Abstract
The biotransformation of β-artemether (1) by cell suspension cultures of Glycyrrhiza glabra and Lavandula officinalis is reported here for the first time. The major biotransformed product appeared as a grayish-blue color spot on thin-layer chromatography (TLC) with transparent crystal-like texture. Based on its infrared (IR) and 1H nuclear magnetic resonance (NMR) spectra, the product was characterized as a tetrahydrofuran (THF)–acetate derivative (2). The highest conversion efficiencies of 57 and 60% were obtained when 8–9-day-old cell suspensions of G. glabra and L. officinalis were respectively fed with 4–7 mg of compound 1 in 40 ml of medium per culture and the cells were harvested after 2–5 days of incubation. The addition of compound 1 at the beginning of the culture cycle caused severe growth depression in a dose-dependent manner, resulting in poor bioconversion efficiency of ~25% at 2–5 mg/culture dose only.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malaria, the most severe parasitic infection around the world, annually results in 1.5–3.0 million deaths [1]. The disease is caused by Plasmodium species that have now developed strong resistance to most of the traditional quinoline and anti-folate-based drug therapies [2, 3]. Artemisinin—a sesquiterpene lactone with an endoperoxide bridge—and its semi-synthetic lactol (dihydroartemisinin), ether (β-artemether), or ester (sodium artesunate) derivatives are becoming increasingly popular as safe and effective components of alternative combination therapy against drug-resistant malaria [4–7]. The oxygen–oxygen unit of these peroxides kills the parasite by inhibiting the polymerization of toxic heme residue of the patient’s hemoglobin into non-toxic hemozoin pigment in the parasite, resulting in the breakdown of the endoperoxide linkage of the trioxane drugs by the available heme that, ultimately, leads to the generation of carbon free radicals that quickly alkylate the cellular proteins of the parasite [8–13]. The practical efficacy of artemisinin and the majority of its first-generation trioxane derivatives is, however, generally impaired by their low solubility in water and oil, poor bioavailability, and shorter half-life, resulting in frequent recrudescence of infection [14–16]. Therefore, intensive efforts are ongoing in many laboratories to synthesize more efficient artemisinin derivatives/analogs with improved clinical efficiency [17–19]. Our group is engaged in plant cell culture-based biotransformations of artemisinin and related molecules [20]. This paper summarizes the results of a study wherein the cell suspension cultures of Glycyrrhiza glabra and Lavandula officinalis were successfully employed for the first time to biotransform β-artemether (1) into a tetrahydrofuran (THF)–acetate derivative (2).
Results and discussion
Effect of β-artemether addition on cell growth
The cell suspension cultures of G. glabra and L. officinalis used in this study entered in their exponential phase of growth from the 6th day of culturing in respective mediums and acquired maximum dry matter accumulation within next 3–4 days, with growth indices of 432 and 238, respectively (Fig. 1a). The cell growth was then steadily maintained till the end of the 15-day-long culture cycle. The addition of increasing concentrations of β-artemether (0–25 mg/40 ml medium/culture) in the medium on day 0 of culturing caused corresponding dose-dependent reduction in cell growth within 10 days in both of the plant species (Fig. 1b). In the case of G. glabra, the growth inhibition was more pronounced when β-artemether addition exceeded the 5.0 mg/culture level. More than 8-fold depression in growth index (GI = 30–70) was evident at 15–20-mg levels of substrate addition in comparison to the untreated control cell cultures. The supplementation of 25.0 mg of substrate proved lethal within 5–6 days of culturing. L. officinalis cultures, on the other hand, showed lesser growth inhibition by β-artemether supplementation up to 10 mg/culture level, with a GI = 196 in comparison to 243 in the non-treated control cultures. However, the addition of 25 mg β-artemether again proved to be highly inhibitory and resulted in a GI value of just 40 after 10 days of growth. This growth inhibitory effect observed in β-artemether-fed cultures might have been a consequence of increasing volumes of ethanol required to dissolve the substrate, as was the case in an earlier study by us on the bioconversion of artemisinin to deoxyartemisinin by Catharanthus roseus cell suspension cultures [20]. When cell and left-over spent medium obtained at the time of harvesting after 10 days of culture cycle were analyzed for bioconversion, a new product could be detected on thin-layer chromatography (TLC) in samples treated with 5–20 mg of substrate addition. The maximum conversion efficiency of ~25% was evident in G. glabra as well as L. officinalis cells treated with 5.0 mg/flask β-artemether. A faint spot corresponding to that of the transformed product was also visible in the spent medium extract of such cultures. Gradual lowering of conversion occurred when the substrate concentration was increased to 10–20 mg in both of the test plant systems. The major bioconverted product appeared as a grayish-blue color spot on TLC and had transparent crystal-like texture. No such transformed product was obtained in the extracts of controls where either no β-artemether was added in the cell cultures or when it was added in the medium devoid of cells. To further optimize the substrate doses and incubation period for biotransformation, 8- or 9-day-old cell suspensions of G. glabra and L. officinalis in the late exponential or early stationary stages of growth were fed with 0–10 mg β-artemether/culture and the cells were harvested after 1–5 days of incubation (Table 1). In the case of G. glabra cultures, the highest bioconversion efficiency of ~57% was obtained when 4 or 7 mg of substrate was added in 8-day-old cultures and cells were harvested after 2–5 days of incubation. The conversion efficiency during the corresponding duration of incubation was reduced to ~35% if β-artemether supplementation was increased to 10 mg/culture dose. The cultures of L. officinalis also depicted similar trends of biotransformation, with a marginally better conversion efficiency of ~60% when fed with 5–7 mg of substrate and incubated for at least 3–5 days. Interestingly, the feeding of β-artemether in such pre-grown cultures of both plant species did not cause any significant growth depression during the bioconversion phase, as evident from the cell dry matter accumulation data obtained under different feeding and incubation regimes in this experiment (Table 1).
Effect of β-artemether supplementation on growth and dry biomass accumulation in cell suspension cultures of G. glabra and L. officinalis. a Growth kinetics of cell suspensions through a 15-day-long culture passage in normal growth medium. b Growth inhibition caused by β-artemether (2–25 mg/culture) supplementation after 10 days of growth. Growth indices were calculated as the percentage dry matter accumulation over the initial inoculum weight. The values in parentheses indicate the conversion efficiency at different β-artemether supplemented doses
Characterization of biotransformed product
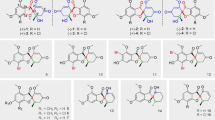
The biotransformed product, mp 96–97°C of β-artemether (1) obtained in this study was characterized as a THF–acetate derivative (2) by the comparison of its physico-spectral data (mp, infrared [IR], 1H, and mass spectrometry [MS]) with the spectral values reported in the literature [21]. Complete structure, assignment for 13/14-methyls (doublets at 0.88/0.91 ppm), an acetate (singlet at 2.13 ppm), O-methyl group (singlet at 3.42 ppm), and all carbons were analyzed by 1D (13C nuclear magnetic resonance [NMR], distortionless enhancement by polarization transfer [DEPT]) and 2D (COSY, HSQC, HMBC) spectra for the first time. Conversion of β-artemether into compound (2) in the present study occurred with 57–60% efficiency, which was sufficiently higher in comparison to the synthetic route from artemisinin (37%), reported earlier [21]. The proposed biocatalytic mechanism of THF–acetate compound (2) is depicted in Fig. 2. It appears that the enzymatic machinery of cultured cells of G. glabra and L. officinalis brought about the homolytic cleavage of the peroxide bridge, giving a diradical which subsequently rearranges to the ring-contracted THF–acetate derivative, as also proposed by Kalita et al. [22]. The presence of an acetate moiety in the THF derivative obtained in our study makes it an interesting synthone for further derivatization into new clinically potent molecules.
Plant cell suspension cultures and cell-free enzyme extracts have been frequently employed as efficient biocatalysts to carry out complex regiospecific or stereospecific chemical reactions that are otherwise difficult or very costly to perform through synthetic mechanisms [23]. Cultured cells of G. glabra have earlier been used to biotransform papaverine, carvone, bornyl acetate, 18β-glycyrrhetinic acid, terpenes, and related compounds by many investigators [24–26], but the use of cell cultures of L. officinalis in biotransformation work is confined to only two investigations wherein their efficacy to either reducing monoterpenoid aldehydes into their corresponding primary alcohols [27] or for the deoxygenation of artemisinin [20] was reported. The present study constitutes the first report on the successful utilization of cell cultures of these two plant systems in the bioconversion of anti-malarial compound β-artemether into its THF–acetate derivative.
Experimental
Raising of plant cell suspension cultures
Cell suspension cultures of L. officinalis and G. glabra were initiated from stem-derived callus in liquid Murashige and Skoog [28] medium supplemented with 0.5 mg/l 2,4-D, 0.5 mg/l NAA, 0.1 mg/l kinetin, 1.0 g/l casein hydrolysate, or 1.0 mg/l 2,4-D and 0.25 mg/l kinetin, respectively. The pH of all the media was adjusted to 5.8 before autoclaving. A constant inoculum to medium ratio of 1:10 was used in all experiments. The suspension cultures were incubated at 26–28°C on a rotary shaker (100 rpm) through a 15- and 12-day culture passage for L. officinalis and G. glabra, respectively. The growth and biomass accumulation in the cultured cells was measured in terms of the growth index, which was calculated as the percent increment in cell weight over the initial inoculums weight per flask.
Substrate addition
Initially, 250 mg of compound 1 was dissolved in 5 ml ethanol and added to the medium in the range of 2–25 mg/40 ml on day 0 of the culture cycle for dose optimization. The optimized dose of 7.0 mg/40 ml medium was then tested for bioconversion using 0- and 8-day and 0- and 9-day-old cultures of G. glabra and L. officinalis, respectively. Cell cultures fed with compound 1 on day 0 of the culture cycle were harvested on 10th day, whereas cultures fed on the 8th or 9th days were harvested at 1-day intervals for five subsequent days till the completion of the incubation period. Substrate evaporation and decomposition controls were run by adding the same amount of compound 1 to flasks containing only the culture medium without cells and incubated under similar conditions throughout the experiment.
Isolation of biotransformed product
The entire incubated mixture of cells and medium was extracted three times with diethyl ether and the combined extract was dried over anhydrous Na2SO4 and evaporated to dryness at 40°C under reduced pressure to afford an orange-brown residue. The residue was purified by column chromatography over a silica gel column (2.5 g, 1 × 8 cm), using a hexane–ethyl acetate (8:2) mixture as an eluting system. The mixture was further purified by preparative TLC to obtain pure metabolite as colorless crystals with Rf = 0.55.
Product identification and characterization
The melting point of biotransformed product 2 was determined on a Toshniwal melting point apparatus and is uncorrected. IR spectra were recorded on a Perkin Elmer 1719 FT-IR spectrophotometer. NMR spectra were obtained in CDCl3 on a Bruker Avance, 300 MHz instrument using TMS as the internal standard. The chemical shift values are reported in ppm and coupling constants in Hz. ESIMS spectra were recorded on a Perkin Elmer Turbo Mass/Shimadzu LC–MS. For column chromatography, the adsorbent used was Si gel 60–120 mesh (Spectrochem).
Spectral data of THF–acetate derivative (2)
Colorless crystals (51% yield), mp 96–97°C (hexane); FT-IR λ max (KBr): 1740(ester CO), 1458, 1360, 1220, 1036, 929 cm−1; ESI–MS (positive): [M + Na]+ 321; 1H NMR (CDCl3,300 MHz,) δ 0.88 (3H, d, J = 7.5 Hz Me-13), 0.91 (3H, d, J = 6.3 Hz Me-14), 2.13 (3H, s, COCH3), 2.41 (1H, m, H-11), 3.42 (3H, s, OCH3), 4.26 (1H, t, J = 5.4, H-3a), 3.91 (1H, q, J = 8.1 Hz, H-3b), 4.62 (1H, d, J = 3.9 Hz, H-12), 6.23 (1H, s, H-5); 13C NMR and DEPT (CDCl3, 75 MHz) δ 169.65 (s, C-4), δ 103.80 (d, C-12), δ 88.58 (d, C-5), δ 169.65 (s, C-4), δ 80.95 (q, C-6), δ 36.25 (t, C-9), δ 68.95 (t, C-3), δ 56.30 (q, C-16), δ 56.05 (d, C-1), δ 47.39 (d, C-7), δ 33.74 (d, C-10), δ 25.11 (t, C-8), δ 30.99 (d, C-11), δ 28.12 (t, C-2), δ 21.97 (q, C-15), δ 20.87 (q, C-14), δ 12.77 (q, C-13).
References
Brisibe EA, Uyoh EA, Brisibe F, Magalhäes PM, Ferreira JFS (2008) Building a golden triangle for the production and use of artemisinin derivatives against falciparum malaria in Africa. Afr J Biotechnol 7(25):4884–4896
Riley EM (2000) The London School of Hygiene and Tropical Medicine: a new century of malaria research. Mem Ins Oswaldo Cruz 95:25–32
Sriram D, Rao VS, Chandrasekhara KVG, Yogeeswari P (2004) Progress in the research of artemisinin and its analogues as antimalarials: an update. Nat Prod Res 18(6):503–527
Bhakuni RS, Jain DC, Sharma RP (2002) Phytochemistry of Artemisia annua and the development of artemisinin-derived antimalarial agents. In: Wright CW (ed) Artemisia. Medicinal and aromatic plants—industrial profiles. Taylor & Francis, London, pp 211–247
Olliaro PL, Taylor WR (2004) Developing artemisinin based drug combinations for the treatment of drug resistant falciparum malaria: a review. J Postgrad Med 50:40–44
Dondorp A, Nosten F, Stepniewska K, Day N, White N (2005) Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 366:717–725
Mårtensson A, Strömberg J, Sisowath C, Msellem MI, Gil JP, Montgomery SM, Olliaro P, Ali AS, Björkman A (2005) Efficacy of artesunate plus amodiaquine versus that of artemether-lumefantrine for the treatment of uncomplicated childhood Plasmodium falciparum malaria in Zanzibar, Tanzania. Clin Infect Dis 41:1079–1086
Asawamahasakda W, Ittarat I, Pu YM, Ziffer H, Meshnick SR (1994) Reaction of antimalarial endoperoxides with specific parasite proteins. Antimicrob Agents Chemother 38:1854–1858
Cumming JN, Ploypradith P, Posner GH (1997) Antimalarial activity of artemisinin (qinghaosu) and related trioxanes: mechanism(s) of action. Adv Pharmacol 37:253–297
Park BK, O’Neill PM, Maggs JL, Pirmohamed M (1998) Safety assessment of peroxide antimalarials: clinical and chemical perspectives. Br J Clin Pharmacol 46:521–529
Avery MA, Alvim-Gaston M, Woolfery JR (1999) Synthesis and structure–activity relationships of peroxidic antimalarials based on artemisinin. Adv Med Chem 4:125–217
Ekthawatchai S, Kamchonwongpaisan S, Kongsaeree P, Tarnchompoo B, Thebtaranonth Y, Yuthavong Y (2001) C-16 Artemisinin derivatives and their antimalarial and cytotoxic activities: syntheses of artemisinin monomers, dimers, trimers, and tetramers by nucleophilic additions to artemisitene. J Med Chem 44:4688–4695
Meshnick SR (2002) Artemisinin: mechanisms of action, resistance and toxicity. Int J Parasitol 32:1655–1660
Lee IS, Hufford CD (1990) Metabolism of antimalarial sesquiterpene lactones. Pharmacol Ther 48:345–355
Posner GH, Oh CH (1992) Regiospecifically oxygen-18 labeled 1,2,4-trioxane: a simple chemical model system to probe the mechanism(s) for the antimalarial activity of artemisinin (qinghaosu). J Am Chem Soc 114(21):8328–8329
Kim BJ, Sasaki T (2006) Recent progress in the synthesis of artemisinin and its derivatives. Org Prep Proc Int 38:1–80
Balint GA (2001) Artemisinin and its derivatives: an important new class of antimalarial agents. Pharmacol Ther 90(2–3):261–265
Jung M, Lee K, Kim H, Park M (2004) Recent advances in artemisinin and its derivatives as antimalarial and antitumor agents. Curr Med Chem 11:1265–1284
Chaturvedi D, Goswami A, Saikia PP, Barua NC, Rao PG (2010) Artemisinin and its derivatives: a novel class of anti-malarial and anti-cancer agents. Chem Soc Rev 39:435–454
Patel S, Gaur R, Verma P, Bhakuni RS, Mathur A (2010) Biotransformation of artemisinin using cell suspension cultures of Catharanthus roseus (L.) G.Don and Lavandula officinalis L. Biotechnol Lett 32(8):1167–1171
Wu W-M, Wu YK, Wu Y-L, Yao Z-J, Zhou C-M, Li Y, Shan F (1998) Unified mechanistic framework for the Fe(II)-induced cleavage of qinghaosu and derivatives/analogues. The first spin-trapping evidence for the previously postulated secondary C-4 radical. J Am Chem Soc 120:3316–3325
Kalita B, Barua NC, Bez G (2003) An unusual outcome in the Wittig olefination of artemisinin and its derivatives under microwave irradiation. Ind J Chem B 42:2622–2624
Giri A, Dhingra V, Giri CC, Singh A, Ward OP, Narasu ML (2001) Biotransformations using plant cells, organ cultures and enzyme systems: current trends and future prospects. Biotechnol Adv 19(3):175–199
Dorisse P, Gleye J, Loiseau P, Puig P, Edy AM, Henry M (1988) Papaverine biotransformation in plant cell suspension cultures. J Nat Prod 51:532–536
Hayashi H, Yamada K, Fukui H, Tabata M (1992) Metabolism of exogenous 18β-glycyrrhetinic acid in cultured cells of Glycyrrhiza glabra. Phytochemistry 31:2729–2733
Shams-Ardakani M, Ghannadi A, Badr P, Mohagheghzadeh A (2005) Biotransformation of terpenes and related compounds by suspension culture of Glycyrrhiza glabra L. (Papilionaceae). Flavour Fragr J 20:141–144
Shams-Ardekani M, Linley PA, Harkiss KJ, Mohagheghzadeh A, Gholami A, Mosaddegh M (2007) Biotransformation of monoterpenoids by suspension culture s of Lavandula angustifolia. Iran J Pharm Sci 3(2):93–100
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Acknowledgments
This work was carried out with the financial grants received under a CSIR Network Research Programme (NWP-09) on “Chemical and Biological Transformations of Plant Compounds for Production of Value Added Products of Therapeutic/Aroma Value”. We also thank the Director of the Central Institute of Medicinal and Aromatic Plants (CIMAP; Lucknow, India) for providing the necessary facilities and constant encouragement to carry out this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, S., Gaur, R., Upadhyaya, M. et al. Glycyrrhiza glabra (Linn.) and Lavandula officinalis (L.) cell suspension cultures-based biotransformation of β-artemether. J Nat Med 65, 646–650 (2011). https://doi.org/10.1007/s11418-011-0539-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-011-0539-5