Abstract
Chronic oxidative stress has been associated with several human ailments including the condition of aging. Extensive studies have shown the causal relationship between oxidative stress, aging, and cellular senescence. In this regard, forestalling or preventing senescence could delay the aging process as well as act as an intervention against premature aging. Hence, in the present study, we investigated the anti-senescence potential of Mangiferin (MGN) against Hydrogen peroxide (H2O2) induced premature senescence using human dermal fibroblast cells. Early passage human dermal fibroblasts cells were exposed to H2O2 (10 μM) for 15 days. In order to assess the anti-senescence property of MGN, cells were preconditioned with MGN (10 μM / 50 μM; 2 h) followed by addition of H2O2 (10 μM). H2O2 mediated induction of premature senescence was accompanied by elevated ROS, lowering of mitochondrial mass and membrane potential, changes in ATP content along with G0/G1 arrest and SA-β-gal expression. While, conditioning the cells with MGN lowered oxidative burden, stabilized mitochondrial membrane potential / mass and protected the cells against cell cycle arrest, ultimately rendering protection against premature senescence. The present findings showed that MGN might act as a potential cytoprotective nutraceutical that can prolong the onset of chronic oxidative stress mediated premature senescence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cellular senescence is a phenomenon wherein cells cease to proliferate and leave the cell cycle. They enter a state of irreversible growth arrest and are distinct from cells that are terminally differentiated or quiescent [1]. Senescence has been evolutionarily conserved and is a classical example of antagonistic pleiotropy [2]. Senescence is broadly divided into two categories, a) replicative and b) premature. Replicative senescence is triggered by the erosion of protective caps present at the ends of chromosomes, called telomeres. When telomeres reach a critical length, then a DNA damage response is triggered, as the eroded chromosomal ends are recognized as nicks [3]. This leads to the activation of a set of cell cycle checkpoint genes which ensure that cells continue to remain in a state of irreversible growth arrest. Alternatively, cells may also undergo senescence on experiencing certain kinds of extrinsic or intrinsic insults such as oxidative stress, culture stress, strong mitogenic signals, loss of tumor suppressor genes, thereby becoming senescent much before they would have otherwise, which is referred as premature senescence [4]. The properties of senescent cells include irregular, flattened appearance, over-express cell cycle checkpoint proteins and senescence-associated β-galactosidase activity [5].
Oxidative stress is the underlying cause of many pathological conditions, including premature senescence. Reactive oxygen species (ROS) function as signaling molecules initiating cascades that are detrimental for cells [6]. They are quenched by anti-oxidant systems present within cells, such as superoxide dismutase, catalase, glutathione peroxidase and vitamin C. When the balance between pro-oxidants and anti-oxidants is disrupted, oxidative stress manifests. Mitochondria, being the powerhouses of cells, are potent endogenous sources of reactive oxygen species. These ROS may be free radicals or ions (superoxide, hydroxyl radicals, H2O2 , peroxyl, nitric oxide etc.) which have the potential of damaging intracellular proteins, lipids and nucleic acids [7]. Exogenous oxidative stress imposed by treatment with oxidants such as H2O2 increases ROS levels within cells which may damage mitochondria and lead to their dysfunction. Thus, a positive feedback loop is setup, where damaged mitochondria produce higher levels of ROS by electron leak that ultimately damages DNA and elicits a damage response cascade. This positive feedback loop is responsible for maintenance of senescent state of cells [6].
MGN is a natural polyphenolic compound that is known to possess several pharmacological and nutraceutical properties. It is mainly found in Mangifera indica, though it is also present in a range of medicinal herbs, for instance, honeybush [8]. MGN serves as an effective antioxidant by, a) interfering with lipid peroxidation as it reduces localized ROS levels to form MGN phenoxy radicals; b) chelating iron (Fe2+/Fe3+) so as to form metal-ligand complexes, thereby preventing Fenton reaction from taking place, and c) extracting membrane hydroxyl/peroxyl radicals to prevent its continued oxidation [9]. Structurally, MGN carries out its antioxidant activities by virtue of the hydroxyl groups present on its heterocyclic ring [8]. Also, a recent report suggests the pleiotropic health promoting potential of MGN in several conditions [10]. Therefore, the present study aims to investigate the anti-senescence potential of MGN in H2O2 induced premature senescence model using human fibroblast cells growing in vitro. It is anticipated that MGN, due to its antioxidant potential might confer protection or prolong the onset of premature senescence mediated by chronic oxidative stress.
Materials and methods
Cell culture and reagents
Mangiferin (MGN), Dulbecco’s Modified Eagle Medium (DMEM), Fetal Bovine Serum (FBS), Rhodamine 123, Acridine orange (AO) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Other chemicals mentioned were procured from HiMedia Laboratories, Mumbai, India. Human dermal fibroblast cells used in this study were previously established at the Manipal School of Life Sciences, from the skin biopsies of healthy individuals with prior consent and approval from Institutional Ethics Committee, Kasturba Hospital, Manipal (approval number IEC 011/2009). Briefly, skin sample was cut into small pieces aseptically and cultured in the presence of DMEM containing 10% FBS and fibroblast cells were allowed to outgrow and routinely passaged by trypsinization. Fibroblast cells were identified by their distinct property of being spindle-shaped, bipolar and retractile (as described in ATCC). The fibroblast cells established were maintained in DMEM supplemented with 10% FBS, along with 1× antibiotics/antimycotic mixture (containing 100 U of Penicillin, 0.1 mg of Streptomycin and 0.25 μg of Amphotericin B) at 37 °C in a 5% CO2 incubator. Cells were maintained at 50–60% confluence and all the experiments were conducted using cells established from multiple donors of below passage 10.
Experimental design
We initially carried out experiments in order to determine the effective concentration at which H2O2 is potent enough to induce senescence. For this, exponentially growing cells (104) growing in 96 well plate were treated with different concentrations of H2O2 (10-500 μM) for 24 h in order to determine the effect of H2O2 on cell viability by MTT assay [11]. Our finding (unpublished data) indicated that treating the fibroblast cells with a low concentration of H2O2 (10 μM) everyday for 15 days could induce the phenotype of premature senescence in fibroblast cells which was confirmed by SA-β Gal staining assay.
For protective studies, two doses of MGN were selected (10 μM and 50 μM) based on MTT assay performed as described earlier [11]. In this case, exponentially growing cells (104) growing on 96-well plate were conditioned with different concentration of MGN (5–100 μM; 2 h) and then challenged with H2O2 (200 μM; 24 h) following which MTT assay was performed.
For senescence related experiments, the exponentially growing cells (6 × 105) were seeded in several T-75 flask with 10 mL of DMEM per flask and were divided as following for treatment with test agents:
1) Control, 2) MGN (10 μM), 3) MGN (50 μM), 4) H2O2 (10 μM), 5) MGN (10 μM) + H2O2 (10 μM) and 6) MGN (50 μM) + H2O2 (10 μM). In the combination treatment group, cells were conditioned with the mentioned concentrations of MGN for 2 h prior to H2O2 exposure. The cells received both the drugs every day up to day 15. Cells were harvested at different days viz., day 1, day 5, day 10, and day 15 of treatment in order to carry out various assays mentioned below. Cells were seeded and maintained at a sub-confluent level throughout the treatment time-period to 15 day with intermittent sub-culturing up in order to prevent replicative senescence due to lack of growing space. Sub-culturing of treatment group was performed once in 3 days after the 2 h of MGN conditioning was over.
Assay for estimation of intracellular ROS
Post-treated cells were harvested and centrifuged at 155×g for 5 min. The pellet was dislodged and washed with sterile PBS at 155×g for 5 min. Cells were stained separately with 5 μM DCF-DA (for cytosolic ROS) and 5 μM of MitoSox Red (for mitochondrial superoxide) and incubated for 30 min at 37 °C, in dark. Acquisition of cells was performed with BD FACS Calibur (USA) flow cytometer using CellQuest software (BD, USA) [12].
Mitochondrial membrane potential assay
Upon termination of the treatment, cells were harvested and centrifuged at 155×g for 5 min. The pellet was dislodged and washed with sterile PBS at 155×g for 5 min. Cells were stained with 5 μM Rhodamine 123 (excitation at 488 nm; emission at 525 nm) and incubated for 30 min at 37 °C, in dark. Acquisition of cells was performed with BD FACS Calibur (USA) flow cytometer using CellQuest software (BD, USA) [11].
Assessment of alterations in mitochondrial mass
After various treatments, cells were harvested and washed with sterile PBS at 155×g for 5 min. Cells were stained with 5 μM nonylacridine orange (excitation at 488 nm; emission at 525 nm) and incubated for 30 min at 37 °C, in dark. Acquisition of cells was performed with BD FACS Calibur (USA) using CellQuest software (BD, USA) [13].
Assessment of G0/G1 arrest of cells
Upon treatment, cells were trypsinized, washed in PBS, fixed in 70% ice-cold alcohol and treated with RNase (100 μg/mL) for 30 min and incubated at 37 °C. Next, the cells were stained with propidium iodide (50 μg/mL in PBS) for 15 min at 4 °C in dark and analyzed in FACS Calibur using Cell Quest software (Becton Dickinson, USA) as described earlier [14]. The percentage of cells in Go/G1 phase was calculated by using Win MDI 2.8 program.
Cellular ATP content
Intracellular ATP content was measured by Adenosine 5′-triphosphate (ATP) Bioluminescent Assay Kit (Sigma, USA) as per the manufacturer’s instructions. Briefly, after treatment cells were suspended in sterile PBS (pH 7.4) and lysed with repeated freeze (in liquid N2)-thaw (in water-bath set to 37 °C) and the protein content in the lysates were quantified by standard Bradford assay. Next, appropriate volumes of cells lysates were mixed with reaction buffer containing luciferin/luciferase provided in the kit and the luminescence was immediately read using a luminometer (F12 luminometer, Berthhold Detection Systems, Germany). Data obtained has been represented as relative luminescence units (RLU) second−1 mg−1 protein for all the samples.
Senescence-associated β-Galactosidase assay
Cells (7.5 × 104) were seeded on 60 mm petri-plates and treated according to the experimental design. At the respective time points, the petri-plates were taken and washed twice with PBS to remove all residual media, following which, the cells were fixed using fixative containing 2% formaldehyde and 0.2% glutaraldehyde prepared in sterile PBS (pH 7.4) for 5 min at room temperature. After fixing, the cultures were rinsed with PBS to make sure to remove the fixative content. Next, the freshly prepared SA-β galactosidase staining solution, comprising 1 mg/mL of X-gal, 40 mM citric acid/sodium phosphate buffer (pH 6), 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 150 mM NaCl, and 2 mM MgCl2, was added to the culture plates and incubated at 37 °C, overnight [15]. Following, the staining solution was removed and the cells were washed twice with PBS and observed under phase-contrast microscope. Senescence-positive cells appear blue in color which was captured at 100× magnification using inverted microscope (Olympus IX 51, Olympus, Japan) fitted with DP73 color camera (Olympus, Japan) using ImagePro Insight Image Acquisition software (MediaCybernetics, USA).
Statistical analysis
Each experiment was performed independently in triplicates using cells of the same passage established from several donors. Data was analyzed by One-way Analysis of Variance and Bonferroni’s post-hoc test using GraphPAD Prism 5 software (CA, USA). The data was expressed as mean ± SD. The “p” value <0.05 was considered significant.
Results
Changes in cell viability in the presence of H2O2/MGN
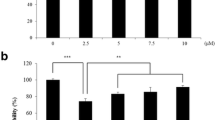
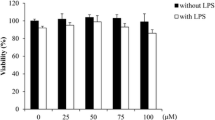
After 24 h treatment with increasing H2O2 doses, viability of cells was assessed using MTT assay. The EC50 value for cells treated with H2O2 for 24 h was found to be 218.2 μM (Fig. 1a). Fibroblasts treated with increasing doses of MGN (5–500 μM) did not show any significant reduction in cell viability indicating its non-toxic nature at the tested concentrations (Fig. 1b). For dose selection, fibroblasts were preconditioned for 2 h with the aforementioned concentrations of MGN, followed by addition of 200 μM H2O2 to each well. MTT results revealed that cell viability with H2O2 alone was 60%, whereas cells pretreated with MGN showed an increase in their viability. There was elevated cell survival (70%) after treatment with 5 μM MGN; viability kept rising with higher concentrations of MGN and stabilized beyond 25 μM (to 85%). Thus, two doses of MGN were selected (10 μM and 50 μM respectively) for further studies to analyze its cytoprotective effects (Fig. 1c). Therefore, from these observations, we used a sub-micromolar concentration of 10 μM of H2O2 for the induction of premature senescence and 10 and 50 μM of MGN for protection studies.
a Effect of H2O2 (10–500 μM) on viability of fibroblasts after 24 h as assessed using MTT assay. The EC50 (24 h) value for H2O2 exposure was calculated by plotting Log10 of concentrations of H2O2 versus viability and performing a non-linear regression analysis using GraphPad Prism 5 (CA, USA). b Viability of fibroblasts after 24 h of treatment with MGN (5–500 μM) assessed using MTT assay. c Changes in viability of fibroblasts on being pretreated for 2 h with MGN (5–500 μM) followed by exposure to a single 200 μM of H2O2. Time-dependent changes in the cytosolic ROS induced by chronic exposure of fibroblast cells with H2O2 (10 μM) and its modulation by MGN conditioning. Graphs indicating levels of cytosolic ROS as measured by DCF fluorescence at d Day 1; e Day 5; f Day 10 and g Day 15. Data has been indicated as Mean ± SD, n = 3; φ,*, ***, # p < 0.05 when compared to respective controls
Measurement of cytosolic and mitochondrial ROS
Cells exposed to H2O2 (10 μM) showed an increase in cytosolic ROS in a time dependent manner as measured on days 1, 5, 10 and 15. Cell conditioned with MGN (10/50 μM; 2 h) aided in lowering of cytosolic ROS indicating its protective effect (Fig. 1d, e, f and g). We also observed an increase in the percentage of cells showing higher florescence for MitoSox red dye indicating the elevation in the number of cells expressing more superoxide levels upon exposure to H2O2 (10 μM) in a time dependent manner as measured on days 1, 5, 10 and 15. On the contrary, cells exposed to MGN (10/50 μM; 2 h) prior to H2O2 (10 μM) exposure lead to a decrease in the percentage of MitoSOX positive cells with 50 μM of MGN showing better protection when compared to 10 μM of MGN (Supplementary Fig. 1; Table 1).
Measurement of mitochondrial membrane potential and mass
Cells chronically exposed to 10 μM H2O2 showed a gradual decline in mitochondrial trans-membrane potential over a period of 15 days. Conditioning the cells with MGN aided in maintenance of membrane potential when compared to H2O2 alone treated groups. Cells conditioned with 50 μM of MGN showed greater protection against H2O2 mediated depletion of membrane potential when compared to 10 μM of MGN (Fig. 2a, b, c and d). Cells chronically exposed to 10 μM H2O2 showed a gradual decline in mitochondrial mass potential over a period of 15 days. Conditioning the cells with MGN aided in maintenance of mitochondrial turnover when compared to H2O2 alone treated groups. Cells conditioned with 50 μM of MGN showed greater protection against H2O2 mediated mitochondrial depletion when compared to 10 μM of MGN (Fig. 2e, f, g and h).
Time-dependent changes in the mitochondrial membrane potential induced by chronic exposure of fibroblast cells with H2O2 (10 μM) and its modulation by MGN conditioning. Graphs indicating levels of mitochondrial membrane potential as measured by Rhodamine 123 fluorescence at (a) Day 1; (b) Day 5; (c) Day 10 and (d) Day 15. Time-dependent changes in the mitochondrial mass induced by chronic exposure of fibroblast cells with H2O2 (10 μM) and its modulation by MGN conditioning. Graphs indicating changes in mitochondrial mass as measured by NAO fluorescence at (e) Day 1; (f) Day 5; (g) Day 10 and (h) Day 15. Data has been shown as Mean ± SD, n = 3; *, φ, ***, # p < 0.05 when compared to respective controls
Estimation of G0/G1 cell population by propidium iodide staining
Cells chronically exposed to H2O2 showed an increase in the % of cells accumulated at G0/G1 phase at day 15. Cells preconditioned with MGN, showed lesser accumulation of cells at G0/G1 phase indicating its ability to ameliorate H2O2 mediated cell cycle arrest (Fig. 3a, b, c and d).
Pre-conditioning the cells with MGN reduced the accumulation of cells at G0/G1 phase of cell cycle induced by chronic exposure of H2O2 (10 μM). Graphs indicating % of cells accumulated at G0/G1 stage of cell cycle as measured by PI staining at (a) Day1; (b) Day 5; (c) Day 10 and (d) Day 15. Data has been shown as Mean ± SD, n = 3; φ, ***, # p < 0.05 when compared to respective controls
Intracellular ATP content
We observed a time dependent decrease in the intracellular ATP content in case of cells exposed to 10 μM H2O2. Cells conditioned with MGN (50 μM) showed higher levels of ATP in comparison to H2O2 alone treated cells at all time points (Fig. 4).
Senescence-associated β-Galactosidase assay
Cells that are senescent in nature exhibit unique morphological features that can be eaasily noted under the microscope. Senescent cells demonstrate structural aberrations that includes more enlarged and flattened form and appear blue under light microscope as they are positve for X-gal. Cells were exposed to a low chronic dose of H2O2 (10 μM) alone or first conditioned with MGN (50 μM; 2 h) followed by H2O2 (10 μM) for 15 days, after which they were stained to check for SA-β-Gal activity. A significant fraction of cells were stained blue, in case of H2O2 (10 μM) alone group indicating that they have undergone senescence. Cells condition with MGN (50 μM; 2 h) prior to H2O2 (10 μM) exposure lead to a decrease in the SA-β-gal activity (Fig. 5).
Chronic exposure to 10 μM H2O2 for 15 days induces premature senescence in fibroblast cells as detected by SA- β gal (blue) positive cells in treated cells versus control cells. Representative images for SA- β gal staining: (a) control at day 15; (b) 50 μM MGN at day 15; (c) 10 μM H2O2 at day 15; (d) 10 μM H2O2 + 50 μM MGN at day 15
Discussion
There exist certain compounds which have the ability to alter the physiology as well as secretome of cells on being administered in sub-lethal doses [16]. H2O2 is one such agent which induces premature senescence in cells by imposing oxidative stress. In our study, we have assessed the ability of MGN to mitigate H2O2-induced premature senescence and prolong its onset. The data obtained indicated that normal human fibroblasts on being exposed to H2O2, an established oxidant, showed a concentration-dependent decrease in viability as compared to untreated control. Our study corroborated with an earlier report [17], according to which increasing doses of H2O2 caused viability of fibroblasts to reduce consistently. Being membrane permeable and diffusible, H2O2 is generally detoxified by the antioxidant systems that exist within cells, though sustained accumulation of H2O2 ultimately brings about oxidative stress and associated damages [18].
Chronic treatment with a sub-lethal dose of H2O2 induces an irreversible growth arrest, leading to senescence [19]. In our study, cells were exposed to a low dose of H2O2 consistently for 15 days. At the end of the treatment period, the fibroblast cells exhibited positive for X-gal staining indicating induction of senescence. A similar finding was reported earlier [20] wherein young human fibroblast cells exhibited senescence like phenotype with chronic exposure to H2O2. Therefore, H2O2 induced senescence model of human fibroblast cells growing in vitro is the widely accepted model in aging research and is extensively used to screen the anti- senescence potential of various agents.
Our data indicated that MGN by itself did not have any detrimental effects over the concentration range selected. There was no significant cell-death after 24 h of treatment with increasing doses of MGN from 5 to 500 μM. Pre-conditioning fibroblasts for 2 h with different doses of MGN, followed by H2O2 treatment showed an increase in cell viability as compared to that of cells treated with H2O2 only. This is in concordance with another study, where MGN attenuated rotenone-induced cytotoxicity [21]. Cells showed increased viability on being pre-treated with MGN, in a dose-dependent manner.
MGN confers protective effects on cells by virtue of its heterocyclic ring structure which facilitates scavenging of ROS, chelating metal ions (thereby interrupting Fenton reaction). Free radicals and ROS participate in intracellular signal transduction cascades and maintain the redox balance within cells under normal circumstances. In our study, it is revealed that ROS levels increase in cells treated with H2O2 as compared to control. MGN-conditioned cells showed reduced levels of ROS as MGN helped in lowering the ROS generated. This is in line with the findings of a study wherein xanthones identified from Garcinia mangostana L. showed similar activity against H2O2-induced oxidative stress [22]. Polyphenols may not always serve as radical scavengers. This was shown in an experiment where resveratrol enhanced premature senescence induced by infrared radiation. It increased the radiosensitivity of tumor cells by increasing ROS generation, thereby facilitating chemotherapy [23]. Another study demonstrated that MGN can act as an effective chemotherapeutic agent by increasing ROS levels within rhabdomyosarcoma cells in a dose-dependent manner [24]. Polyphenols often behave as double-edged swords; at higher doses they may exert a pro-oxidant effect on cells, as was seen in the experiment carried out by Padma and colleagues [24]. They have a paradoxical behavior which is primarily dependent on administered dose as well as the physiological status of cells to which are being treated [25]. Since in our study cell lines were normal and not cancerous, MGN did not display any pro-oxidant attribute.
Mitochondria are major sites of ROS generation, hence they are extremely susceptible to its damaging effects [6]. Mitochondrial membrane potential is an electrochemical gradient that exists across the two mitochondrial membranes and is an important biomarker of the functional status of mitochondria. Disruption of this gradient leads to dysfunctional mitochondria [26]. In our study, mitochondrial membrane potential has been shown to decrease in cells treated with H2O2. Restoration of transmembrane potential occurs when cells are pretreated with MGN as it serves as a free-radical scavenger and reduces damage to the mitochondrial membrane. Our findings are in line with a research conducted by Zhou and co-workers [27] where mussel oligopeptides shielded fibroblasts from oxidative-stress induced premature senescence. Data obtained during this study indicated that pretreatment with these oligopeptides restored mitochondrial membrane potential which had reduced significantly due to H2O2 addition. Another study successfully indicated that various high-molecular tea polyphenols extracted from oolong and black tea increased the mitochondrial membrane potential of cells, thereby proving that polyphenols protect mitochondrial membranes from free radical attacks [28].
Treatment of fibroblast cells with H2O2 induced a decrease in the mitochondrial mass in the present study, which is in contrast to a previous study that showed elevation in mitochondrial mass upon oxidative stress [29]. Although this observation made warrants further investigation, it might be attributed to various mechanisms such as clearance of damaged mitochondria in due course of senescence by mitophagy, increased fusion process of residual mitochondria population due to it s dynamic nature [30] or deranged mitochondrial biogenesis. Cells conditioned with MGN prior to H2O2 exposure aided in maintenance of mitochondrial mass indicating its protective potential. As described previously, nutraceuticals have been confirmed to enhance cell’s physiology by aiding in redox balance and maintaining mitochondrial function and biogenesis [31] and we envisage that MGN could act similary in this case.
Cell cycle arrest is another hallmark featured by cells undergoing senescence. In the present study, H2O2 treatment led to G0/G1 arrest in human fibroblast cells. This observation is supported by a previously published report where H2O2-induced oxidative stress led to the induction of G0/G1 arrest in normal human epidermal keratinocytes [32] and in normal dermal fibroblast cells [20]. This could arise due to several factors including persistent DNA damage as an outcome of chronic oxidative stress preventing cell proliferation further leading to senescence [5]. Conditioning the cells with MGN resulted in lowering of % of cells blocked at G0G1 phase of the cell cycle. This could be an outcome of MGN mediated decline in oxidative DNA damage due to its antioxidant property, thus preventing cell cycle arrest.
Our results indicated that MGN could forestall H2O2 mediated alterations in cellular redox status, mitochondrial transmembrane potential, mitochondrial mass and reduced G0/G1 arrest of cells. To the best of our knowledge, this is a first report indicating the ability of MGN to forestall the senescence-inducing property of low chronic oxidative stress which can be attributed to its free-radical scavenging property. Although, lack of precise molecular mechanism of MGN’s anti-senescence property limits this present study, but it opens a platform for future investigations aiming towards deciphering the molecular mechanism of action of phytochemicals like MGN. As MGN is an activator of Nrf-2 [33, 34], which is a chief regulator of molecules such as GSH, GST, and other enzymes responsible for oxidative homeostasis and detoxification [35], we envisage that MGN-mediated delay in H2O2-induced premature senescence could be linked with Nrf-2 mediated signaling responses, which warrants further investigation.
Conclusion
To conclude, from the data obtained in this study it is quite evident that MGN may be a potential nutraceutical in ameliorating chronic oxidative stress mediated premature senescence. These evidences further warrant investigations related to dietary intake of MGN at physiologically relevant doses that may contribute to slow down premature senescence thereby thwarting aging process.
References
Childs BG, Gluscevic M, Baker DJ, Laberge RM, Marquess D, Dananberg J, van Deursen JM (2017) Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov 16(10):718–735
Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL (2013) Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 123(3):966–972
d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426(6963):194–198
Herranz N, Gil J (2018) Mechanisms and functions of cellular senescence. J Clin Invest 128(4):1238–1246
Hernandez-Segura A, Nehme J, Demaria M (2018) Hallmarks of cellular senescence. Trends Cell Biol 28(6):436–453
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P (2018) Oxidative stress, aging, and diseases. Clin Interv Aging 13:757–772
Lushchak VI (2014) Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact 224:164–175
Matkowski A, Kus P, Goralska E, Wozniak D (2013) Mangiferin - a bioactive xanthonoid, not only from mango and not just antioxidant. Mini Rev Med Chem 13(3):439–455
Masibo M, He Q (2008) Major mango polyphenols and their potential significance to human health. Compr Rev Food Sci Food Saf 7(4):309–319
Du S, Liu H, Lei T, Xie X, Wang H, He X, Tong R, Wang Y (2018) Mangiferin: an effective therapeutic agent against several disorders (review). Mol Med Rep 18(6):4775–4786
Ramesh G, Das S, Bola Sadashiva SR (2020) Berberine, a natural alkaloid sensitizes human hepatocarcinoma to ionizing radiation by blocking autophagy and cell cycle arrest resulting in senescence. J Pharm Pharmacol. https://doi.org/10.1111/jphp.13354 Online ahead of print
Mukhopadhyay P, Rajesh M, Yoshihiro K, Hasko G, Pacher P (2007) Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun 358(1):203–208
Widlansky ME, Wang J, Shenouda SM, Hagen TM, Smith AR, Kizhakekuttu TJ, Kluge MA, Weihrauch D, Gutterman DD, Vita JA (2010) Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl Res 156(1):15–25
Rao BSS, Kumar MRS, Das S, Aithal K, Udupa N (2015) Radiosensitizing potential of Plumbagin in B16F1 melanoma tumor cells through mitochondrial mediated programmed cell death. J Appl Biomed 13(4):279–288
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O et al (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 92(20):9363–9367
Matos L, Gouveia A, Almeida H (2012) Copper ability to induce premature senescence in human fibroblasts. Age 34(4):783–794
Dash R, Acharya C, Bindu PC, Kundu SC (2008) Antioxidant potential of silk protein sericin against hydrogen peroxide-induced oxidative stress in skin fibroblasts. BMB Rep 41(3):236–241
Cui H, Kong Y, Zhang H (2012) Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct 2012:646354
Chen Q, Ames BN (1994) Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci U S A 91(10):4130–4134
Duan J, Zhang Z, Tong T (2005) Irreversible cellular senescence induced by prolonged exposure to H2O2 involves DNA-damage-and-repair genes and telomere shortening. Int J Biochem Cell Biol 37(7):1407–1420
Kavitha M, Manivasagam T, Essa MM, Tamilselvam K, Selvakumar GP, Karthikeyan S, Thenmozhi JA, Subash S (2014) Mangiferin antagonizes rotenone: induced apoptosis through attenuating mitochondrial dysfunction and oxidative stress in SK-N-SH neuroblastoma cells. Neurochem Res 39(4):668–676
Lee Y, Kim S, Oh Y, Kim YM, Chin YW, Cho J (2019) Inhibition of oxidative neurotoxicity and scopolamine-induced memory impairment by γ-Mangostin: in vitro and in vivo evidence. Oxidative Med Cell Longev 2019:3640753
Luo H, Yang A, Schulte BA, Wargovich MJ, Wang GY (2013) Resveratrol induces premature senescence in lung cancer cells via ROS-mediated DNA damage. PLoS One 8(3):e60065
Padma VV, Kalaiselvi P, Yuvaraj R, Rabeeth M (2015) Mangiferin induces cell death against rhabdomyosarcoma through sustained oxidative stress. Integr Med Res 4(2):66–75
Martin KR, Appel CL (2009) Polyphenols as dietary supplements: a double-edged sword. Nutr Diet Suppl 2:1–12
Maharjan S, Oku M, Tsuda M, Hoseki J, Sakai Y (2014) Mitochondrial impairment triggers cytosolic oxidative stress and cell death following proteasome inhibition. Sci Rep 4:5896
Zhou Y, Dong Y, Xu QG, Zhu SY, Tian SL, Huo JJ, Hao TT, Zhu BW (2014) Mussel oligopeptides protect human fibroblasts from hydrogen peroxide (H2O2)-induced premature senescence. Arch Gerontol Geriatr 58(2):293–299
Fujihara T, Nakagawa-Izumi A, Ozawa T, Numata O (2007) High-molecular-weight polyphenols from oolong tea and black tea: purification, some properties, and role in increasing mitochondrial membrane potential. Biosci Biotechnol Biochem 71(3):711–719
Lee HC, Yin PH, Lu CY, Chi CW, Wei YH (2000) Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem J 348(Pt 2):425–432
Biala AK, Dhingra R, Kirshenbaum LA (2015) Mitochondrial dynamics: orchestrating the journey to advanced age. J Mol Cell Cardiol 83:37–43
Naoi M, Wu Y, Shamoto-Nagai M, Maruyama W (2019) Mitochondria in neuroprotection by phytochemicals: bioactive polyphenols modulate mitochondrial apoptosis system, function and structure. Int J Mol Sci 20(10):2451
Sasaki M, Kajiya H, Ozeki S, Okabe K, Ikebe T (2014) Reactive oxygen species promotes cellular senescence in normal human epidermal keratinocytes through epigenetic regulation of p16(INK4a.). Biochem Biophys Res Commun 452(3):622–628
Liu YW, Cheng YQ, Liu XL, Hao YC, Li Y, Zhu X, Zhang F, Yin XX (2017) Mangiferin upregulates glyoxalase 1 through activation of Nrf2/ARE signaling in central neurons cultured with high glucose. Mol Neurobiol 54(6):4060–4070
Mahalanobish S, Saha S, Dutta S, Sil PC (2019) Mangiferin alleviates arsenic induced oxidative lung injury via upregulation of the Nrf2-HO1 axis. Food Chem Toxicol 126:41–55
Bellezza I, Giambanco I, Minelli A, Donato R (2018) Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta, Mol Cell Res 1865(5):721–733
Acknowledgements
The authors are indebted to Manipal School of Life Sciences, MAHE for providing the infrastructure and laboratory facilities. The authors are thankful to Dr. K. Satyamoorthy, Director, Manipal School of Life Sciences, MAHE for his support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The above study utilized early passage primary human dermal fibroblast cells that were established from skin samples obtained from healthy donors with the prior approval from the institutional ethical clearance (approval number IEC 011/2009).
Informed consent
The skin samples were collected after obtaining written consent from the donors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kanoi, R., Loachan, P., Das, S. et al. Mangiferin, a naturally occurring polyphenol, mitigates oxidative stress induced premature senescence in human dermal fibroblast cells. Mol Biol Rep 48, 457–466 (2021). https://doi.org/10.1007/s11033-020-06074-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-06074-2