Abstract
In the present study, siaresinolic acid (siaresinol, SA) was isolated from the leaves of Sabicea grisea and studied to evaluate its antinociceptive and anti-inflammatory activity. The antinociceptive effect of SA was investigated in mice using different animal models to study pain. In the acetic acid-induced writhing test, intraperitoneal (i.p.) injection of SA (0.1, 1, and 10 mg/kg, i.p.) 1 h before a pain stimulus significantly reduced the nociceptive response (by 42.3, 68.2, and 70.9 %, respectively). Pretreatment with glibenclamide, but not with yohimbine, metoclopramide, ketanserin, or naloxone, restored the antinociceptive effect induced by SA in the writhing test, suggesting that the K+ATP channel pathway might be involved in its mechanism of action. In the formalin test, SA (1 mg/kg, i.p.) decreased licking time in the second phase only, thereby indicating an anti-inflammatory effect. In the hot plate test, there was no significant difference in nociceptive behavior. In the rota-rod test, it was verified that a high dose of SA (10 mg/kg, i.p.) did not affect the locomotor activity of mice. In the pleurisy model, induced by carrageenan, treatment with SA inhibited important events involved in inflammatory responses, namely leukocyte influx, plasma leakage, and increased inflammatory mediators (TNF-α, IL-1β, and chemokine CXCL1), in the pleural exudate. Additionally, SA itself was not cytotoxic when evaluated using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in macrophages cultured for 24 h at concentrations ranging from 1 to 200 μg/mL. These results suggest, for the first time, that SA attenuates nociceptive behavior through mechanisms involving receptors for ATP-dependent potassium channels, in addition to suppressing acute inflammatory responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Rubiaceae family contains species of great economic importance, which are utilized as food, for ornamental purposes, and in the pharmaceutical industry. This family contains about 650 genera with 13,000 species, which are distributed mainly in tropical and subtropical regions [1–3]. The plants belonging to the genus Sabicea, a member of this family, grow in Africa and South America and consist of approximately 146 species. Some of these species are used in traditional medicine to treat various diseases, including epilepsy, stomach disorders, fever, and malaria [4–6]. Their wide use in traditional medicine has encouraged phytochemical investigation and pharmacological study of their active components [7]. In this regard, the observed effects of the Sabicea species-derived medicines on fever and malaria suggest that their chemical components may be effective for the treatment of pain and inflammation.
Previous studies have identified the presence of active components in the leaves of Sabicea grisea Cham. & Schltdl. var. grisea [8], but its phytochemical profile has not yet been completely characterized. However, it is known that plants of the Rubiaceae family produce a wide variety of secondary metabolites, including triterpenoids [9–12].
A growing interest has been focused on triterpenoids over the past decade due to their beneficial effects on human health [13, 14]. Triterpenoids have been reported to exhibit a wide range of biological activities, including cytotoxic [15], antimalarial [16], antinociceptive [17], and anti-inflammatory [18] effects. Siaresinolic acid (SA), also known as siaresinol, is a triterpene derivative of oleanolic acid and exhibits antidiabetic activity [19], in addition to exerting antiproliferative effects on human leukemia cells and inhibiting their differentiation [20]. In our study, this compound was isolated from the chloroform fraction of an ethanol extract from S. grisea var. grisea leaves. To date, there have been no previous reports on the antinociceptive and anti-inflammatory properties of this triterpene. Thus, this study was conducted to evaluate the effects of SA in nociception and acute inflammation using experimental animal models.
Materials and methods
Animals
Male Swiss mice weighing 18–22 g were obtained from the breeding colonies of the Universidade Federal de Alagoas. Animals were allowed free access to food and water and maintained at 22 ± 2 °C with a controlled 12 h light–dark cycle at the Instituto de Ciências Biológicas e da Saúde animal housing facility. Experiments were performed during the light phase of the cycle. The animals were allowed to adapt to the laboratory for at least 2 h before testing and used only once. This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals by the Brazilian Society of Laboratory Animal Science (SBCAL). The protocol was approved by the Committee on the Ethics of Animal Experiments of the Federal University of Alagoas (Comissão de Ética no Uso de Animais—CEUA, license no. 014512/2011-65). All efforts were made to minimize the suffering of the animals.
Plant material
Leaves of S. grisea var. grisea were collected in May 2008, at Horto Municipal de Maceió, Alagoas State, Brazil, and identified by Rosangela P. Lyra Lemos of the Instituto do Meio Ambiente do Estado de Alagoas, where a voucher specimen was deposited (MAC-11356).
Reagents
The drugs used were formalin and acetic acid (both from Vetec, Rio de Janeiro, RJ, Brazil), indomethacin, yohimbine, metoclopramide, glibenclamide, λ-carrageenan, phosphate-buffered saline (PBS), dimethyl sulfoxide (DMSO), and Roswell Park Memorial Institute (RPMI-1640) medium (Sigma Chemical Co., St. Louis, MO, USA). Immediately before use, SA was dissolved in 200 μL of absolute ethanol and 3,800 μL of saline (NaCl, 0.9 %) to give a concentration of 2 mg/mL, which was diluted to achieve the required concentrations for subsequent experiments. Other drugs and reagents used were of analytical grade.
Isolation and characterization of SA
Air-dried and powdered leaves of S. grisea var. grisea (515 g) were exhaustively extracted with 90 % ethanol at room temperature and the solvent evaporated under vacuum (71.8 g). This extract was suspended in methanol–water (MeOH–H2O) solution (3:2) and successively extracted with hexane, chloroform, and ethyl acetate (EtOAc). The chloroform fraction (4.4 g), which gave positive results in a preliminary antinociceptive assay, afforded SA (38 mg) as an amorphous solid after successive chromatographic fractionations over silica gel using a gradient of EtOAc in hexane and Sephadex LH-20 with MeOH. The melting point was measured using an MQAPF-302 apparatus. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance 400, operating with either 1H and 13C at 400 and 100 MHz, respectively, in CDCl3/CD3OD solutions. The 1H and 13C chemical shifts were referenced to the residual solvent peak at δ H 3.32 and δ C 49.1 for CD3OD or at δ H 7.27 and δ C 77.0 for CDCl3, respectively. Infrared (IR) spectra were obtained on a Perkin-Elmer model 1750 FTIR spectrometer. Silica gel (70–230 mesh, Merck) and Sephadex LH-20 (Pharmacia) were used for column chromatography separations, while analytical thin layer chromatography (TLC; 0.5 mm thickness) was carried out on the silica gel plates (60 F254, Merck). The structure of SA was identified by interpretation of its spectroscopic data and comparison with those reported in the published literature [21, 22]. The percentage purity of SA used in the biological experiments was determined as 95 %.
Acetic acid-induced writhing
The writhing test was carried out in mice according to the method previously described by de Barros et al. [23]. Abdominal writhing was caused by intraperitoneal (i.p.) injection of 0.1 mL of 0.6 % acetic acid/10 g body weight. Control mice received an equivalent volume of saline (NaCl, 0.9 %). Animals were administered i.p. SA (0.01, 0.1, 1, and 10 mg/kg) 1 h before stimulation with acetic acid. Five minutes after the acetic acid injection, the number of times that each animal displayed abdominal constriction was counted for 10 consecutive minutes. The percentage of inhibition was determined for each experimental group using the following formula: inhibition (%) = 100 – [(T × 100)]/C, where C and T indicate nontreated (vehicle) and drug-treated, respectively.
To assess the possible mechanisms involved in the antinociceptive effect of SA, mice were pretreated subcutaneously (s.c.) or intraperitoneally (i.p.) with different antagonists, yohimbine (5 mg/kg, s.c.), metoclopramide (1 mg/kg, s.c.), glibenclamide (5 mg/kg, s.c.), ketanserin (0.3 mg/kg, i.p.), and naloxone (5 mg/kg, i.p.), in the acetic acid animal model. After 15 min, the animals were administered SA (1 mg/kg, i.p.).
Formalin-induced nociception
The procedure described by de Souza Ferro et al. [24] was used, with slight modifications. Mice were injected in the subplantar area of the right hind paw with formalin (2.5 %, 20 μL). The duration of paw licking, a nociception index, was measured from 0 to 5 min (neurogenic phase) and from 15 to 30 min (inflammatory phase) after formalin administration. Animals were treated with SA (1 mg/kg, i.p.) 1 h before intraplantar injection of the stimulus. Indomethacin (10 mg/kg, i.p.) was used as the standard comparator drug, and control animals were treated with sterile saline (vehicle).
Hot plate test
Animals were placed individually on a hot plate metallic surface (Insight®, Brazil; model EFF-361) maintained at 54 ± 1 °C, and the time between placement of the animal on the hot plate and occurrence of either licking of the hind paws, shaking, or jumping off the surface was recorded in terms of the reaction time (s). The reaction time was measured 1 h following treatment with saline (i.p.), SA (1 mg/kg, i.p.), or morphine (5 mg/kg, i.p.), with a cut-off time of 20 s. Each experimental group contained at least six animals.
Rota-rod apparatus
To investigate if the treatments could influence the motor activity of the animals and, consequently, impair assessment of nociceptive behavior in experimental models, the motor activity of the animals was evaluated on a rota-rod apparatus, according to a procedure proposed by de Oliveira et al. [8]. The animals were trained on the rota-rod apparatus (Insight®, Brazil; model EFF-412) for 2 days before the experiment. On the day of the experiment, mice were treated with SA (10 mg/kg, i.p.) or diazepam (10 mg/kg, i.p.) and tested on the rota-rod apparatus, starting 1 h after drug administration. After treatment, the motor performance time (s) was recorded with a stopwatch for up to 240 s.
Carrageenan-induced pleurisy in mice
Pleurisy was induced in mice by intrapleural administration of 100 μL of 1 % (w/v) carrageenan suspension in sterile saline solution [8]. A specially adapted 13 × 5 needle was introduced into the right side of the thoracic cavity for injection of the carrageenan solution, and an equal volume of saline was injected into the controls. The animals were sacrificed in a carbon dioxide (CO2) gas chamber 4 h after the carrageenan injection, and the thoracic cavity was opened and washed with 1 mL of PBS containing ethylenediaminetetraacetic acid (EDTA; 10 mM). Pleural wash samples were diluted in Turk fluid (2 % acetic acid) to measure total leukocytes, using Neubauer chambers. Cytospin preparations of the samples were stained with May–Grünwald–Giemsa to obtain the differential leukocyte count, which was performed under the immersion objective of a light microscope. Subsequently, groups of animals were pretreated (1 h before pleurisy induction) with SA (0.01, 0.1, and 1 mg/kg, i.p.) and 4 h later, the same inflammatory parameters as above were evaluated.
Cytokine/chemokine analysis
Cytokines were measured using a commercially available MagPix Milliplex kit (Merck-Millipore). The immune kit comprised analyte-specific components for simultaneous measurement of the following cytokines from mice: tumor necrosis factor-α (TNF-α), interleukin-1 beta (IL-1β), and chemokine (C-X-C motif) ligand 1 (CXCL1). A minimum of 100 positive beads for each cytokine was acquired with a Luminex MagPix instrument (Luminex Corporation, Austin, TX, USA). All samples were run in duplicate and analyzed on the same day to minimize day-to-day variation. Manufacturer-supplied controls were used to monitor the coefficients of variation, which were <10 %.
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay
The cytotoxic effect of SA on macrophages was determined using the MTT assay method according to Guimarães et al. [25]. Murine peritoneal macrophages (2.5 × 105 cells) were treated with SA at concentrations ranging from 1 to 200 μg/mL and further cultured in RPMI-1640 media supplemented with 10 % fetal bovine serum (FBS) for 24 h. Thereafter, the medium was replaced with fresh RPMI containing 5 mg/mL of MTT. After an additional 4-h incubation period at 37 °C, the supernatant was discharged and DMSO solution (150 μL/well) added to each culture plate. After 15 min of incubation at room temperature, the absorbance of solubilized MTT formazan product was spectrophotometrically measured at 540 nm. Five individual wells were assayed per treatment, and the percentage of viability was determined in relation to the controls [(absorbance of treated cells/absorbance of untreated cells) × 100].
Statistical analysis
Data are reported as mean ± standard error of the mean (SEM) and were analyzed using the GraphPad Prism® software, version 5.0 (GraphPad Software Inc., San Diego, CA, USA). Statistical differences between the groups were determined using the Student’s t test or via two-way analysis of variance followed by the Student–Neuman–Keuls test. p-Values <0.05 were considered statistically significant. All statistical analyses were done using GraphPad Prism® software, version 5.0.
Results
Isolation and identification of SA
SA was obtained as an amorphous solid, melting point 279–281 °C (literature value: 275–278 °C), and gave a positive response to the Liebermann–Burchard test, suggesting a triterpenoid nature [26]. Its IR spectrum showed absorption bands characteristic of hydroxyl (3425 cm−1) and carboxyl (1687 cm−1) groups and a double bond (1608 cm−1). A detailed analysis of the NMR spectral data of this compound revealed features of an olean-12-ene type triterpene containing a hydroxyl group at C-3 (δ H 3.15 [dd, J = 10.2 and 5.0 Hz]; δ C 79.21 [CH]), an α-hydroxyl group at C-19 (δ 3.27 [d, J = 3.5 Hz]; δ C 82.02 [CH]) and a carboxyl group at C-28 (δ C 181.75 [C]) [21]. The 13C NMR spectrum revealed 30 carbon signals to a triterpenoid skeleton. Assignments for all hydrogen and carbon resonances were achieved by a combination of distortionless enhancement by polarization transfer (DEPT), heteronuclear single quantum correlation (HSQC), and heteronuclear multiple-bond correlation (HMBC) experiments. The Δ12 functionality was deduced from the resonance of the sp2 carbons C-12 (δ H 5.35 [sl] and δ C 124.97 [CH]; δ C 143.48 [C, C-13]), as well as a methine hydrogen (δ H 3.05 [sl, H-18]; δ C 44.30 [C-18]) and signals for seven tertiary methyl groups (δ H 0.71 [correlated in the HSQC spectrum with δ C 17.23], 0.74 [δ C 15.88], 0.87 [δ C 28.31], 0.92 [δ C 15.46], 0.93 [δ C 25.02 and 28.33], and 1.23 [δ C 24.85]). The HMBC experiment exhibited correlations particularly between H-19 (δ 3.27) and methyl groups at C-20 (δ 28.31 [C-29] and δ 25.02 [C-30]). These results and comparison with those reported in the literature [21, 22] supported the elucidation of the structure of SA (Fig. 1).
Acetic acid-induced writhing
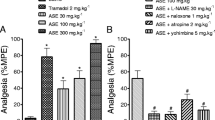
An acetic acid-induced writhing test was used to detect possible antinociceptive effects. In this model, the animals were pretreated with SA (0.01, 0.1, 1, and 100 mg/kg, i.p.) and nociceptive behavior was evaluated 1 h post-acetic acid injection. The administration of this acid caused stereotypical behavior, characterized by abdominal contractions. At 0.01 mg/kg, SA produced no alteration in nociceptive behavior, but pretreatment with SA at 0.1, 1, and 10 mg/kg reduced the number of abdominal writhes by 42.3, 58.2, and 70.9 %, respectively. Indomethacin (10 mg/kg, i.p.), used as the positive control, also significantly inhibited abdominal writhing (by 57.3 %; Fig. 2).
Effects of SA on acetic acid-induced writhing. Mice were treated with SA (0.01, 0.1, 1, and 10 mg/kg) or vehicle (5 % ethanol in saline; control group) by the intraperitoneal (i.p.) route 1 h before acetic acid 0.8 % (injected at time zero). Indomethacin (10 mg/kg, i.p.) was the positive control. Data are expressed as mean ± standard error of the mean (SEM); n = 6 mice per group. **p < 0.01 and ***p < 0.001 indicate statistically significant differences compared to the vehicle-treated group
In another set of experiments, a lower SA dose (1 mg/kg, i.p.) was able to induce a maximum effect in the writhing test. The possible mechanism of action involved in the antinociceptive response of SA was evaluated by subcutaneously treating animals with an α2-adrenergic antagonist (yohimbine; 5 mg/kg), serotonergic and dopaminergic receptor antagonists (metoclopramide; 1 mg/kg), and a selective K+ATP channel antagonist (glibenclamide; 5 mg/kg) 15 min before the administration of SA. As shown in Fig. 3, pretreatment with metoclopramide or yohimbine produced no significant changes in SA-induced antinociception (1 mg/kg), while pretreatment with glibenclamide resulted in full impediment of the antinociceptive effect of SA. Additionally, ketanserin (22.0 ± 0.7 writhes) and naloxone (22.7 ± 2.2 writhes) were unable to reverse the antinociceptive effects of SA (28.0 ± 2.0 writhes) compared to saline-treated mice (40.2 ± 3.3 writhes). Individually, glibenclamide (31.0 ± 1.4 writhes), metoclopramide (33.0 ± 2.7 writhes), yohimbine (31.7 ± 1.7 writhes), ketanserin (37.3 ± 3.1 writhes), and naloxone (39.8 ± 2.4 writhes) did not alter the nociceptive behaviors induced by acetic acid (31.5 ± 1.8 writhes).
Effect of metoclopramide (1 mg/kg, i.p.), yohimbine (5 mg/kg, i.p.), and glibenclamide (5 mg/kg, i.p.) against the antinociceptive action of SA (1 mg/kg) in acetic-induced writhing in mice. Data are expressed as mean ± SEM; n = 6 mice per group. ***p < 0.001 and # p < 0.01 indicate statistically significant differences compared to the vehicle-treated group and the SA-treated group, respectively. Under the graph, the signs + and − indicate the presence or absence of the respective treatment
Formalin-induced nociception
As shown in Table 1, intraplantar injection of 2.5 % formalin evoked a characteristic biphasic licking response. The duration of licking for the first phase (0–5 min, neurogenic) was 49.4 ± 1.1 s and for the second phase (15–30 min, inflammatory) it was 234.3 ± 9.2 s in the control groups treated with the vehicle. Pretreatment of animals for 1 h with SA (1 mg/kg, i.p.) had no significant effect on the licking activity duration in the first phase (57.6 ± 3.5 s), while in the second phase, the SA-treated mice showed a significant reduction (186.0 ± 22.6 s) in the licking activity induced by nociceptive stimuli. The reference drug indomethacin (10 mg/kg) significantly inhibited pain only in the second phase (98.7 ± 15.1 s).
Hot plate test
Intraperitoneal administration of SA (1 mg/kg) was not effective in blocking the nociceptive response to thermal stimuli (8.8 ± 0.8 s) when compared to the saline-treated group (7.0 ± 0.8 s). On the other hand, morphine (5 mg/kg, i.p.; positive control) significantly increased the response latency (18.9 ± 1.4 s).
Evaluation of motor activity
In the rota-rod test, mice in the vehicle group remained on the rota-rod apparatus for 205.3 ± 37.9 s in a period of 1 h. This performance was not affected by the administration of SA (1 mg/kg, i.p.), in which case the animals remained on the rota-rod for 226.0 ± 45.6 s, whereas diazepam (10 mg/kg, i.p.) pretreatment significantly changed the motor response of the animals, decreasing the time spent on the rota-rod by 68 % (66.2 ± 15.3 s).
Carrageenan-induced pleurisy
The intrapleural injection of carrageenan produced an acute inflammation within 4 h characterized by plasma leakage and considerable leukocyte migration, represented by neutrophils and mononuclear cells (Fig. 4a–d). The administration of SA (0.01, 0.1, and 1 mg/kg, i.p.) 1 h prior to carrageenan significantly decreased the total cell count and number of neutrophils, but not the mononuclear cell count (Fig. 4a, b, d). The same doses of SA also reduced plasma leakage (Fig. 4c).
Effects of treatment with SA on the inflammation induced by carrageenan in the mouse pleurisy model. Effects of SA (0.01, 0.1, and 1 mg/kg, i.p.) upon total leukocytes (a), neutrophils (b), total protein (c), and mononuclear cells (d). The open bars represent animals that were stimulated by vehicle (saline, control group). The hatched bars represent animals that were stimulated by carrageenan. Data are expressed as mean ± SEM; n = 6 mice per group. +++ p < 0.001 indicate statistically significant differences compared to vehicle-treated groups. *p < 0.05, **p < 0.01, and ***p < 0.001 indicate statistically significant differences compared to carrageenan-stimulated groups
A substantial increase in TNF-α, IL-1β, and CXCL1 production was found in pleural exudates collected from mice 4 h after carrageenan injection (Table 2). At a dose of 1 mg/kg, SA significantly reduced the levels of TNF-α, IL-1β, and CXCL1 with inhibitions of 82.3, 95.7, and 89.3 %, respectively (Table 2).
Evaluation of cytotoxicity
Its direct effect on cell viability in vitro revealed that SA (1, 10, 100, and 200 µg/mL) did not cause significant cytotoxic effects on murine peritoneal macrophages after a 24-h exposure (96.1 ± 0.2, 96.1 ± 0.2, 98.0 ± 0.4, 94.4 ± 0.2 % of RPMI control, respectively). This finding clearly indicates that SA treatment did not affect the mitochondrial reduction of MTT to formazan, resulting in no detection of any cytotoxic effect.
Discussion
In the present study, we demonstrated that systemic administration of SA, a pentacyclic triterpene isolated from S. grisea var. grisea, significantly inhibited nociceptive behavior and acute inflammatory responses in mice. The possible involvement of ATP-sensitive potassium channel receptors in SA-induced antinociception in mice was also demonstrated. Initially, the effects of SA on peripheral nociception were determined using the acetic acid-induced writhing model, which is frequently used to estimate both the central and peripheral analgesic effects of drugs [27]. The acetic acid-induced writhing test has been associated with an increased level of prostaglandins and cytokines in peritoneal fluids [28]. These mediators induce abdominal constrictions by activating and sensitizing peripheral chemosensitive nociceptors, which are largely associated with the development of inflammatory pain [29]. The administration of SA clearly showed a dose-related antinociceptive effect when assessed in the writhing test, with greater effectiveness than indomethacin [a nonsteroidal anti-inflammatory drug (NSAID) with a broad analgesic spectrum]. This analgesic potential might be related to the inhibition of prostanoids, suggesting that the antinociceptive effect of SA could be associated with an anti-inflammatory action. Additionally, different triterpenoids have been found to be antinociceptive and anti-inflammatory agents due to their ability to inhibit arachidonic acid metabolism [30, 31].
In the present study, an attempt was made to pharmacologically characterize and investigate the possible pathways involved in the analgesic effect of SA. The involvement of neurotransmitter systems, such as the dopaminergic and adrenergic systems, as well as ATP-gated potassium channels, were evaluated using the acetic acid-induced writhing test. The roles of these receptors in regulating the modulation of nociceptive processing have been demonstrated in several previous studies [32–34]. In the present study, metoclopramide, a D2-receptor antagonist, failed to alter the antinociceptive effect of SA. We also noted that yohimbine, an α2-adrenergic receptor antagonist, failed to interfere with the antinociception induced by SA. These observations seem to eliminate the involvement of the dopaminergic and adrenergic systems in the antinociceptive actions of SA. Other neurotransmission systems able to modulate pain threshold, such as opioid and serotoninergic, are not involved in SA antinociception, since the opioid antagonist naloxone and the 5-HT(2A) antagonist ketanserin were unable to prevent the effect of SA. However, antinociception induced by SA was prevented by the administration of glibenclamide, a blocker of the K+ATP channel, suggesting that the antinociceptive effect of SA involves ATP-gated potassium channels. Previous reports showed that triterpenoids achieve their effects in pathological conditions by engaging ATP-sensitive potassium channels [35, 36]. Corroborating these data, Longhi-Balbinot and coworkers demonstrated that glibenclamide also reversed the antinociceptive action of the triterpene 3β,6β,16β-trihydroxylup-20(29)-ene in mice [37].
Another interesting finding in the present study was the demonstration that SA caused significant antinociception only in the second phase of the formalin test. The formalin test produces a distinct biphasic nociceptive response. A first phase (neurogenic pain), occurring within seconds of formalin injection, is elicited by direct chemical activation of nociceptive primary afferent fibers. A second phase (inflammatory pain), occurs as a result of ongoing activity in the primary afferents and increased sensitivity of the dorsal horn neurons [28]. Central analgesic drugs, such as morphine, inhibited both phases equally, while peripherally acting drugs, such as NSAIDs, suppressed mainly the second phase [38]. Thus, the inhibition of nociception in the second phase in the formalin test suggests a probable anti-inflammatory action of SA, thus indicating that this triterpene acts peripherally, similarly to NSAIDs, and corroborating the results of the tests for abdominal contortions.
Seeking to dismiss a possible central involvement in the antinociceptive effect of SA, a hot plate assay was conducted. This model is known to evaluate the possible specific central actions by which analgesic effects are exerted through opioid agents acting on supraspinal and spinal receptors [39]. This test, however, is insensitive to NSAIDs such as cyclooxygenase inhibitors [28]. Our results showed that SA did not alter the latency time for reaction responses, indicating that the antinociceptive action of SA occurs via a peripheral rather than a centrally acting mechanism.
Because the antinociceptive effect of SA was evidenced by a change in the nociceptive behavior of mice, caution was taken to discard possible interfering variables. Thus, in order to determine if SA affected motor coordination, mice were exposed to its highest concentration tested and evaluated in the rota-rod test. Our data showed that SA did not alter the motor performance of mice in the rota-rod test, thereby validating the specificity of its antinociceptive effect.
The effects induced by SA on the acute inflammatory response induced by carrageenan were also evaluated. A pleurisy model has been used for decades to induce inflammation and study its mediators, along with the effectiveness of anti-inflammatory agents [40, 41]. Carrageenan is a phlogistic agent that, when injected into the pleural cavity, promotes a severe inflammation, resulting in an intense leukocyte migration and pleural exudate formation caused by protein extravasation. Our data supported a previous study showing that the injection of carrageenan into the pleural cavity of mice elicited an acute inflammatory response [40], characterized by infiltration and accumulation of fluid (edema) containing high levels of polymorphonuclear leukocytes (PMNs). Treatment of the animals with SA attenuated total leukocytes, polymorphonuclear cell counts, and the pleural volume of exudate after carrageenan challenge; a phenomena that may be related to the lower amount of inflammatory mediators at the inflammation site. In addition, macrophages are specialized cells, with a key role in innate and acquired defense process by releases of proinflammatory mediators. Thus, potential toxic effects on this cell can result in severe problems to the patient. Here, we observed that the concentrations of SA used in vitro did not affect the reduction of MTT in murine macrophages.
In the present study, SA significantly attenuated the production of TNF-α, IL-1β, and CXCL1 in the pleural exudates of carrageenan-injected mice. These proinflammatory molecules are released into the pleural exudates of rodents in response to carrageenan [42]. In addition, these mediators can cause chemotaxis, attracting granulocytes and monocytes that, in turn, stimulate production of additional cytokines and other proinflammatory mediators [43]. There are several studies demonstrating that TNF-α and IL-1β are involved in the upregulation of adhesion molecules on the surface of leukocytes [44]. Moreover, CXCL1 is known as a neutrophil chemotactic factor, which promotes neutrophil chemotaxis and degranulation in rodents. Thus, as a result, the anti-inflammatory effect of SA may be due to suppression of cell recruitment and subsequent generation of proinflammatory mediators. Therefore, we cannot rule out the possibility that SA could produce its effects by inhibiting the synthesis of other proinflammatory mediators. However, this proposal still needs to be evaluated.
In summary, the results of the present study show for the first time that SA, isolated from S. grisea var. grisea, produces peripheral antinociception when assessed in models of nociception in mice. This antinociceptive action seems to be mediated by ATP-gated potassium channels, but not by pathways involving dopaminergic, adrenergic, serotoninergic, and opioid receptors, or through motor impairment. In addition, SA showed significant anti-inflammatory effects on carrageenan-induced pleurisy in mice. Based on these results, this compound may hold great promise for treating painful conditions and inflammatory diseases. However, its molecular mechanism remains unknown. The mechanisms of action of SA on cell activation and other proinflammatory mediators will be explored in future studies.
References
Bremer B, Manen JF (2000) Phylogeny and classification of the subfamily Rubioideae (Rubiaceae). Plant Syst Evol 225:43–72
Taylor CM, Steyermark JA, Delprete PG, Vicentini A, Cortés R, Zappi D, Persson C, Costa CB, Anunciação E (2004) Rubiaceae. In: Berry PE, Yatskievych K, Holst BK (eds) Flora of the Venezuelan Guayana. Missouri Botanical Garden Press, St. Louis, pp 497–848
Pereira ZV, Carvalho-Okano RM, Garcia FC (2006) Rubiaceae Juss. da Reserva Florestal Mata do Paraíso, Viçosa, MG, Brasil. Acta Bot Brasilica 20:207–224
Roumy V, Garcia-Pizango G, Gutierrez-Choquevilca AL, Ruiz L, Jullian V, Winterton P, Fabre N, Moulis C, Valentin A (2007) Amazonian plants from Peru used by Quechua and Mestizo to treat malaria with evaluation of their activity. J Ethnopharmacol 112:482–489
Hirschmann GS, De Arias AR (1990) A survey of medicinal plants of Minas Gerais, Brazil. J Ethnopharmacol 29:159–172
Awad R, Ahmed F, Bourbonnais-Spear N, Mullally M, Ta CA, Tang A, Merali Z, Maquin P, Caal F, Cal V, Poveda L, Vindas PS, Trudeau VL, Arnason JT (2009) Ethnopharmacology of Q’eqchi’ Maya antiepileptic and anxiolytic plants: effects on the GABAergic system. J Ethnopharmacol 125:257–264
Comini LR, Fernandez IM, Rumie Vittar NB, Núñez Montoya SC, Cabrera JL, Rivarola VA (2011) Photodynamic activity of anthraquinones isolated from Heterophyllaea pustulata Hook f. (Rubiaceae) on MCF-7c3 breast cancer cells. Phytomedicine 18:1093–1095
de Oliveira AM, Conserva LM, de Souza Ferro JN, de Almeida Brito F, Lyra Lemos RP, Barreto E (2012) Antinociceptive and anti-inflammatory effects of octacosanol from the leaves of Sabicea grisea var. grisea in mice. Int J Mol Sci 13:1598–1611
Suksamrarn A, Tanachatchairatana T, Kanokmedhakul S (2003) Antiplasmodial triterpenes from twigs of Gardenia saxatilis. J Ethnopharmacol 88:275–277
Heitzman ME, Neto CC, Winiarz E, Vaisberg AJ, Hammond GB (2005) Ethnobotany, phytochemistry and pharmacology of Uncaria (Rubiaceae). Phytochemistry 66:5–29
Karou SD, Tchacondo T, Ilboudo DP, Simpore J (2011) Sub-Saharan Rubiaceae: a review of their traditional uses, phytochemistry and biological activities. Pak J Biol Sci 14:149–169
Conserva LM, Ferreira JC Jr (2012) Borreria and Spermacoce species (Rubiaceae): a review of their ethnomedicinal properties, chemical constituents, and biological activities. Pharmacogn Rev 6:46–55
Wu GS, Lu JJ, Guo JJ, Li YB, Tan W, Dang YY, Zhong ZF, Xu ZT, Chen XP, Wang YT (2012) Ganoderic acid DM, a natural triterpenoid, induces DNA damage, G1 cell cycle arrest and apoptosis in human breast cancer cells. Fitoterapia 83:408–414
Chung PY, Chung LY, Navaratnam P (2014) Potential targets by pentacyclic triterpenoids from Callicarpa farinosa against methicillin-resistant and sensitive Staphylococcus aureus. Fitoterapia 94:48–54
Zhang Y, Peng Y, Li L, Zhao L, Hu Y, Hu C, Song S (2013) Studies on cytotoxic triterpene saponins from the leaves of Aralia elata. Food Chem 138:208–213
Gnoatto SC, Susplugas S, Dalla Vechia L, Ferreira TB, Dassonville-Klimpt A, Zimmer KR, Demailly C, Da Nascimento S, Guillon J, Grellier P, Verli H, Gosmann G, Sonnet P (2008) Pharmacomodulation on the 3-acetylursolic acid skeleton: design, synthesis, and biological evaluation of novel N-{3-[4-(3-aminopropyl)piperazinyl]propyl}-3-O-acetylursolamide derivatives as antimalarial agents. Bioorg Med Chem 16:771–782
Kinoshita K, Akiba M, Saitoh M, Ye Y, Koyama K, Takahashi K, Kondo N, Yuasa H (1998) Antinociceptive effect of triterpenes from cacti. Pharm Biol 36:50–55
Akihisa T, Yasukawa K, Oinuma H, Kasahara Y, Yamanouchi S, Takido M, Kumaki K, Tamura T (1996) Triterpene alcohols from the flowers of compositae and their anti-inflammatory effects. Phytochemistry 43:1255–1260
Song Y, Xu B, Cai W (2013) Active substances and in vitro anti-diabetic effects of a traditional folk remedy Bian-Que Triple-Bean Soup as affected by the boiling time. Food Funct 4:635–643
Teles HL, Hemerly JP, Paulettit PM, Pandolfi JR, Araujot AR, Valentini SR, Young MC, Bolzani VS, Silva DH (2005) Cytotoxic lignans from the stems of Styrax camporum (Styracaceae). Nat Prod Res 19:319–323
Mahato SB, Kundu AP (1994) 13C NMR spectra of pentacyclic triterpenoids—a compilation and some salient features. Phytochemistry 37:1517–1575
Wang XL, Hay AE, Matheeussen A, Gupta MP, Hostettmann K (2011) Structure elucidation and NMR assignments of two new triterpenoids from the stems of Paragonia pyramidata (Bignoniaceae). Magn Reson Chem 49:184–189
de Barros BS, da Silva JP, de Souza Ferro JN, Agra IK, de Almeida Brito F, Albuquerque ED, Caetano LC, Barreto E (2011) Methanol extract from mycelium of endophytic fungus Rhizoctonia sp. induces antinociceptive and anti-inflammatory activities in mice. J Nat Med 65:526–531
de Souza Ferro JN, da Silva JP, Conserva LM, Barreto E (2013) Leaf extract from Clusia nemorosa induces an antinociceptive effect in mice via a mechanism that is adrenergic systems dependent. Chin J Nat Med 11:385–390
Guimarães AG, Xavier MA, de Santana MT, Camargo EA, Santos CA, Brito FA, Barreto EO, Cavalcanti SC, Antoniolli AR, Oliveira RC, Quintans-Júnior LJ (2012) Carvacrol attenuates mechanical hypernociception and inflammatory response. Naunyn Schmiedebergs Arch Pharmacol 385:253–263
Reddy GC, Rangaswami S, Sunder R (1977) Triterpenoids of the stem bark of Gardenia gummifera. Planta Med 32:206–211
Guimarães AG, Oliveira GF, Melo MS, Cavalcanti SC, Antoniolli AR, Bonjardim LR, Silva FA, Santos JP, Rocha RF, Moreira JC, Araújo AA, Gelain DP, Quintans-Júnior LJ (2010) Bioassay-guided evaluation of antioxidant and antinociceptive activities of carvacrol. Basic Clin Pharmacol Toxicol 107:949–957
Le Bars D, Gozariu M, Cadden SW (2001) Animal models of nociception. Pharmacol Rev 53:597–652
Dirig DM, Isakson PC, Yaksh TL (1998) Effect of COX-1 and COX-2 inhibition on induction and maintenance of carrageenan-evoked thermal hyperalgesia in rats. J Pharmacol Exp Ther 285:1031–1038
Ling H, Jia X, Zhang Y, Gapter LA, Lim YS, Agarwal R, Ng KY (2010) Pachymic acid inhibits cell growth and modulates arachidonic acid metabolism in nonsmall cell lung cancer A549 cells. Mol Carcinog 49:271–282
Xiao ZY, Zheng QY, Jiang YY, Zhou B, Yin M, Wang HB, Zhang JP (2004) Effects of esculentoside A on production of interleukin-1, 2, and prostaglandin E2. Acta Pharmacol Sin 25:817–821
Katyal J, Gupta YK (2012) Dopamine release is involved in antinociceptive effect of theophylline. Int J Neurosci 122:17–21
Shannon HE, Lutz EA (2002) Comparison of the peripheral and central effects of the opioid agonists loperamide and morphine in the formalin test in rats. Neuropharmacology 42:253–261
Meotti FC, Fachinetto R, Maffi LC, Missau FC, Pizzolatti MG, Rocha JB, Santos AR (2007) Antinociceptive action of myricitrin: involvement of the K+ and Ca2+ channels. Eur J Pharmacol 567:198–205
Ramachandran V, Saravanan R (2013) Efficacy of asiatic acid, a pentacyclic triterpene on attenuating the key enzymes activities of carbohydrate metabolism in streptozotocin-induced diabetic rats. Phytomedicine 20:230–236
Han XH, Liu P, Zhang YY, Zhang N, Chen FR, Cai JF (2011) Astragaloside IV regulates expression of ATP-sensitive potassium channel subunits after ischemia-reperfusion in rat ventricular cardiomyocytes. J Tradit Chin Med 31:321–326
Longhi-Balbinot DT, Martins DF, Lanznaster D, Silva MD, Facundo VA, Santos AR (2011) Further analyses of mechanisms underlying the antinociceptive effect of the triterpene 3beta, 6beta, 16beta-trihydroxylup-20(29)-ene in mice. Eur J Pharmacol 653:32–40
Yamamoto T, Nozaki-Taguchi N (2002) The role of cyclooxygenase-1 and -2 in the rat formalin test. Anesth Analg 94:962–967
Staahl C, Drewes AM (2004) Experimental human pain models: a review of standardised methods for preclinical testing of analgesics. Basic Clin Pharmacol Toxicol 95:97–111
Farias JAC, Ferro JNS, Silva JP, Agra IKR, Oliveira FM, Candea ALP, Conte FP, Ferraris FK, Henriques Md, Conserva LM, Barreto E (2012) Modulation of inflammatory processes by leaves extract from Clusia nemorosa both in vitro and in vivo animal models. Inflammation 35(2):764–771
Lopes-Martins RA, Albertini R, Martins PS, Bjordal JM, Faria Neto HC (2005) Spontaneous effects of low-level laser therapy (650 nm) in acute inflammatory mouse pleurisy induced by carrageenan. Photomed Laser Surg 23:377–381
Ferreira RG, Matsui TC, Godin AM, Gomides LF, Pereira-Silva PE, Duarte ID, Menezes GB, Coelho MM, Klein A (2012) Neutrophil recruitment is inhibited by nicotinamide in experimental pleurisy in mice. Eur J Pharmacol 685:198–204
Yao L, Yago T, Shao B, Liu Z, Silasi-Mansat R, Setiadi H, Lupu F, McEver RP (2013) Elevated CXCL1 expression in gp130-deficient endothelial cells impairs neutrophil migration in mice. Blood 122:3832–3842
Carrero R, Cerrada I, Lledó E, Dopazo J, García-García F, Rubio MP, Trigueros C, Dorronsoro A, Ruiz-Sauri A, Montero JA, Sepúlveda P (2012) IL1beta induces mesenchymal stem cells migration and leucocyte chemotaxis through NF-kappaB. Stem Cell Rev 8:905–916
Acknowledgments
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Fundação de Amparo à Pesquisa do Estado de Alagoas (FAPEAL) (Brazil).
Conflict of interest
There is no conflict of interest among the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Oliveira, A.M., de Araújo, A.F., Lyra Lemos, R.P. et al. Antinociceptive and anti-inflammatory activity of the siaresinolic acid, a triterpene isolated from the leaves of Sabicea grisea Cham. & Schltdl. var. grisea . J Nat Med 69, 232–240 (2015). https://doi.org/10.1007/s11418-014-0883-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-014-0883-3