Abstract
Cellular senescence contributes to tissue and organismal aging, tumor suppression and progress, tissue repair and regeneration, and age-related diseases. Thus, aging intervention might be a promising target for treatment and prevention of diverse age-related diseases. In the present study, we investigated whether juglanin purified from the crude extract of Polygonum aviculare exerted inhibitory activity against cellular senescence in human dermal fibroblasts (HDFs). Juglanin decreased senescence-associated β-galactosidase activity (SA-β-gal) and the level of reactive oxygen species in senescent cells induced by adriamycin treatment. Juglanin also repressed SA-β-gal activity in HDFs under replicative senescence. These results suggest that juglanin represses cellular senescence in HDFs and might be useful for the development of dietary supplements or cosmetics that alleviate tissue aging or age-related diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cellular senescence can be defined as irreversible growth arrest of normal somatic cells [1] induced by telomere shortening [2], activation of tumor suppressor genes and oncogenes, chronic inflammation, oxidative stress, chemotherapeutic agents, and UV or ionizing irradiation [3]. In addition to growth arrest, as cells senesce they become enlarged and flattened and reveal DNA damage foci in the nucleus, senescence-associated secretory phenotypes (SASP), and senescence-associated β-galactosidase (SA-β-gal) activity [4, 5]. Even though many factors are involved in cellular senescence, the well-known tumor suppressor pathways p53/p21 and Rb/p16 are important in the regulation of cellular senescence [6]. A variety of evidence indicates that cellular senescence contributes to tissue and organismal aging, tissue repair and regeneration, and cancer progression and protection, as well as the pathogenesis of diverse age-related diseases, including cancer, atherosclerosis, skin aging, neurodegenerative disease, muscle atrophy, osteoporosis, and benign prostate hyperplasia [4]. Inhibition of cellular senescence or removal of senescent cells in vivo was recently shown to preclude or delay age-related tissue dysfunction and lengthen health span. Restoration of telomerase in telomerase null mice, which show premature aging phenotypes, decreased DNA damage signaling and rescued degenerative phenotypes across multiple organs [7]. Elimination of senescent cells with high levels of p16INK4a in BubR1 progeroid mice delayed onset of age-associated tissue phenotypes and retarded progression of already initiated age-related disorders [8].
Some extracts prepared from medicinal plants used in East Asia have been shown to exert inhibitory effects against cellular senescence [9–11]. Additionally, chemicals such as ascorbic acid [12], N-acetylcysteine and NS398 [13], and epifriedelanol [14] have been reported to rescue cellular senescence in human primary cells. Rapamycin, an mTOR inhibitor, elongated lifespan in mice by delaying death from cancer, retarding mechanisms of ageing, or both [15]. DDS (4,4′-diaminodiphenylsulfone), which is commonly used to treat Hansen disease patients, was shown to increase lifespan in nematodes by retarding the aging process and reducing the levels of a mitochondrial complex [16].
Based on a free radical-scavenging assay (DPPH assay), juglanin (kaempferol-3-O-α-l-arabinofuranoside) has been reported to have antioxidant activity [17, 18] as well as neuroprotective activities against glutamate-induced toxicity in the mouse hippocampal neuronal cell line, HT22 [19], and cytotoxic activity against the human hepatoma cell line, HepG2 [20]. However, the effects of juglanin on cellular senescence have not yet been determined.
In this study, we investigated whether juglanin exerted inhibitory activity against adriamycin-induced cellular senescence and replicative senescence in human dermal fibroblasts and found that juglanin inhibited cellular senescence in human dermal fibroblasts.
Materials and methods
Materials
Human dermal fibroblasts (HDFs), human umbilical vein endothelial cells (HUVECs), and endothelial cell growth medium-2 (EGM-2) were purchased from Lonza (Walkersville, MD, USA). Dulbecco’s Modified Eagle medium (DMEM), fetal bovine serum (FBS), and penicillin–streptomycin solution were obtained from WelGene (Daegu, Republic of Korea). Antibodies against p53 and p21 were acquired from Santa Cruz Biotech Inc. (Santa Cruz, CA, USA) and antibody against phosphorylated S6 kinase was obtained from Cell Signaling Technology Inc. (Beverly, MA, USA). Additionally, rabbit polyclonal antibody against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was kindly provided by Dr. K. S. Kwon (KRIBB, Daejeon, Republic of Korea). Adriamycin was obtained from Ildong Pharmaceutical Co. Ltd. (Seoul, Republic of Korea). N-Acetylcysteine (purity >99 %) and rapamycin, as positive controls, were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA).
Melting points were measured using a Fisher-Johns melting point apparatus and reported uncorrected. Optical rotations were measured using a JASCO DIP-1000 (Tokyo, Japan) automatic digital polarimeter. NMR spectra were acquired using a Bruker 250 MHz (DMX 250, Germany) spectrometer with Bruker’s standard pulse program. Samples were dissolved in methanol-d 4 and chemical shifts were reported in ppm downfield from TMS. FABMS was obtained using a JEOL JMS700 spectrometer (JEOL, Japan). The stationary phases used for column chromatography (silica gel 60, 70–230 and 230–400 mesh, Lichroprep Rp-18 gel) and TLC plates (silica gel 60 F254 and Rp-18 F254, 0.25 mm) were purchased from Merck KGaA (Darmstadt, Germany). All other chemicals and solvents were of analytical grade and used without further purification.
Isolation of juglanin from P. aviculare
The dried aerial parts of P. aviculare L. (stems and leaves, 9 kg) were extracted with 70, 90, and 100 % MeOH by reflux for 12 h, successively, after which the combined MeOH solution was evaporated to dryness (1.4 kg). The dried MeOH extract (1.4 kg) was resuspended in 1.5 L distilled water and the solution was partitioned with n-hexane (1.5 L × 3) and ethyl acetate (EtOAc, 1.5 L × 3), successively. After drying, solvent fractions of n-hexane (120 g) and EtOAc (65 g) were obtained. The EtOAc fraction (50 g) was loaded onto a silica gel column (50 × 12 cm, silica gel 70–230 mesh), which was subsequently eluted with n-hexane–EtOAc (step-wise gradient from n-hexane 100 % to EtOAc 100 %) and then EtOAc–MeOH (step-wise gradient from EtOAc 100 % to MeOH 100 %). The eluent was combined on the basis of TLC, giving 29 fractions (PAE 1–29). Juglanin (29 mg) was obtained from PAE 20 (1 g) by reverse-phase column chromatography (4 × 50 cm, MeOH–H2O, gradient from 35:65 to 100 % MeOH).
Spectroscopic data of juglanin
Yellow powder; C20H18O10; m.p. 181–183 °C; 1H-NMR (250 MHz, methanol-d 4) δ 5.47 (1H, brs, H–1″), 6.20 (1H, d, J = 2.0 Hz, H-6), 6.40 (1H, d, J = 2.1 Hz, H-8), 6.93 (2H, d, J = 8.8 Hz, H-3′, 5′), 7.97 (2H, d, J = 8.8 Hz, H-2′, 6′); 13C-NMR (62.9 MHz, methanol-d 4) δ 62.7 (C-5″) 78.8 (C-3″), 83.5 (C-2″), 88.1 (C-4″), 94.9 (C-8), 100.0 (C-6), 105.8 (C-10), 109.8 (C-1″), 116.7 (C-3′, 5′), 122.9 (C-1′), 132.2 (C-2′, 6′), 135.1 (C-3), 158.7 (C-2), 159.6 (C-9), 161.8 (C-4′), 163.3 (C-5), 166.2 (C-7), 180.1 (C-4); positive FABMS m/z 419.0 [M+H]+; \(\left[ \alpha \right]_{\text{D}}^{23}\): −162.7° (c 0.1 methanol). The purity of juglanin measured based on the 1H-NMR spectrum was 98 %.
Cell culture
HDFs in DMEM with 10 % FBS and 1 % antibiotics (penicillin 10,000 unit/mL and streptomycin 10,000 μg/mL) were seeded at 1 × 105 cells per 100-mm culture plate and incubated at 37 °C in 5 % CO2 humidified air. When subcultures reached 80–90 % confluence, serial passaging was performed by trypsinization. HUVECs in EGM-2 media were cultured under the same conditions. The number of population doublings (PDs) was monitored for further experiments. PD was calculated using the geometric equation: PD = log2 F/log2 I, where F = final cell number and I = initial cell number. HDFs in PD <35 and HUVECs in PD <30 were used for adriamycin-induced cellular senescence. HDFs in PD >75 and HUVECs in PD >50 were used as old cells under replicative senescence [21, 22].
Induction of cellular senescence by adriamycin treatment
HDFs in DMEM media or HUVECs in EGM-2 media were plated at 1.5 × 105 cells per 100-mm culture plate. After incubation at 37 °C in a CO2 incubator for 3 days, cells were washed twice with DMEM containing 1 % antibiotics and then treated with 500 nM adriamycin for 4 h. Following three rinses with DMEM containing 1 % antibiotics, HDFs in DMEM containing 10 % FBS and 1 % antibiotics and HUVECs in EGM-2 were incubated in a 5 % CO2 incubator for 4 days. Adriamycin-induced cellular senescence was confirmed by senescence-associated β-galactosidase (SA-β-gal) activity staining.
Treatment with juglanin
To determine whether juglanin would inhibit adriamycin-induced cellular senescence, adriamycin-treated cells were dissociated by trypsinization. Juglalin was dissolved in DMSO. HDFs and HUVECs were then plated at 500 and 1,000 cells/well, respectively, in 96-well plates. Cells were incubated at 37 °C in 5 % CO2 humidified air for 24 h, after which they were further treated with 10 μg/mL of juglanin, 0.5 % DMSO, 5 mM NAC, or 500 nM rapamycin for 3 days. Cell toxicity was measured by MTT assay and cellular senescence by SA-β-gal activity staining.
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay
Cells were treated with 0.1 % MTT solution for 3 h, after which the media was discarded and the resulting formazan crystals were solubilized with 100 μL DMSO. Viability was assessed by measuring the absorbance at 550 nm using a microplate reader.
Senescence-associated β-galactosidase (SA-β-gal) activity assay
SA-β-gal activity was determined as previously described [5]. Briefly, cells were fixed with 3.7 % paraformaldehyde in PBS and then incubated in staining solution (40 mM citric acid/phosphate, pH 5.8, 5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 150 mM NaCl, 2 mM MgCl2, 1 mg/mL X-gal) at 37 °C for 16–18 h. Next, cells were washed twice with PBS, after which they were counterstained with 1 % eosin solution for 5 min. Finally, a total of 100 cells were counted in five randomized fields and the percentage of blue cells was calculated.
Protein extraction
Cells were plated at a density of 1 × 105 in 60-mm culture dishes and incubated for 24 h, after which they were treated with the indicated concentrations of single compounds for 1 h prior to adriamycin treatment and incubated for 4 h. Cells were then lysed in 50 μL of ice-cold RIPA buffer (25 mM Tris–HCl, pH 7.6, 150 mM NaCl, 1 % Triton X-100, 0.5 % sodium deoxycholate, 0.1 % SDS, 1 mM sodium vanadate, 5 mM NaF, protease inhibitor or 1 mM PMSF). The particulate debris was removed by centrifugation at 12,000g for 10 min at 4 °C. Finally, the protein concentration in the supernatants was quantified by the bicinchoninic acid (BCA) method (Pierce Biotechnology Inc., Rockford, IL, USA) using bovine serum albumin as a standard.
Western blot analysis
Proteins (30 μg) were separated on 10 % SDS–polyacrylamide gels and then transferred to nitrocellulose membranes. Following blocking of membranes with Tween-20 Tris-buffered saline (TTBS) containing 5 % skim milk for 1 h, the membranes were incubated overnight with antibodies against p53, p21, phosphorylated S6 kinase (pS6K), and GAPDH. The membranes were then washed three times in TTBS, after which horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit antibodies were applied for 1.5 h. Antigen–antibody complexes were detected using Western Blotting Luminol Reagent (Santa Cruz Biotech Inc.) and images of membranes were obtained using a LAS-3000 imaging system (Fujifilm Inc., Stamford, CT, USA). Equal loading of proteins was verified with GAPDH antibody.
Measurement of intracellular ROS level
Adriamycin-treated cells were seeded at 1.5 × 105 in 60-mm culture dishes and incubated for 24 h at 37 °C in a 5 % CO2 incubator. Cells were then treated with 10 μg/mL of juglanin, 0.5 % DMSO, 5 mM NAC, or 500 nM rapamcyin for 3 days, after which they were washed twice with DMEM containing antibiotics and then treated with 250 μM 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) for 20 min at 37 °C in a 5 % CO2 incubator. Following incubation, cells were harvested by trypsinization, washed twice with PBS containing 2 % FBS, and then suspended in 1 % paraformaldehyde (pH 7.4). Finally, the DCF fluorescence intensity of each population of 10,000 cells was measured using a BD FACS Canto II flow cytometer (BD Biosciences, San Jose, CA, USA).
Statistical analysis
All data in the present study are presented as mean ± SD from three independent experiments measured in triplicate. Statistical significance was measured by a paired Student’s t test and a p value of <0.05 was considered statistically significant.
Results
Juglanin was purified from MeOH extract of P. aviculare L. and its chemical structure was determined by comparison of melting points, optical rotation values, 1H- and 13C-NMR, and mass spectral data [23] with those of previously reported values (Fig. 1).
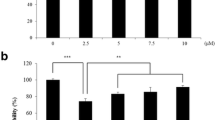
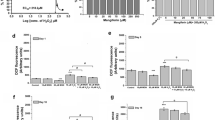
We initially attempted to determine whether juglanin exerts cytotoxicity toward HDFs and HUVECs by MTT assay. Treatment with 10 μg/mL juglanin revealed no cytotoxicity in HDFs or HUVECs (data not shown). We then tested the effects of juglanin on cellular senescence in HDFs and HUVECs induced by treatment with adriamycin by measuring SA-β-gal activity [5]. Juglanin significantly decreased SA-β-gal activity in HDFs that had been treated with adriamycin (Fig. 2a, b). However, juglanin did not repress SA-β-gal activity which had been upregulated in HUVECs by adriamycin treatment (Fig. 3a, b). Therefore, we used HDFs to measure the effects of juglanin on cellular senescence in subsequent experiments. Since juglanin exerted inhibitory activity against cellular senescence induced by adriamycin, we further tested its dose-dependent effects on cellular senescence and confirmed that juglanin reduced SA-β-gal activity in a dose-dependent manner (Fig. 4a, b). The expression levels of p53 and p21 proteins were reported to increase during cellular senescence [22]; we therefore measured whether juglanin decreased the p53 and p21 levels in adriamycin-treated cells. As expected, juglanin reduced the levels of p53 and p21 proteins that were upregulated in HDFs in response to adriamycin treatment (Fig. 5).
Effects of juglanin on adriamycin-induced cellular senescence in HDFs. Cells treated with 500 nM adriamycin for 4 h were seeded at 500 cells/well (HDFs) in 96-well plates. After treatment with 10 μg/mL juglanin, cells were incubated for 3 days and cellular senescence was assessed by SA-β-gal activity staining. a SA-β-gal activity staining in HDFs (×100). b Percentages of SA-β-gal positive cells. Representative SA-β-gal staining pictures of three independent experiments are shown. Values are the mean ± SD from three independent experiments measured in triplicate. C control, D 0.5 % DMSO, N 5 mM N-acetylcysteine, R 500 nM rapamycin, J juglanin. *p < 0.05 or **p < 0.01 vs. D
Effects of juglanin on adriamycin-induced cellular senescence in HUVECs. Cells treated with 500 nM adriamycin for 4 h were seeded at 1,000 cells/well (HUVECs) in 96-well plates. After treatment with increasing concentrations of juglanin, cells were incubated for 3 days and cellular senescence was assessed by SA-β-gal activity staining. a SA-β-gal activity staining (×100). b Percentages of SA-β-gal positive cells. Representative SA-β-gal staining pictures of three independent experiments are shown. Values are the mean ± SD from three independent experiments measured in triplicate. C control, D 0.5 % DMSO, N 5 mM N-acetylcysteine, R 500 nM rapamycin, J juglanin. *p < 0.05 or **p < 0.01 vs. D
Dose-dependent effects of juglanin on adriamycin-induced cellular senescence in HDFs. Cells treated with 500 nM adriamycin for 4 h were seeded at 500 cells/well (HDFs) in 96-well plates. After treatment with 1–10 μg/mL juglanin, cells were incubated for 3 days and cellular senescence was assessed by SA-β-gal activity staining. a SA-β-gal activity staining in HDFs (×100). b Percentages of SA-β-gal positive cells. Representative SA-β-gal staining pictures of three independent experiments are shown. Values are the mean ± SD from three independent experiments measured in triplicate. C control, D 0.5 % DMSO, N 5 mM N-acetylcysteine, R 500 nM rapamycin, J juglanin. *p < 0.05 or **p < 0.01 vs. D
Effects of juglanin on the expression levels of p53, pS6K, and p21 proteins in HDFs treated with adriamycin. Cells were treated with the indicated concentrations of juglanin for 1 h prior to adriamycin treatment and incubated for 4 h. Proteins from cells were extracted and separated. The expression levels of each protein were analyzed by Western blotting. Representative data of three independent experiments are shown. NT not treated with adriamycin, ADR adriamycin, C control, D 0.5 % DMSO, N 5 mM N-acetylcysteine, R 500 nM rapamycin, J juglanin
Intracellular ROS are known to increase in cells during adriamycin-induced premature cellular senescence [14]; we therefore evaluated whether juglanin would reduce the upregulation of ROS in HDFs caused by adriamycin treatment by measuring the intracellular DCF fluorescence intensity. The results revealed that juglanin attenuated the increase in intracellular ROS induced by adriamycin treatment in HDFs (Fig. 6).
Effects of juglanin on intracellular ROS levels upregulated in response to adriamycin treatment in HDFs. Young cells (1.5 × 105) treated with or without adriamycin were seeded in 60-mm culture dishes and incubated for 24 h. Following treatment of cells with 10 μg/mL juglanin, 0.5 % DMSO, 5 mM N-acetylcysteine, or 500 nM rapamcyin for 3 days, cells were loaded with 250 μM H2DCFDA for 20 min. The DCF fluorescence intensity of each population of 10,000 cells was then measured by flow cytometry. Representative data from three independent experiments are shown. Median fluorescence intensities were obtained and compared. Values are the mean ± SD from three independent experiments. NT not treated with adriamycin, ADR adriamycin, C control, D 0.5 % DMSO, N 5 mM N-acetylcysteine, R 500 nM rapamycin, J juglanin. *p < 0.05 vs. D
Since juglanin inhibited adriamycin-induced cellular senescence in HDFs, we investigated whether it also rescues replicative senescence in HDFs. Juglanin led to a significant decrease in SA-β-gal activity in HDFs under replicative senescence, and this decrease occurred in a dose-dependent manner (Fig. 7).
Effects of juglanin on replicative senescence of HDFs. Old cells (3 × 104/well) were seeded in 6-well plates and incubated with or without 10 μg/mL juglanin for 3 days. Cellular senescence was assessed by SA-β-gal activity staining. Representative pictures of three independent experiments are shown (×100). Percentages of SA-β-gal positive cells were measured. Values are the mean ± SD from three independent experiments. O old cells, D 0.5 % DMSO, N 5 mM N-acetylcysteine, R 500 nM rapamycin, J juglanin. *p < 0.05 or **p < 0.05 vs. D
Overall, these results suggest that juglanin represses adriamycin-induced premature senescence as well as replicative senescence in HDFs.
Discussion
The results of this study revealed that juglanin repressed premature senescence induced by adriamycin treatment as well as replicative senescence, which was confirmed based on the SA-β-gal activity, levels of p53 and p21 proteins, and intracellular ROS.
Juglanin is known to exert antioxidant activity [17, 18], neuroprotective activities against glutamate-induced toxicity in HT22 cells [19], and cytotoxic activity against HepG2 cells [20]. However, to the best of our knowledge this is the first report to identify anti-senescence activity of juglanin based on SA-β-gal activity and the levels of p53 and p21 proteins in HDFs.
In this study, we attempted to elucidate the inhibitory effects of juglanin on premature-cell senescence induced in HDFs and HUVECs by adriamycin treatment. However, the inhibitory effects of juglanin on cellular senescence were only observed in HDFs (Figs. 2, 3). These findings suggest that the inhibitory effects of juglanin on cellular senescence could be cell context-dependent or specific regulatory target-dependent, which is in accordance with the results of previous studies that revealed differential effects of plant extracts on cellular senescence among cell types [9]. Additional investigations should be conducted to determine the mechanisms by which juglanin inhibits cellular senescence in HDFs.
It is well known that reactive oxygen species (ROS) function in the regulation of cellular senescence [3, 14]. Intracellular ROS levels increase in premature and replicative senescence, and treatment with antioxidants such as N-acetylcysteine has been reported to inhibit cellular senescence [24, 25]. ROS induces cellular senescence through activation of the DNA damage signaling cascade [24, 25]. Our study revealed that juglanin decreased the ROS level in adriamycin-treated HDFs (Fig. 6), implying that its inhibitory effects on cellular senescence might be due to its antioxidant activity.
Our data were obtained from experiments conducted using an in vitro cellular model. Accordingly, further study is needed to determine the mechanism by which juglanin rescues cellular senescence and to demonstrate its inhibitory effects using in vivo animal models.
Overall, we found that juglanin purified from P. aviculare exerts an inhibitory effect on cellular senescence in HDFs, suggesting that it may be a useful candidate for development of dietary supplements or cosmetics to modulate tissue aging or aging-associated diseases.
Abbreviations
- HDF:
-
Human dermal fibroblast
- HUVEC:
-
Human umbilical vein endothelial cell
- EGM-2:
-
Endothelial cell growth medium-2
- SA-β-gal:
-
Senescence-associated β-galactosidase
- mTOR:
-
Mammalian target of rapamycin
- X-gal:
-
5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside
- PD:
-
Population doubling
- DMEM:
-
Dulbecco’s modified Eagle medium
- H2DCFDA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- DMSO:
-
Dimethyl sulfoxide
References
Hayflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25:585–621
Collado M, Blasco MA, Serrano M (2007) Cellular senescence in cancer and aging. Cell 130:223–233
Kuilman T, Michaloglou C, Mooi WJ, Peeper DS (2010) The essence of senescence. Genes Dev 24:2463–2479
Rodier F, Campisi J (2011) Four faces of cellular senescence. J Cell Biol 192:547–556
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O et al (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92:9363–9367
Campisi J (2011) Cellular senescence: putting the paradoxes in perspective. Curr Opin Genet Dev 21:107–112
Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadinanos J, Horner JW, Maratos-Flier E, Depinho RA (2011) Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 469:102–106
Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM (2011) Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479:232–236
Yang HH, Jung B, Kim JR (2010) Identification of plant extracts that inhibit cellular senescence in human fibroblasts, endothelial cells, and vascular smooth muscle cells. J Korean Soc Appl Biol Chem 53:584–592
Yang J, Lei Y, Cui W, Fang S, Chen K (2009) Mechanisms of delay endothelial cell replicative senescence by extracts from Panax ginseng, Panax notoginseng and Ligusticum chuanxiong. Zhongguo Zhong Yao Za Zhi 34:1544–1548
Im W, Chung J-Y, Bhan J, Lim J, Lee S-T, Chu K, Kim M (2012) Sun ginseng protects endothelial progenitor cells from senescence associated apoptosis. J Ginseng Res 36:78–85
Hwang W-S, Park S-H, Kim H-S, Kang H-J, Kim M-J, Oh S-J, Park J-B, Kim J, Kim SC, Lee J-Y (2007) Ascorbic acid extends replicative life span of human embryonic fibroblast by reducing DNA and mitochondrial damages. Nutr Res Pract 1:105–112
Kim SR, Park JH, Lee ME, Park JS, Park SC, Han JA (2008) Selective COX-2 inhibitors modulate cellular senescence in human dermal fibroblasts in a catalytic activity-independent manner. Mech Ageing Dev 129:706–713
Yang HH, Son JK, Jung B, Zheng M, Kim JR (2011) Epifriedelanol from the root bark of Ulmus davidiana inhibits cellular senescence in human primary cells. Planta Med 77:441–449
Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460:392–395
Cho SC, Park MC, Keam B, Choi JM, Cho Y, Hyun S, Park SC, Lee J (2010) DDS, 4,4′-diaminodiphenylsulfone, extends organismic lifespan. Proc Natl Acad Sci USA 107:19326–19331
Phan VK, Nguyen TM, Minh CV, Nguyen HK, Nguyen HD, Nguyen PT, Nguyen XC, Nguyen HN, Nguyen XN, Heyden YV, Quetin-Leclercq J, Kim GN, Jang HD, Kim YH (2010) Two new C-glucosyl benzoic acids and flavonoids from Mallotus nanus and their antioxidant activity. Arch Pharm Res 33:203–208
Nguelefack TB, Mbakam FH, Tapondjou LA, Watcho P, Nguelefack-Mbuyo EP, Ponou BK, Kamanyi A, Park HJ (2011) A dimeric triterpenoid glycoside and flavonoid glycosides with free radical-scavenging activity isolated from Rubus rigidus var. camerunensis. Arch Pharm Res 34:543–550
Yang H, Sung SH, Kim J, Kim YC (2011) Neuroprotective diarylheptanoids from the leaves and twigs of juglans sinensis against glutamate-induced toxicity in HT22 cells. Planta Med 77:841–845
Liu JX, Di DL, Wei XN, Han Y (2008) Cytotoxic diarylheptanoids from the pericarps of walnuts (Juglans regia). Planta Med 74:754–759
Yoon IK, Kim HK, Kim YK, Song IH, Kim W, Kim S, Baek SH, Kim JH, Kim JR (2004) Exploration of replicative senescence-associated genes in human dermal fibroblasts by cDNA microarray technology. Exp Gerontol 39:1369–1378
Kim KS, Seu YB, Baek SH, Kim MJ, Kim KJ, Kim JH, Kim JR (2007) Induction of cellular senescence by insulin-like growth factor binding protein-5 through a p53-dependent mechanism. Mol Biol Cell 18:4543–4552
An R-B, Kwon J-W, Kwon T-O, Chung W-T, Lee H-S, Kim Y-C (2007) Chemical constituents from the whole plants of Euphorbia supina Rafin. Kor J Pharmacogn 38:291–295
Kim HJ, Kim KS, Kim SH, Baek SH, Kim HY, Lee C, Kim JR (2009) Induction of cellular senescence by secretory phospholipase A2 in human dermal fibroblasts through an ROS-mediated p53 pathway. J Gerontol A Biol Sci Med Sci 64:351–362
Kim KS, Kang KW, Seu YB, Baek SH, Kim JR (2009) Interferon-gamma induces cellular senescence through p53-dependent DNA damage signaling in human endothelial cells. Mech Ageing Dev 130:179–188
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (2005-0049417).
Author information
Authors and Affiliations
Corresponding author
Additional information
H. H. Yang, K. Hwangbo and M. S. Zheng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yang, H.H., Hwangbo, K., Zheng, M.S. et al. Inhibitory effects of juglanin on cellular senescence in human dermal fibroblasts. J Nat Med 68, 473–480 (2014). https://doi.org/10.1007/s11418-014-0817-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-014-0817-0