced in oxidative metabolism in biological system. It is well known that oxidative deterioration is accelerated in oilseeds when the seed integrity has been compromised, underscoring the importance of seed selection to the initial quality and stability of the extracted oil [29].
Conclusion
Fourteen cyclolinopeptides were separated, identified, and quantified from flaxseed oil and meal by an optimized HPLC–MS method. The relative oxidative stability of the cyclolinopeptides both in the bulk oil and the meal were assessed. Significant changes in cyclolinopeptides were observed in the oven stored oils but not in the meals, and only affected those cyclolinopeptides containing the easily oxidizable methionine residue. Due to the high predisposition of the methionine containing cyclolinopeptides to oxidation and the ease of CLPs’ quantification with the proposed method, it is possible to reliably assess the extent of flaxseed meal and oil oxidation. There was also evidence of cyclolinopeptide oxidation occurring in the seed prior to or during oil extraction, emphasizing the importance of good seed selection and adequate control of the conditions that encourage oxidation during oil extraction.
References
Kailash P (2009) Flaxseed and cardiovascular health. J Cardiovasc Pharm 54:369–377
Adkins Y, Kelley DS (2010) Mechanism underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem 21:781–792
Leiba A, Amital H, Gershwin ME, Shoenfeld Y (2001) Diet and lupus. Lupus 10:246–248
Bergman JM, Thompson LU, Dabrosin C (2007) Flaxseed and its lignans inhibit estradiol-induced growth, angiogenesis, and secretion of vascular endothelial growth factor in human breast cancer xenografts in vivo. Clin Cancer Res 13:1061–1067
Wieczorek Z, Bengtsson B, Trojnar J, Siemion IZ (1991) Immunosuppressive activity of cyclolinopeptide A. Pept Res 4:275–283
Picur B, Cebrat M, Zabrocki J, Siemion IZ (2006) Cylolinopeptides of Linum usitatissimum. J Pept Sci 12:569–574
Gaymes TJ, Cebrat M, Siemion IZ, Kay JE (1997) Cyclolinopeptide A (CLP) mediates its immunosuppressive activity through cyclophilin-dependent calcineurin inactivation. FEBB Lett 418:224–227
Gaymes TJ, Carrett NJ, Patel N, Kay JE, Siemion IZ (1996) Effects of cyclolinopeptide A on T lymphocyte activation and peptidyl prolyl isomerase activity. Biochem Soc Trans 24:90S
Bell A, McSteen PM, Cebrat M, Picur B, Siemion IZ (2000) Antimalarial activity of cyclolinopeptide A and its analogues. Acta Pol Pharm 57:481–490
Morita H, Shishido A, Matsumoto T, Itokawa H, Takeya K (1999) Cyclolinopeptides B – E, new cyclic peptides from Linum usitatissimum. Tetrahedron 55:967–976
Wiesenborn D, Kangas N, Tostenson K, Hall C, Chang K (2005) Sensory and oxidative quality of screw-pressed flaxseed oil. J Am Oil Chem Soc 82:887–892
Abuzaytoun R, Shahidi F (2006) Oxidative stability of flax and hemp oils. J Am Oil Chem Soc 83:855–861
Kumarathasan R, Rajkumar AB, Hunter NR, Gesser HD (1992) Autoxidation and yellowing of methyl linolenate. Prog Lipid Res 31:109–126
Brühl L, Matthäus B, Fehling B, Wiege B, Lehmann B, Luftmann H, Bergander K, Quiroga K, Scheipers A, Frank O, Hofmann T (2007) Identification of bitter off-taste compounds in the stored cold pressed linseed oil. J Agric Food Chem 55:7864–7868
Brühl L, Matthäus B, Scheipers A, Hofmann T (2008) Bitter off-taste in stored cold-pressed linseed oil obtained from different varieties. Eur J Lipid Sci Technol 110:625–631
Stefanowicz P (2004) Electrospray mass spectrometry and tandem mass spectrometry of the natural mixture of cyclic peptides from linseed. Eur J Mass Spectrom 10:665–671
Bagonluri MT, Okinyo-Owiti DP, Burnett PG, Olivia C, Gui B, Reaney MJT (2011) High throughput HPLC analysis of cyclolinopeptides in flax seeds. A paper presented at the CAOCS conference, Edmonton
Gui B, Shim YY, Datla RSS, Covello PS, Stone SL, Reaney MJT (2012) Identification and quantification of cyclolinopeptides in five flaxseed cultivars. J Agric Food Chem. doi:10.1021/jf301847u
Firestone D (2009) Official methods and recommended practices of the American Oil Chemists`Society, 6th edn. AOCS, Champaign
International Organization for Standardization (2008) Animal and vegetable fats and oils -Determination of peroxide value—potentiometric end-point determination. ISO, Geneva 27107
International Organization for Standardization (2004) Animal and vegetable fats and oils—determination of anisidine value. ISO, Geneva 6885
Marr J, Tremblay P, Picard P, Auger S, Burnett PG, Okinyo-Owiti DP, Reaney MJT (2010) High throughput cyclolinopeptide and triacyglycerol (TAG) profiling of Linum usitatissimum using LDTD-MS/MS. A paper presented at The American Society for Mass Spectrometry (ASMS) conference, Salt Lake City, Utah
Jadhav P, Schatte G, Labiuk S, Burnett PG, Li B, Okinyo-Owiti DP, Reaney MJT, Grochulski P, Fodje M, Sammynaiken R (2011) Cyclolinopeptide K butanol disolvate monohydrate. Acta Cryst E67:2360–2361
Gasparetto JC, Smolarek FSF, de Francisco TMG, Miranda LC, Pontarolo R, Siqueira PF (2012) Development and validation of an HPLC-DAD method for analysis of the six major isoflavones in extracts from soybean processing. J Am Oil Chem Soc 89:1211–1222
Przybylski R, Eskin NAM (1988) A comparative study on the effectiveness of nitrogen and carbon dioxide flushing in preventing oxidation during the heating of oil. J Am Oil Chem Soc 65:629–633
Malcolmson LJ, Przybylski R, Daun JK (2000) Storage stability of milled flaxseed. J Am Oil Chem Soc 77:235–238
Przybylski R, Daun JK (2001) Additional data on the storage stability of milled flaxseed. J Am Oil Chem Soc 78:105–106
Vogt W (1995) Oxidation of methionyl residues in proteins: tools, targets and reversal. Free Radic Biol Med 18:93–105
Frankel EN (2005) Lipid oxidation. Oily Press Lipid Library, vol 18. Oily Press, Bridgewater, pp 28–52
Ngoka LCM, Gross ML (1999) A nomenclature system for labeling cyclic peptide fragments. J Am Soc Mass Spectrom 10:360–363
Ngoka LCM, Gross ML (1999) Multistep tandem mass spectrometry for sequencing cyclic peptides in ion-trap mass spectrometer. J Am Soc Mass Spectrom 10:732–746
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Aladedunye, F., Sosinska, E. & Przybylski, R. Flaxseed Cyclolinopeptides: Analysis and Storage Stability. J Am Oil Chem Soc 90, 419–428 (2013). https://doi.org/10.1007/s11746-012-2173-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-012-2173-0