Abstract
Marine microorganisms have long been recognized as potential sources for drug discovery. Griseofulvin was one of the first antifungal natural products and has been used as an antifungal agent for decades. In this study, 12 new griseofulvin derivatives [(±)-1−2, (+)-3, (±)-4, 10−12, and 14−15] and two new griseofulvin natural products (9 and 16) together with six known analogues [(−)-3, 5−8, and 13] were isolated from the mangrove-derived fungus Nigrospora sp. QQYB1 treated with 0.3% NaCl or 2% NaBr in rice solid medium. Their 2D structures and absolute configurations were established by extensive spectroscopic analysis (1D and 2D NMR, HRESIMS), ECD spectra, computational calculation, DP4 + analysis, and X-ray single-crystal diffraction. Compounds 1−4 represent the first griseofulvin enantiomers with four absolute configurations (2S, 6'S; 2R, 6'R; 2S, 6'R; 2R, 6'S), and compounds 9−12 represent the first successful production of brominated griseofulvin derivatives from fungi via the addition of NaBr to the culture medium. In the antifungal assays, compounds 6 and 9 demonstrated significant inhibitory activities against the fungi Colletotrichum truncatum, Microsporum gypseum, and Trichophyton mentagrophyte with inhibition zones varying between 28 and 41 mm (10 μg/disc). The structure−activity relationship (SAR) was analyzed, which showed that substituents at C-6, C-7, C-6' and the positions of the carbonyl and double bond of griseofulvin derivatives significantly affected the antifungal activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Griseofulvin, a spirocyclic benzofuran-3-one fungal metabolite, was first isolated from Penicillium griseofulvum in 1939 by Oxford et al. (Oxford et al. 1939), used clinically for the treatment of tinea capitis and other superficial mycoses as a classic antifungal agent (Gentles et al. 1958; Williams et al. 1958). Tinea capitis was a common dermatophyte infection caused predominantly by Trichophyton or Microsporum species and the clinical presentations were seborrheic-like scale, ‘black dot’ pattern, inflammatory tinea capitis with kerion, and tiny pustules in the scalp (Gupta et al. 2000; Seebacher et al. 2007). Griseofulvin was the only drug available for treatment of tinea capitis before the approval of Terbinafine by the US Food and Drug Administration in 2007 (Rønnest et al. 2012).
In addition to antifungal effects, various other biological activities of griseofulvin were reported, including antitumor, anti-HIV, and marinated shrimp lethality (Rønnest et al. 2009; Panda et al. 2005; Wei et al. 2016; Zhang et al. 2017). More than 400 griseofulvin analogues have been synthesized for drug screening since 1950 (Rønnest et al. 2009, 2012), but fewer than 20 new natural products of griseofulvin were reported in recent decades. Furthermore, the absolute configuration of most natural griseofulvin analogues was identified as 2S, 6'R, except for 6'-hydroxygriseofulvin (2S, 6'S) and leptosphaerin C (2S, 2'S, 6'S) (Lin et al. 2010; Shang et al. 2012).
Marine microorganisms have been considered as potential sources of structurally novel and biologically active secondary metabolites for drug discovery (Hai et al. 2021; Xu et al. 2022). The expressions of marine microorganism biosynthetic gene clusters, which are often silent under experimental laboratory culture conditions, impose restrictions on the discovery of new cryptic natural products. The addition of NaBr or other halogens to the culture medium, an important strategy of the OSMAC approach, may trigger fungal biosynthetic pathways to restore osmotic imbalances, which could activate different silent gene clusters for the discovery of new metabolites (Pan et al. 2019; Pinedo-Rivilla et al. 2022). For example, adding KBr to the rice medium led to the isolation of the brominated metabolite 2-bromo-gentisylalcohol from cultures of Penicillium concentricum (Ali et al. 2017) and adding NaBr to the rice medium led to the isolation of two new brominated azaphilones, bromophilones A and B from Penicillium canescens 4.14.6a (Frank et al. 2019). It is worth noting that four griseofulvin derivatives were reported from the two strains, but no brominated griseofulvin analogue was obtained after adding KBr or NaBr to the rice medium. In our group’s previous research, one brominated cytochalasin, phomopchalasins E, and two iodized cytochalasin, phomopchalasins F and H, were isolated from Phomopsis sp. QYM-13 by adding NaBr or KI to the potato liquid medium, respectively (Chen et al. 2022).
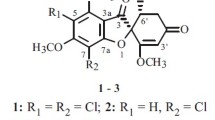
Inspired by this approach, NaCl or NaBr was added to the rice solid medium of the mangrove-derived fungus Nigrospora sp. QQYB1 in an attempt to acquire halogen-substituted griseofulvin derivatives. Subsequently, LC–MS analysis of two extracts revealed the production of different halogen-substituted griseofulvin derivatives (Supplementary Fig. S56). The follow-up fermentation led to the isolation of four pairs of griseofulvin enantiomers (1−4) and 12 griseofulvin derivatives (5−16) (Fig. 1), including four bromide derivatives (9−12). Compounds 1−4 represented the first griseofulvin enantiomers with four absolute configurations (2S, 6’S; 2R, 6’R; 2S, 6'R; 2R, 6’S), and compounds 9−12 were the first successful production of brominated griseofulvin derivatives. Herein, we report the details of isolation, structure elucidation, antifungal and antitumor activities, and the structure−activity relationship (SAR) of these metabolites.
Materials and methods
General experimental procedure
Optical rotations were measured on an MCP 300 (Anton Paar, Shanghai, China). UV spectra were performed in MeOH using a Shimadzu UV-2600 spectrophotometer (Shimadzu, Kyoto, Japan). ECD data were measured on a J-810 spectropolarimeter (JASCO, Tokyo, Japan). IR spectra were performed on IR Affinity-1 spectrometer (Shimadzu, Kyoto, Japan). Melting points were recorded on a Fisher–Johns hot-stage apparatus. NMR spectra were tested on a Bruker Avance spectrometer (Bruker, Beijing, China) (compounds 3 − 4: 600 MHz for 1H and 150 MHz for 13C, respectively; compounds 1 − 2, 10 − 12, and 14 − 15: 400 MHz for 1H and 100 MHz for 13C). HR-ESI-MS spectra were obtained on a ThermoFisher LTQ-Orbitrap-LC–MS spectrometer (Palo Alto, CA, USA). LC–MS analysis was recorded on a Q-TOF manufactured by Waters and a Waters acquity UPLC BEH C18 column (1.7 µm, 2.1 mm × 100 mm) was used for analysis. Silica gel (200–300 mesh, Marine Chemical Factory, Qingdao, China) and sephadex LH-20 (Amersham Pharmacia, Piscataway, NJ, USA) were used for column chromatography (CC). Silica gel plates (Qingdao Huang Hai Chemical Group Co., G60, F-254) were used for thin-layer chromatography (TLC). Semi-preparative HPLC (Ultimate 3000 BioRS, Thermo Scientific, Germany) was conducted using a Daice Chiralcel AD-H column (5 μm, 4.6 mm × 250 mm, Daicel, Japan), a Chiral INA column (5 μm, 4.6 mm × 250 mm, Phenomenex, USA), and a Chiral ND column (5 μm, 4.6 mm × 250 mm, Phenomenex, USA) for chiral separation. Single-crystal data were performed on an Agilent Gemini Ultra diffractometer (Cu Kα radiation, Agilent, Santa Clara, CA, USA).
Fungal material
The fungus Nigrospora sp. QQYB1 was isolated from healthy leaves of Kandelia candel, which were collected in March 2019 from Huizhou East Mangrove National Nature Reserve in Guangdong Province, China. The strain was identified as a Nigrospora sp. (GenBank No. OQ380944) by a BLAST search, which showed 99% identity to the sequence of a Nigrospora sp. (GenBank No. MT123052.1). The strain was deposited in the School of Chemistry, Sun Yat-sen University.
Fermentation
The fungus Nigrospora sp. QQYB1 was fermented on potato dextrose agar (PDA) for 5 days. The mycelia of the strain were cultivated on potato dextrose broth (PDB) for 3 days to prepare the seed culture. Then, the culture was inoculated into solid cultured medium (sixty 1000 mL Erlenmeyer flasks, each containing 50 g of rice, and 50 mL of distilled water with 0.3% NaCl or 2% NaBr) for 30 days at 25 °C.
Extraction and isolation
After the fermentation (adding 0.3% NaCl), the cultures were extracted three times with MeOH to yield 23.6 g of residue. Then, the crude extract was eluted using gradient elution with petroleum ether/EtOAc from 9:1 to 0:10 (v/v) on silica gel CC to get six fractions (Fr. A–F). Fr. C was applied to Sephadex LH-20 CC (CH2Cl2/MeOH, v/v, 1:1) to give three fractions (Fr. C1−C3). Fr. C1 was subjected to silica gel CC (petroleum ether/EtOAc, v/v, 8:2) to yield compound 16 (23.4 mg). Fr. D was applied to Sephadex LH-20 CC (CH2Cl2/MeOH, v/v, 1:1) to give three fractions (Fr. D1 − D3). Fr. D1 was subjected to silica gel CC (CH2Cl2/MeOH, v/v, 200:1) to yield compound 5 (128 mg). Fr. E was subjected to silica gel CC (petroleum ether/EtOAc, v/v, 6:4) to afford three fractions (Fr. E1−E2). Fraction E1 was applied to Sephadex LH-20 CC (CH2Cl2/MeOH, v/v, 1:1) to give compound 6 (639 mg). Fr. E2 was applied to silica gel CC (CH2Cl2/MeOH, v/v, 200:1 to 100:1) to give compounds 7 (30.2 mg) and 8 (29.6 mg). Fr. F was subjected to silica gel CC (petroleum ether/EtOAc, v/v, 5:5) to afford three fractions (Fr. F1−F2). Fr. F2 (23 mg) was separated by normal phase HPLC on a chiral column (AD-H) (flow rate: 1.0 mL/min; solvent: n-hexane–isopropanol = 7:3) to yield (−)-4 (2.2 mg, tR 10.0 min), (+)-3 (1.9 mg, tR 22.5 min) and additional fraction Fr. F2.2 (10 mg, tR 19.0 min). Fr. F2.2 was accomplished over a chiral column (INA) (flow rate: 1.0 mL/min; solvent: n-hexane–isopropanol = 7:3) to yield (+)-4 (2.8 mg, tR 11.7 min) and (−)-3 (3.4 mg, tR 13.5 min).
After the fermentation (adding 2% NaBr), the cultures were extracted using the same methods as above. The residue (15.8 g) was subjected to silica gel CC (200−300 mesh silica) with petroleum ether/EtOAc (9:1 to 1:9) to afford six fractions (Fr. A−F). Fr. C was subjected to Sephadex LH-20 CC (CH2Cl2/MeOH, v/v, 1:1) to obtain compound 15 (32.6 mg). Fr. D was subjected to silica gel CC (petroleum ether/EtOAc, v/v, 7:3), affording compound 12 (8.5 mg) and additional fraction Fr. D1, which was subjected to silica gel CC (CH2Cl2/MeOH, v/v, 200:1) to yield compound 11 (4.3 mg), compounds 13 (4.2 mg) and 14 (3.4 mg). Fr. E was subjected to silica gel CC (petroleum ether/EtOAc, v/v, 6:4 to 5:5) to afford three fractions (Fr. E1−E4). Fr. E1 was subjected to Sephadex LH-20 CC (CH2Cl2/MeOH, v/v, 1:1) to obtain compound 9 (24.3 mg). Fr. E2 was subjected to Sephadex LH-20 CC (CH2Cl2/MeOH, v/v, 1:1) to obtain compound 10 (42.5 mg). Fr. E3 was subjected to silica gel CC (petroleum ether/EtOAc, v/v, 6:4 to 5:5) to obtain compound (±)-2 (15.7 mg). The chiral HPLC separation of (±)-2 was accomplished over a chiral column (INA) (flow rate: 1.0 mL/min; solvent: n-hexane–isopropanol = 8:2) to yield (−)-2 (5.9 mg, tR 12.5 min) and (+)-2 (6.2 mg, tR 16.0 min). Fr. E4 was subjected to silica gel CC (CH2Cl2/MeOH, v/v, 100:1) to obtain compound (±)-1 (18.2 mg). In the same way, (±)-1 was also purified by HPLC on a chiral column (ND) (flow rate: 1.0 mL/min; solvent: n-hexane–isopropanol = 7:3) to obtain (−)-1 (8.3 mg, tR 17.0 min) and (+)-1 (6.1 mg, tR 21.2 min).
(±)-6'-Hydroxy-7-dechlorogriseofulvin (1): White powder; mp 183.8−184.5 °C; UV (MeOH) λmax (log ε): 211 (1.34), 288 (1.19) nm; IR νmax 3397, 2922, 2851, 1697, 1616, 1587, 1506, 1456, 1348, 1219, 1157, 1114, 1039 cm−1; 1H NMR (400 MHz, CDCl3) data, Table 1; 13C NMR (100 MHz, CDCl3) data, Table 2; HRESIMS m/z 335.11221 [M + H] + (calcd. for C17H19O7, 355.11253). (+)-1, \([\alpha ]_{D}^{25}\) + 350 (c 0.1 MeOH); ECD (c = 0.21 mg/mL, MeOH) λmax (Δε) 220 (− 19.5), 235 (+ 12.9), 292 (+ 14.6). (−)-1, \([\alpha ]_{D}^{25}\)− 340 (c 0.1 MeOH); ECD (c = 0.22 mg/mL, MeOH) λmax (Δε) 221 218 (+ 26.4), 236 (− 17.4), 294 (− 19.6).
(±)-6'-Hydroxy-7-dechloroepigriseofulvin (2): White powder; mp 183.8−184.5 °C; UV (MeOH) λmax (log ε): 212 (1.46), 287 (1.19) nm; IR νmax 3397, 2922, 2851, 1697, 1616, 1587, 1506, 1456, 1348, 1219, 1157, 1114, 1039 cm−1; 1H NMR (400 MHz, CDCl3) data, Table 1; 13C NMR (100 MHz, CDCl3) data, Table 2; HRESIMS m/z 335.11221 [M + H] + (calcd. for C17H19O7, 355.11253). (+)-2, \([\alpha ]_{D}^{25}\)+ 306 (c 0.1 MeOH); ECD (c = 0.19 mg/mL, MeOH) λmax (Δε) 219 (− 34.9), 236 (+ 13.7), 296 (+ 12.6). ( −)-2, \([\alpha ]_{D}^{25}\)− 310 (c 0.1 MeOH); ECD (c = 0.20 mg/mL, MeOH) λmax (Δε) 218 (+ 52.2), 236 (− 20.1), 297 (− 18.8).
( ±)-6'-Hydroxygriseofulvin (3): White powder; mp 153.4 − 114.9 °C; UV (MeOH) λmax (log ε): 210 (1.70), 292 (1.39) nm; IR νmax 3373, 2951, 2926, 2851, 1714, 1653, 1614, 1589, 1456, 1350, 1219, 1139, 1101 cm−1; 1H NMR (600 MHz, CDCl3) data, Table 1; 13C NMR (150 MHz, CDCl3) data, Table 2; HRESIMS m/z 369.07324 [M + H] + (calcd. for C17H18ClO7, 369.07356). ( +)-3, \([\alpha ]_{D}^{25}\)+ 286 (c 0.03 MeOH); ECD (c = 0.18 mg/mL, MeOH) λmax (Δε) 221 (− 28.2), 236 (+ 40.9), 297 (+ 24.5). ( −)-3, \([\alpha ]_{D}^{25}\)− 270 (c 0.05 MeOH); ECD (c = 0.17 mg/mL, MeOH) λmax (Δε) 221 (+ 12.2), 234 (− 29.6), 296 (− 19.3).
( ±)-6'-Hydroxyepigriseofulvin (4): White powder; mp 153.4−154.9 °C; UV (MeOH) λmax (log ε): 213 (1.17), 291 (0.92) nm; IR νmax 3373, 2951, 2926, 2851, 1714, 1653, 1614, 1589, 1456, 1350, 1219, 1139, 1101 cm−1; 1H NMR (600 MHz, CDCl3) data, Table 1; 13C NMR (150 MHz, CDCl3) data, Table 2; HRESIMS m/z 369.07324 [M + H] + (calcd. for C17H18ClO7, 369.07356). ( +)-4, \([\alpha ]_{D}^{25}\)+ 310 (c 0.1 MeOH); ECD (c = 0.19 mg/mL, MeOH) λmax (Δε) 220 (− 49.9), 238 (+ 38.2), 299 (+ 16.9). ( −)-4, \([\alpha ]_{D}^{25}\)− 304 (c 0.1 MeOH); ECD (c = 0.20 mg/mL, MeOH) λmax (Δε) 220 (+ 49.5), 238 (− 38.3), 295 (− 15.4).
6-O-Desmethyl-7-bromogriseofulvin (10): White powder, mp 278.3−279.8 °C; \([\alpha ]_{D}^{25}\) +298.9 (c 0.08 MeOH); UV (MeOH) λmax (log ε): 213 (1.50), 238 (1.38), 292 (1.24) nm; ECD (c = 0.19 mg/mL, CD3OD) λmax (Δε) 221 (− 51.7), 238 (+ 49.7), 295 (+ 17.1); IR νmax 3331, 2964, 2922, 2852, 1697, 1603, 1408, 1359, 1223, 1130, 1018 cm−1; 1H NMR (400 MHz, MeOH) data, Table 3; 13C NMR (100 MHz, CD3OD) data, Table 4; HRESIMS m/z 383.01215 [M + H] + (calcd. for C16H16BrO6, 383.01248).
5-Bromo-6-O-desmethyl-7-dechlorogriseofulvin (11): White powder, mp 264.8−266.2 °C; \([\alpha ]_{D}^{25}\)+ 298,5 (c 0.06 MeOH); UV (MeOH) λmax (log ε): 218 (1.35), 240 (1.22), 285 (0.82) nm; ECD (c = 0.19 mg/mL, MeOH) λmax (Δε) 221 (− 29.8), 245 (+ 33.7), 325 (+ 14.6); IR νmax 3304, 2964, 2926, 2849, 1697, 1605, 1573, 1355, 1226, 1093 cm−1; 1H NMR (400 MHz, CD3OD) data, Table 3; 13C NMR (100 MHz, CD3OD) data, Table 4; HRESIMS m/z 383.01221 [M + H] + (calcd. for C16H16BrO6, 383.01248).
5,7-Dibromo-6-O-desmethylgriseofulvin (12): Colorless oil, \([\alpha ]_{D}^{25}\)+ 269.8 (c 0.08 MeOH); UV (MeOH) λmax (log ε): 224 (1.32), 242, (1.23), 340 (0.84) nm; ECD (c = 0.18 mg/mL, CD3OD) λmax (Δε) 218 (− 19.3), 249 (+ 24.9), 329 (+ 11.4); IR νmax 3373, 2941, 2849, 1647, 1595, 1456, 1360, 1231, 1180, 1055 cm−1; 1H NMR (400 MHz, MeOH) data, Table 3; 13C NMR (100 MHz, CD3OD) data, Table 4; HRESIMS m/z 460.92255 [M + H] + (calcd. for C16H15Br2O6, 460.92299).
3',4'-Dihydroeupenigriseofulvin (14): White powder, mp 217.4 − 218.5 °C; \([\alpha ]_{D}^{25}\)− 36.9 (c 0.08 MeOH); UV (MeOH) λmax (log ε): 211 (1.66), 282 (1.05) nm; ECD (c = 0.17 mg/mL, MeOH) λmax (Δε) 214 (+ 17.6), 248 (− 5.24), 286 (+ 3.04); IR νmax 3428, 2959, 2926, 2859, 1659, 1616, 1585, 1456, 1217, 1155, 1120 cm−1; 1H NMR (400 MHz, CDCl3) data, Table 3; 13C NMR (100 MHz, CDCl3) data, Table 4; HRESIMS m/z 293.13800 [M + H] + (calcd. for C16H21O5, 293.13835).
4'-Demethoxyl-7-dechloroisogriseofulvin (15): White powder, mp 191.3−192.7 °C; \([\alpha ]_{D}^{25}\)+ 326 (c 0.12 MeOH); UV (MeOH) λmax (log ε): 212 (1.53), 287 (1.02) nm; ECD (c = 0.20 mg/mL, MeOH) λmax (Δε) 235 (+ 44.3), 224 (− 39.6), 314 (+ 25.3); IR νmax 2960, 2922, 2851, 1697, 1683, 1616, 1591, 1506, 1456, 1215, 1157, 1120 cm−1; 1H NMR (400 MHz, CDCl3) data, Table 3; 13C NMR (100 MHz, CDCl3) data, Table 4; HRESIMS m/z 289.10687 [M + H] + (calcd. for C16H17O5, 289.10705).
X-ray crystallographic data
Colorless crystals of compounds ( +)-1, 10, 13, 15 were obtained from n-hexane − EtOAc at room temperature by slow evaporation and measured on an Agilent Xcalibur Nova single-crystal diffractometer with Cu Kα radiation.
Crystal data of ( +)-1: C17H18O7, Mr = 334.31, tetragonal, a = 8.76640(10) Å, b = 8.76640(10) Å, c = 19.7986(2) Å, α = 90°, β = 90°, γ = 90°, V = 1521.52(4) Å3, T = 100 K, space group P41, Z = 4, μ(Cu Kα) = 0.964 mm–1, 11,684 reflections collected, 3039 independent reflections (Rint = 0.0225, Rsigma = 0.0170). The final R1 values were 0.0241, wR2 = 0.0622 [I≥2σ (I)]. The final R1 values were 0.0243, wR2 = 0.0623 (all data). The goodness of fit on F2 was 1.077. The flack parameter was 0.05(4). CCDC number: 2240676.
Crystal data of 10: C16H15BrO6, Mr = 383.20, orthorhombic, a = 11.22120(10) Å, b = 11.38150(10) Å, c = 12.33490(10) Å, α = 90°, β = 90°, γ = 90°, V = 1575.34(2) Å3, T = 100 K, space group P212121, Z = 4, μ(Cu Kα) = 3.827 mm–1, 15,338 reflections collected, 3153 independent reflections (Rint = 0.0207, Rsigma = 0.0133). The final R1 values were 0.0185, wR2 = 0.0494 [I≥2σ (I)]. The final R1 values were 0.0186, wR2 = 0.0495 (all data). The goodness of fit on F2 was 1.052. The flack parameter was 0.011 (11). CCDC number: 2240679.
Crystal data of 13: C16H18O5, Mr = 290.30, monoclinic, a = 6.02584(5) Å, b = 10.47414(10) Å, c = 11.75725(9) Å, α = 90°, β = 103.3219(8)°, γ = 90°, V = 722.096(11) Å3, T = 100 K, space group P21, Z = 2, μ(Cu Kα) = 0.823 mm–1, 13,155 reflections collected, 2734 independent reflections (Rint = 0.0205, Rsigma = 0.0129). The final R1 values were 0.0264, wR2 = 0.0693 [I ≥2σ (I)]. The final R1 values were 0.0265, wR2 = 0.0693 (all data). The goodness of fit on F2 was 1.104. The flack parameter was − 0.07(4). CCDC number: 2240681.
Crystal data of 15: (C16H16O6)2, Mr = (288.29)2, orthorhombic, a = 9.59580(10) Å, b = 11.28320(10) Å, c = 25.9281(2) Å, α = 90°, β = 90°, γ = 90°, V = 2807.27(4) Å3, T = 100 K, space group P212121, Z = 4, μ(Cu Kα) = 0.846 mm–1, 27,716 reflections collected, 5658 independent reflections (Rint = 0.0384, Rsigma = 0.0238). The final R1 values were 0.0244, wR2 = 0.0622 [I≥2σ (I)]. The final R1 values were 0.0251, wR2 = 0.0625 (all data). The goodness of fit on F2 was 1.040. The flack parameter was − 0.01(4). CCDC number: 2240682.
LC–MS analysis
The LC–MS fingerprints of extracts were recorded with gradient elution using Bruker times TOF. The gradient was kept for 1 min at 10% MeOH–H2O, then changed from 10% MeOH–H2O to 40% MeOH–H2O in 2 min, then 40% MeOH–H2O was changed to 100% MeOH in 12 min, was maintained at 100% MeOH for the next 3 min, and then, 100% MeOH was transformed into 10% MeOH–H2O within 0.1 min and maintained for 1.9 min. The elution rate was kept at 0.3 mL/min. The detection wavelength was 190–400 nm. The test samples are as follows: (A) Nigrospora sp. QQYB1, cultured with 0.3% NaCl in the medium; (B) Nigrospora sp. QQYB1, cultured with 2% NaBr in the medium.
ECD calculations, 1H, 13C NMR calculations, and DP4 + analysis
1H, 13C NMR calculations and ECD calculations were optimized with the Spartan’14 and Gaussian 09 software programs (Supplementary Tables S1–S6). The conformers with a Boltzmann population greater than 1% were selected for optimization at B3LYP/6–31 + G (d, p) and calculation at mPW1PW91-SCRF/6–311 + g (2d,p) (1H and 13C NMR) and B3LYP/6–31 + G (d, p) (ECD) (Frisch et al. 2009; Zhang et al. 2017). All calculations were carried out by the high-performance grid computing platform of Sun Yat-Sen University. The ECD spectra and DP4 + analysis were performed as described previously (Cui et al. 2018; Yang et al. 2021).
Antifungal assay
The disc diffusion assays of compounds 1−16 were measured against seven fungi, including C. truncatum, P. expansum, A. flavus, A. niger, M. gypseum, T. mentagrophytes, and C. albicans (Dahiya et al. 2016; Wang et al. 2018; Fang et al. 2022). Briefly, compounds 1−16 were dissolved in DMSO to final concentrations of 0.1−1 mg/mL. The spores of seven indicator fungi were resuspended in normal saline (0.91% w/v of NaCl) at a concentration of ∼1 × 106 colonies mL−1 and spread on Sabouraud’s dextrose agar plates. Then, the 6 mm paper discs saturated with 10 μL of compounds were placed on the petri plates. These plates were incubated for 48 h at 28 °C. After incubation, the presence/absence of fungal growth was observed, and the average diameter of the inhibition zone was calculated from triplicate sets. The results are given in Fig. 6 and Supplementary Table S7. Methanol and ketoconazole were used as negative and positive controls, respectively.
Cytotoxicity assay
The cytotoxicity of all compounds against HeLa (cervical), HepG2 (hepatocellular carcinoma), HCT-116 (human colorectal adenocarcinoma), and MCF-7 (breast cancer) human cancer cell lines were assessed using the MTT assay as previously described (Chen et al. 2016). Cisplatin was taken as the positive control.
Results and discussion
The strain Nigrospora sp. QQYB1 was isolated from the healthy leaves of Kandelia candel, collected from Huizhou East Mangrove National Nature Reserve in Guangdong Province, China. A further chemical investigation of Nigrospora sp. QQYB1, treated with 0.3% NaCl or 2% NaBr in rice solid medium, was carried out and led to isolation and identification of four pairs of griseofulvin enantiomers (1−4) and 12 griseofulvin derivatives (5−16), including four bromide derivatives (9−12). Compounds [( ±)-1−2, ( +)-3, ( ±)-4, 10−12, 14−15] were determined as new griseofulvin derivatives by extensive spectroscopic analysis (1D and 2D NMR, HRESIMS), ECD spectra, computational calculation, DP4 + analysis, and X-ray single-crystal diffraction.
Compound ( ±)-1 was obtained as white amorphous powder. Its molecular formula was assigned as C17H18O7 on the basis of HRESIMS analysis (Supplementary Fig. S6) at m/z 335.11221 [M + H] + (calcd. for C17H19O7, 335.11253), which was determined to possess 9 degrees of unsaturation. The 1H NMR data (Supplementary Fig. S1; Table 1) of ( ±)-1 showed two aromatic protons at δH 6.30 (1H, d, J = 1.7 Hz, H-7) and 6.07 (1H, d, J = 1.7 Hz, H-5), one olefinic proton at δH 5.56 (1H, s, H-3'), one methylene proton at δH 3.23 (1H, d, J = 16.6 Hz, Ha-5') and 2.57 (1H, d, J = 16.6 Hz, Hb-5'), three methoxyls at δH 3.90 (3H, s, 4-OCH3), 3.90 (3H, s, 6-OCH3) and 3.62 (3H, s, 2'-OCH3), and one methyl δH 1.19 (3H, s, 6'-CH3). Subsequently, the 13C NMR data (Supplementary Fig. S2; Table 2) showed the presence of 17 carbon signals, including nine nonprotonated carbons (δC 195.7, 190.8, 175.9, 170.7, 168.7, 159.6, 104.1, 92.3, and 74.8), three methine carbons (δC 104.0, 93.9 and 89.0), one methylene carbon (δC 46.1), three methoxyl carbons (δC 56.8, 56.3, and 56.3), and one methyl carbon (δC 23.3). The 1H and 13C NMR spectra of ( ±)-1 were similar to those of 7-dechlorogriseofluvin (5) (Park et al. 2005), except for the absence of the methine moiety (δH 3.75, δC 37.2) and the presence of a nonprotonated carbon (δC 74.8). Moreover, the chemical shifts of C-2, C-5' and 6'-CH3 in (±)-1 were moved downfield within a range of 1.6 to 9.1 × 10–6 with compound 5, and the HMBC correlations (Fig. 2; Supplementary Fig. S5) from H-5' to C-2, C-3' and 6'-CH3 and 6'-CH3 to C-2, C-5' and C-6' (Fig. 2), suggesting that the hydroxyl group was substituted at C-6' and the planar structure of ( ±)-1 was determined as 6'-hydroxy-7-dechlorogriseofulvin.
Subsequent chiral HPLC purification of ( ±)-1 led to the separation of the two enantiomers (+)-1 and (−)-1 (Supplementary Fig. S57), which displayed opposite Cotton effects in their CD spectra and opposite optical rotations. The structure of (+)-1 was subsequently confirmed by a single-crystal X-ray diffraction experiment using Cu Kα radiation [flack parameter 0.05(4)] (Fig. 3). Compared with experimental and calculated ECD curves (Fig. 4A), the absolute configurations of (+)-1 and (−)-1 were determined as 2S, 6'S and 2R, 6'R, respectively.
Compound (±)-2 was isolated as a white amorphous powder, showing the same molecular formula as that of (+)-1 (Supplementary Fig. S12). The 1H and 13C NMR spectra (Supplementary Figs. S7 and S8; Tables 1 and 2) of ( ±)-2 were similar to those of ( ±)-1, except for the methylene proton at δH 3.23 (1H, d, J = 16.6 Hz, Ha-5'), 2.57 (1H, d, J = 16.6 Hz, Hb-5') in (+)-1 was changed to δH 2.82 (1H, d, J = 16.4 Hz, Ha-5'), and 2.69 (1H, d, J = 16.4 Hz, Hb-5') in (±)-2. Comparing the same HMBC correlations (Fig. 2; Supplementary Fig. S11), compounds (±)-2 and (±)-1 were identified as epimers and compound (±)-2 was named as 6'-hydroxy-7-dechloroepigriseofulvin. The separation of (±)-2 on a chiral column was conducted to afford (+)-2 and (−)-2 (Supplementary Fig. S58). The absolute configurations of (+)-2 and (−)-2 were assigned as 2R, 6'S and 2S, 6'R by comparing the experimental and calculated ECD spectra (Fig. 4B).
Compound (±)-3 was acquired as a white amorphous powder. The molecular formula was determined as C17H17ClO7 based on the HRESIMS data (Supplementary Fig. S17). The 1H and 13C NMR spectra (Supplementary Figs. S13 and S14; Tables 1 and 2) of (±)-3 were similar to those of (±)-1 except the hydrogen atom in the C-7 was replaced by a chlorine atom. The relative configuration of (±)-3 was determined as 2S*, 6'S* based on the similar chemical shifts at δH 3.19 (1H, d, J = 16.6 Hz, Ha-5'), 2.62 (1H, d, J = 16.6 Hz, Hb-5') in (±)-3 and δH 3.23 (1H, d, J = 16.6 Hz, Ha-5'), and 2.57 (1H, d, J = 16.6 Hz, Hb-5') in (±)-1. The absolute configurations of (+)-3 and (−)-3 were determined as 2S, 6'S and 2R, 6'R by comparing the experimental ECD spectra (Fig. 4A) between ( ±)-3 and (±)-1, respectively. In addition, (−)-6'-hydroxygriseofulvin was tentatively assigned as 2S, 6'S (Shang et al. 2012), but its 1H and 13C NMR spectra and optical rotation were consistent with those of (−)-1 and (−)-3. Consequently, (−)-6'-hydroxygriseofulvin was further confirmed as 2R, 6'R.
Similarly, compound (±)-4 showed the same molecular formula as that of (+)-3 (Supplementary Fig. S22). Compounds (±)-4 and (±)-3 were identified as epimers according to the similar 1H and 13C NMR spectra of (±)-4 with those of ( ±)-3 (Supplementary Figs. S18 and S19; Tables 1 and 2), and the relative configuration of (±)-4 was determined 2S*, 6'R* based on the similar chemical shifts at H-5 of (±)-4 with that of (±)-2. The absolute configurations of (+)-4 and (−)-4 were assigned as 2R, 6'S and 2S, 6'R by the similar experimental ECD curves (Fig. 4B) of (±)-4 and (±)-2.
Compound 10 was isolated as a white amorphous powder. The molecular formula was determined to be C16H15BrO6 based on the HRESIMS data (Supplementary Fig. S28), and the bromine isotope pattern at m/z 383.01215 [M + H] + and 385.00977 [M + 2 + H] + (a ratio of 1:1) showed the presence of one bromine atom in the molecule. The 1H and 13C NMR spectra (Supplementary Figs. S23 and S24; Tables 3 and 4) of 10 were similar to those of 6-desmethylgriseofulvin (Belofsky et al. 1998). The difference between them was the chlorine atom at C-7 in 8 was replaced by a bromine atom in 10. The absolute configuration of 10 was determined as 2S, 6'R by X-ray diffraction analysis [flack parameter 0.011(11)] (Fig. 3).
Compound 11 gave the same molecular formula as that of 10 based on the (+)-HRESIMS data (Supplementary Fig. S34). The 1H and 13C NMR data (Supplementary Figs. S29 and S30; Tables 3 and 4) suggested that 11 was structurally similar to 10, and the main difference was the bromine atom was changed from C-7 to C-5, which was confirmed by the HMBC correlations (Fig. 2; Supplementary Fig. S33) from H-7 to C-3a, C-5, C-6, and C-7a. The coupling constant values of 3JH‑5′, H‑6′ (13.4 Hz, 4.4 Hz) of compound 11 were consistent to reported coupling constant values of 3JH‑5′, H‑6′ (13.2 Hz, 3.5 Hz) of compound 10, and the relative configuration of 11 was determined as 2S*, 6'R* (Zhang et al. 2017). The absolute configuration of 11 was determined as 2S, 6'R by the similar experimental ECD curve (Fig. 4C) of 11 to that of 10.
Compound 12 was isolated as a colorless oil. The molecular formula was determined as C16H14Br2O6 based on the HRESIMS data (Supplementary Fig. S40), and the bromine isotope pattern at m/z 460.92255 [M + H] +, 462.92035 [M + 2 + H] +, and 464.91833 [M + 4 + H] + (a ratio of 1:2:1) showed the presence of two bromine atoms in the molecule. The two-dimensional structure of 12 was similar to 10 by comparison of their NMR data (Supplementary Figs. S35 and S36; Tables 3 and 4). The distinction was the hydrogen atom in the C-5 was replaced by a bromine atom. The relative and absolute configurations were identical to 10 based on similar coupling constant values of 3JH‑5′, H‑6′ and ECD spectra (Fig. 4C).
Compound 14 was obtained as a white amorphous powder and had a molecular formula of C16H20O5 based on the positive HRESIMS data (Supplementary Fig. S46). The 1H and 13C NMR data (Supplementary Figs. S41 and S42; Tables 3 and 4) suggested that 14 was structurally similar to 13 (Li et al. 2020). The main difference between them was the absence of two olefin protons in 13 and the presence of two methylene protons at δH 2.30 (1H, m, Ha-3') and 1.86 (1H, m, Hb-3'), 1.83 (1H, m, Ha-4'), and 1.39 (1H, m, Hb-4') in 14, which was confirmed by the COSY correlations (Fig. 2; Supplementary Fig. S44) of H-2'/Ha, b-3'/Ha, b-4'/Ha, b-5'/H-6'/CH3-6'. Thus, the planar structure of 14 was determined. To determine the stereostructure of C-2, C-2', and C-6', computational calculation and DP4 + probability were used to calculate the four possible relative configurations of 14, and the relative configuration 2S*, 2'S*, 6'R*-14 matched with the experimental one (Supplementary Fig. S53 and S54). Comparing the experimental and calculated ECD spectra (Fig. 4D), 14 has the opposite rotation to 13, and thus, the stereoconfiguration of 14 was assigned as 2R, 2'R, 6'S.
Compound 15 was purified as a white amorphous powder with a molecular formula of C16H16O5 based on the HRESIMS data (Supplementary Fig. S52). The 1H and 13C NMR data (Supplementary Figs. S47 and S48; Tables 4 and 5) of 15 showed close resemblances to those of 16 (Levine et al. 1975), with the difference being the presence of one olefin proton (δH 6.28, d, J = 2.0 Hz, H-7). The HMBC correlations (Fig. 2; Supplementary Fig. S51) from H-7 to C-3a, C-5, C-6, and C-7a indicated that the chlorine atom at C-7 in 16 was replaced by a hydrogen atom in 15. The relative and absolute configurations of 15 were clearly deduced under the guidance of single-crystal X-ray diffraction with Flack parameter − 0.01(4) (Fig. 3). Hence, the absolute configuration of 15 was determined as 2S, 6'R.
Seven known analogues were characterized as 7-dechlorogriseofulvin (5) (Park et al. 2005), griseofulvin (6) (Park et al. 2005), 6-O-desmethyl-7-dechlorogriseofulvin (7) (Shang et al. 2012), 6-O-desmethylgriseofulvin (8) (Belofsky et al. 1998), 7-bromogriseofulvin (9) (Schneck et al. 1968), eupenigriseofulvin (13) (Li et al. 2020), and 4'-demethoxylisogriseofulvin (16) (Levine et al. 1975) through comparison of the spectroscopic data with the literature data. Moreover, compounds 9 and 16 were reported as new natural products, and it was the first time the stereostructure of 13 was determined using the single-crystal X-ray diffraction experiment with a Flack parameter of − 0.07(4) (Fig. 3). In addition, (−)-6'-hydroxygriseofulvin was further confirmed as 2R, 6'R (Shang et al. 2012).
Eight dechlorogriseofulvin analogues [(±)-1−2, 5, 7, 14−15] and four brominated griseofulvin derivatives (9−12) were obtained from Nigrospora sp. treated with 2% NaBr in rice solid medium. According to literature research, addition of NaBr or other halogens in the medium maybe triggers fungal biosynthetic pathways to restore osmotic imbalance, which could activate different silent gene clusters for the discovery of new metabolites (Pan et al. 2019; Pinedo-Rivilla et al. 2022). Moreover, the total biosynthetic pathway of griseofulvin (6) was mapped out (Fig. 5) (Cacho et al. 2013): griseophenone D underwent chlorination by the flavin-dependent halogenase GsfI to form griseophenone B and chloride ions were involved in the biological reaction directly. Similarly, compounds 9−12 were speculated to be formed from griseofulvin precursors through bromination with bromine ions.
Compounds 1−16 were tested for their antifungal activities against seven fungi, including four plant pathogenic fungi (Colletotrichum truncatum, Penicillium expansum, Aspergillus flavus, Aspergillus niger), two dermatophytes (Microsporum gypseum, Trichophyton mentagrophytes), and one deep infection yeast Candida albicans (Fig. 6; Supplementary Table S7). Compounds 6 and 9 demonstrated significant inhibitory activities against one plant pathogenic fungus (C. truncatum) and two dermatophytes (M. gypseum, T. mentagrophyte), with the inhibition zones varying between 28 and 41 mm (10 μg/disc), and showed weak or no antifungal activities against P. expansum, A. flavus, A. niger, and C. albicans. Other compounds exhibited weak or no inhibitory activity against these seven fungi with zones of inhibition ≤ 14 mm. According to the literature, griseofulvins were reported to exhibit in vitro fungistatic effects against dermatophytes, such as Microsporum, Epidermophyton, and Trychophyton genera, and the activities were restricted to yeast, actimomyces and Nocardia (Katrsev et al. 2019), which coincided with the experiments. The antifungal activity of griseofulvin against the plant pathogenic fungus C. truncatum has not been reported previously.
Comparing the activities of compounds 6−8 and 13−15 (or 9−12), the methoxyl group at C-6 (R1) improves the antifungal activity, and the antifungal activity was greatly reduced when R1 was replaced by a hydroxyl group. Comparing the activities of compounds 5−6 and 9, the halogen atom at C-7 (R2) makes a contribution to antifungal activity; when the chlorine atom at C-7 was substituted by a bromine atom, the antifungal activity varied little. However, comparing the activities of compounds 1−4 and 6, when R3 was substituted with a hydroxyl, the activity decreased significantly. Comparing the activities of compounds 6 and 15−16, the position of the carbonyl and double bond also have an important effect on activity. The SAR information mentioned above is generalized in Fig. 7.
In addition, all compounds were evaluated for their cytotoxicity against the HeLa (cervical), HepG2 (hepatocellular carcinoma), HCT-116 (human colorectal adenocarcinoma), and MCF-7 (breast cancer) human cancer cell lines. None of the compounds displayed cytotoxicity against any of the four cell lines at 50 μmol/L.
Conclusions
In summary, processing of the mangrove-derived fungus Nigrospora sp. QQYB1, cultured with 0.3% NaCl or 2% NaBr in rice solid medium, led to the isolation of 12 new griseofulvin derivatives [(±)-1−2, (+)-3, (±)-4, 10−12, 14−15], two new natural products (9 and 16), and six known analogues [(−)-3, 5−8, and 13]. Their 2D structures and absolute configurations were established by extensive spectroscopic analysis (1D and 2D NMR, HRESIMS), ECD spectra, computational calculation, DP4 + analysis, and X-ray single-crystal diffraction experiments. Compounds 1−4 represented the first natural griseofulvin enantiomers with four absolute configurations (2S, 6'S; 2R, 6'R; 2S, 6'R; 2R, 6'S), and compounds 9− 12 were the first successfully produced brominated griseofulvin derivatives from fungi. Compounds 6 and 9 demonstrated significant inhibitory activity against one plant pathogenic fungus (C. truncatum) and two dermatophytes (M. gypseum, T. mentagrophyte) with inhibition zones ranging from 28 to 41 mm (10 μg/disc). The structure − activity relationship (SAR) indicated that the substituents at C-6, C-7, C-6' and the positions of the carbonyl and double bond were the main antifungal active sites of the griseofulvin derivatives.
Data availability
The data that support the findings of this study are included in this published article (and its supplementary information files).
References
Ali T, Inagaki M, Chai HB, Wieboldt T, Rapplye C, Rakotondraibe LH (2017) Halogenated compounds from directed fermentation of Penicillium concentricum, an endophytic fungus of the liverwort Trichocolea tomentella. J Nat Prod 80:1397–1403
Belofsky GN, Gloer KB, Gloer JB, Wicklow DT, Dowd PF (1998) New p-terphenyl and polyketide metabolites from the sclerotia of Penicillium raistrickii. J Nat Prod 61:1115–1119
Cacho RA, Chooi YH, Zhou H, Tang Y (2013) Complexity generation in fungal polyketide biosynthesis: a spirocycle-forming P450 in the concise pathway to the antifungal drug griseofulvin. ACS Chem Biol 8:2322–2330
Chen SH, Chen DN, Cai RL, Cui H, Long YH, Lu YJ, Li CY, She ZG (2016) Cytotoxic and antibacterial preussomerins from the mangrove endophytic fungus Lasiodiplodia theobromae ZJ-HQ1. J Nat Prod 79:2397–2402
Chen Y, Yang WC, Zou G, Wang GS, Kang WY, Yuan J, She ZG (2022) Cytotoxic bromine- and iodine-containing cytochalasins produced by the mangrove endophytic fungus Phomopsis sp. QYM-13 using the OSMAC approach. J Nat Prod 85:1229–1238
Cui H, Liu YN, Li J, Huang XS, Yan T, Cao WH, Liu HJ, Long YH, She ZG (2018) Diaporindenes A-D: four unusual 2,3-dihydro-1H-indene analogues with anti-inflammatory activities from the mangrove endophytic fungus Diaporthe sp. SYSU-HQ3. J Org Chem 83:11804–11813
Dahiya R, Singh S, Sharma A, Chennupati SV, Maharaj S (2016) First total synthesis and biological screening of a proline-rich cyclopeptide from a caribbean marine sponge. Mar Drugs 14:228
Fang CY, Zhang QB, Zhang WJ, Zhang CS, Zhu YG (2022) Discovery of efrotomycin congeners and heterologous expression-based insights into the self-resistance mechanism. J Nat Prod 85:2865–2872
Frank M, Hartmann R, Plenker M, Mándi A, Kurtán T, Özkaya FC, Müller WEG, Kassack MU, Hamacher A, Lin WH, Liu Z, Proksch P (2019) Brominated azaphilones from the sponge-associated fungus Penicillium canescens strain 4.14.6a. J Nat Prod 82:2159–2166
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M et al (2009) Gaussian 09, Revision A.1. Gaussian Inc., Wallingford, CT
Gentles JC (1958) Experimental ringworm in guinea pigs: oral treatment with griseofulvin. Nature 182:476–477
Gupta AK, Summerbell RC (2000) Tinea capitis. Med Mycol 38:255–287
Hai Y, Wei MY, Wang CY, Gu YC, Shao CL (2021) The intriguing chemistry and biology of sulfur-containing natural products from marine microorganisms (1987–2020). Mar Life Sci Tech 3:488–518
Kartsev V, Geronikaki A, Petrou A, Lichitsky B, Kostic M (2019) Griseofulvin derivatives: synthesis, molecular docking and biological evaluation. Curr Top Med Chem 19:1145–1161
Levine SG, Hicks RE, Gottlieb HE, Wenkert E (1975) Carbon-13 nuclear magnetic resonance spectroscopy of naturally occurring substances. XXX Griseofulvin J Org Chem 40:2540–2542
Li YQ, Tan YH, Liu J, Zhou XF, Zeng SQ, Dong JD, Liu YH, Yang B (2020) A new griseofulvin derivative from a soft coral derived fungus Eupenicillium sp. SCSIO41208. Nat Prod Res 34:2971–2975
Lin J, Liu SC, Sun BD, Niu SB, Li EW, Liu XZ, Che YS (2010) Polyketides from the ascomycete fungus Leptosphaeria sp. J Nat Prod 73:905–910
Oxford AE, Raistrick H, Simonart P (1939) Studies in the biochemistry of micro-organisms: griseofulvin, C17H17O6Cl, a metabolic product of Penicillium griseofulvum Dierckx. Biochem J 33:240–248
Pan R, Bai X, Chen JM, Zhang HW, Wang H (2019) Exploring structural diversity of microbe secondary metabolites using OSMAC strategy: a literature review. Front Microbiol 10:294
Panda D, Rathinasamy K, Santra MK, Wilson L (2005) Kinetic suppression of microtubule dynamic instability by griseofulvin: Implications for its possible use in the treatment of cancer. P Natl Acad Sci USA 102:9878–9883
Park JH, Choi GJ, Lee SW, Kim KM, Jung HS, Jang KS, Cho KY, Kim JC (2005) Griseofulvin from Xylaria sp. strain F0010, an endophytic fungus of abies holophylla and its antifungal activity against plant pathogenic fungi. J Microbiol Biotechn 15:112–117
Pinedo-Rivilla C, Aleu J, Durán-Patrón R (2022) Cryptic metabolites from marine-derived microorganisms using OSMAC and epigenetic approaches. Mar Drugs 20:84
Rønnest MH, Rebacz B, Markworth L, Terp AH, Larsen TO, Krämer A, Clausen MH (2009) Synthesis and structure-activity relationship of griseofulvin analogues as inhibitors of centrosomal clustering in cancer cells. J Med Chem 52:3342–3347
Rønnest MH, Raab MS, Anderhub S, Boesen S, Krämer A, Larsen TO, Clausen MH (2012) Disparate SAR data of griseofulvin analogues for the dermatophytes Trichophyton mentagrophytes, T. rubrum, and MDA-MB-231 cancer cells. J Med Chem 55:652–660
Schneck DW, Racz WJ, Hirsch GH, Bubbar GL, Marks GS (1968) Studies of the relationship between chemical structure and porphyria-inducing activity—IV: Investigations in a cell culture system. Biochem Pharmacol 17:1385–1399
Seebacher C, Abeck D, Brasch J, Cornely O, Daeschlein G, Effendy I, Ginter-Hanselmayer G, Haake N, Hamm G, Hipler C, Hof H, Korting HC, Kramer A, Mayser P, Ruhnke M, Schlacke KH, Tietz HJ (2007) Tinea capitis: ringworm of the scalp. Mycoses 50:218–226
Shang Z, Li XM, Li CS, Wang BG (2012) Diverse secondary metabolites produced by marine-derived fungus Nigrospora sp. MA75 on various culture media. Chem Biodivers 9:1338–1348
Wang JF, Cong ZW, Huang XL, Hou CX, Chen WH, Tu ZC, Huang DY, Liu YH (2018) Soliseptide A, a cyclic hexapeptide possessing piperazic acid groups from Streptomyces solisilvae HNM30702. Org Lett 20:1371–1374
Wei MY, Xu RF, Du SY, Wang CY, Xu TY, Shao CL (2016) A new griseofulvin derivative from the marine-derived Arthrinium sp. fungus and its biological activity. Chem Nat Compd 52:1011–1014
Williams DI, Marten RH, Sarkany I (1958) Oral treatment of ringworm with griseofulvin. Lancet 2:1212–1213
Xu WF, Wu NN, Wu YW, Qi YX, Wei MY, Pineda LM, Ng MG, Spadafora C, Zheng JY, Lu L, Wang CY, Gu YC, Shao CL (2022) Structure modification, antialgal, antiplasmodial, and toxic evaluations of a series of new marine-derived 14-membered resorcylic acid lactone derivatives. Mar Life Sci Tech 4:88–97
Yang WC, Yuan J, Tan Q, Chen Y, Zhu YJ, Jiang HM, Zou G, Zang ZM, Wang B, She ZG (2021) Peniazaphilones A-I, produced by co-culturing of mangrove endophytic fungi, Penicillium sclerotiorum THSH-4 and Penicillium sclerotiorum ZJHJJ-18. Chin J Chem 39:3404–3412
Zhang DW, Zhao LL, Wang LN, Fang XM, Zhao JY, Wang XW, Li L, Liu HY, Wei YZ, You XF, Cen S, Yu LY (2017) Griseofulvin derivative and indole alkaloids from Penicillium griseofulvum CPCC 400528. J Nat Prod 80:371–376
Acknowledgements
This research was funded by the Guangdong Marine Economy Development Special Project (GDNRC[2022]35, GDNRC[2023]39) and the National Natural Science Foundation of China (U20A2001, 42276114).
Author information
Authors and Affiliations
Contributions
ZG performed the experiments and wrote the paper; LZ provided the pathogenic fungi; YW, CT, and LT participated in the experiments; LZ and CY analyzed the data and discussed the result; SG revised the manuscript; SB and WB reviewed the manuscript and SZ designed and supervised the experiments. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. Zhigang She is one of the Editorial Board Members, but he was not involved in the journal’s review of, or decision related to, this manuscript.
Animal and human rights statement
This article does not contain any studies with human participants or animals performed by the authors.
Additional information
Edited by Chengchao Chen.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zou, G., Yang, W., Chen, T. et al. Griseofulvin enantiomers and bromine-containing griseofulvin derivatives with antifungal activity produced by the mangrove endophytic fungus Nigrospora sp. QQYB1. Mar Life Sci Technol 6, 102–114 (2024). https://doi.org/10.1007/s42995-023-00210-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42995-023-00210-0