Abstract
As part of our chemical studies on the medical plants in Uzbekistan aimed at searching for new drug leads, we have examined the aerial parts of Mediasia macrophylla. This has resulted in the isolation of four new glucosides, together with 30 known compounds. The structures of new compounds were elucidated as (1′S)-(4-hydroxyphenyl) ethane-1′,2′-diol 2′-O-β-glucopyranoside (1), 3-(4′-methoxyphenyl)-propanol 1-O-β-glucopyranoside (2), 2-methoxy-3-hydroxy-5-(E)-propenyl-phenol 1-O-β-glucopyranoside (3), 1-O-angeloyl-β-glucopyranose (4), on the basis of spectral analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
We have been investigating the herbal medicines of Uzbekistan with the aim of searching for novel drug leads (Umbelliferae [1–10], Compositae [11–14], Guttiferae [15, 16], Polygonaceae [17], Cupressaceae [18], Paeoniaceae [19]). The aerial parts of Mediasia macrophylla (Umbelliferae) have been used traditionally as a perfume; an appetite enhancer; a natural preservative; and for treatment of rheumatism, nephritis, eczema, herpes, and injury [20]. It is also used for treatment of hepatopathy as a decoction mixed with four other medicinal plants in Uzbekistan. As part of our study of medicinal plants in Uzbekistan, we previously investigated the constituents of M. macrophylla, and reported the isolation and structure determination of one structurally unique new C14-polyacetylene glucoside possessing an α-pyrone moiety and four new C10-polyacetylene glucosides from the MeOH extract of M. macrophylla [21]. The isolation of C10-polyacetylene glucosides from the family Umbelliferae was the first example of this type of compound from this source. Further chemical examination of this plant has now resulted in the isolation of four new glucosides (1–4), together with 30 known compounds. We now report on the isolation and structure elucidation of these compounds.
Results and discussion
The MeOH extract of the aerial parts of M. macrophylla (530 g) was successively partitioned with n-hexane, EtOAc, n-BuOH and H2O. Repeated chromatography of the n-BuOH and EtOAc-soluble fractions on Diaion HP-20, Sephadex LH-20, MCI-gel CHP-20P, Toyopearl HW-40, silica gel, YMC-ODS-A, and reverse-phase HPLC gave four new glucosides (1–4) along with 30 known compounds.
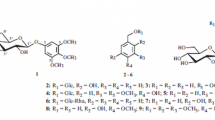
The molecular formula of compound 1 was established as C14H19O8 by HRESIMS. The glycosidic nature of 1 was indicated by anomeric resonances [δH 4.37 (1H, d, J = 8.0 Hz); δC 104.8]. The 1H NMR spectrum revealed an oxygen-bearing methine signal [δH 4.24 (1H, dd, J = 9.6, 2.8 Hz)], and methylene signals [δH 4.01 (1H, dd, J = 9.6, 2.8 Hz) and 3.57 (1H, t, J = 9.6 Hz)], coupled with each other, and A2B2-type aromatic signals [δH 7.24 and 6.79 (each 2H, d, J = 8.4 Hz)], suggesting the existence of a 4-hydroxyphenyl ethane-1,2-diol structure. Enzymatic hydrolysis of 1 gave glucose and an aglycone, which was identified as (1′S)-(4-hydroxyphenyl) ethane-1′,2′-diol [22] from spectroscopic evidence (1H NMR, MS, specific optical rotation). The location of the glucosyl moiety was assigned to be the C-2′ hydroxy group from the HMBC correlation of the anomeric proton with C-2′. Based on these examinations, the structure of 1 was elucidated as shown (Fig. 1).
Compound 2 gave a pseudo molecular ion peak at m/z 351.1446 ([M+Na]+, calcd for 351.1420) in positive-ion HRESIMS, suggesting the molecular formula C16H25O7. The anomeric resonances [δH 4.83 (1H, d, J = 8.0 Hz); δC 104.8] indicated that 2 was also a glycoside. The 1H NMR spectrum of 2 showed the presence of one 1,4-substituted aromatic ring [δH 7.15 and 6.91 (each 2H, d, J = 8.8 Hz)], an oxygenated methylene [δH 4.11 and 3.67 (each 1H, dt, J = 9.6, 6.4 Hz)], two methylenes [δΗ 2.67 and 1.95 (each 2H, m)], and a methoxy group [δH 3.64 (3H, s)]. The 13C NMR spectrum displayed 16 carbon signals including six aromatic carbons [δC 158.4, 134.4, 129.8 (2C), 114.3 (2C)], an oxygen-bearing methylene (δC 68.9) and a methoxy group (δC 55.1), along with six carbon resonances assignable to a glucosyl moiety. The structure of the aglycone of 2 was elucidated as 3-(4′-methoxyphenyl)-propanol from the H–H COSY correlations of H2-1–H2-2–H2-3, together with the HMBC correlations of H-3 with C-1′, C-2′ and C-6′, and of OMe with C-4′. The location of the glucosyl moiety was assigned as C-1 from the HMBC cross peak of the anomeric proton signal (H-1″) with C-1, and its mode of the linkage was concluded to be β from the J value (8.0 Hz) of H-1″. On the basis of these observations, the structure of 2 was assigned as shown in Fig. 1.
Compound 3 had the molecular formula of C16H20O8 on the basis of the HRESIMS. The 1H NMR spectrum of 3 displayed meta-coupled aromatic protons [δH 6.71 and 6.53 (each 1H, d, J = 1.9 Hz)], trans-coupled olefinic protons [δH 6.24 (1H, dt, J = 15.7, 1.3 Hz) and 6.12 (δH 1H, dt, J = 15.7, 6.4 Hz)], and a vinylic methyl group [δH 1.81 (3H, dd, J = 6.4, 1.3 Hz)], suggesting the presence of a 1,3,4,5-tetrasubstituted aromatic ring and an E-propenyl group, along with a methoxy signal [δH 3.82 (3H, s)]. The existence of a sugar moiety was indicated by an anomeric proton signal [δH 4.91 (1H, d, J = 7.4 Hz)]. The carbon resonances (δC 102.8, 78.3, 78.1, 75.0, 71.5 and 62.6) arising from the sugar moiety coincided with the presence of a glucopyranosyl moiety. The structure of the aglycone was elucidated as 1,3-dihydroxy-2-methoxy-4-(E)-propenyl benzene from the HMBC correlations of H-2 with C-3, H-6 with C-4, C-5 and C-1′, H-2′ with C-1, and of OMe with C-4. The location of the glucosyl moiety of 3 was assigned to be the C-1 hydroxy group from the HMBC cross peak of H-1″ with C-1 and the β-linkage was concluded from the coupling constant value (7.4 Hz) of the anomeric proton signal. From this evidence, the structure of 3 was characterized as shown in Fig. 1.
The molecular formula of compound 4 was established as C11H18O7 by HRESIMS. The 1H and 13C NMR spectra of 4 indicated the presence of one α,β-unsaturated carboxyl group, two vinylic methyl groups and a glucosyl moiety. The existence of either angeloyl or tigloyl moiety was deduced from the HMBC correlations of Me-4 with C-2 and C-3, and of Me-5 with C-1, C-2 and C-3. The geometry of the double bond was established as Z by a NOE difference spectroscopy experiment, in which an NOE enhancement was observed in Me-5 upon irradiation of H-3, thus confirming the angeloyl group in 4. The HMBC cross peak of the anomeric proton [δH 5.51 (1H, d, J = 8.0 Hz)] with C-1 (δC 167.8) indicated the location of the angeloyl group at the glucosyl C-1 position and its β-linkage was concluded from the J value (8.0 Hz) of the anomeric proton signal. From these observations, the structure of 4 was elucidated as shown in Fig. 1.
The structure of compound 5 was assigned as 2-O-β-glucopyranosyl-5-methoxy-benzoic acid methyl ester [23] by spectroscopic analysis, since the NMR spectroscopic data for this compound has not been reported previously.
The following known compounds were identified by comparison of their spectral data with those described in the literature: mudanpinoside F [24], (1R,4R,5S)-5-hydroxyfenchone 5-O-β-d-glucopyranoside [25], (1R,4S,6R)-6-hydroxyfenchone 6-O-β-d-glucopyranoside [25], (1R,2R,4S)-1,8-epoxy-2-hydroxy-p-menthan 2-O-β-d-glucopyranoside [26], (1S,2S,4R)-1,8-epoxy-2-hydroxy-p-menthan 2-O-β-d-glucopyranoside [26], betulalbuside A [27], 8-hydroxy-6,7-dihydrolinalol 8-O-glucopyranoside [28], 1-(3,4,5-trimethoxy-phenyl)-propane 1R,2R diol [29], 3-(3′,4′,5′-trimethoxyphenyl) prop-2-en-1-ol [30], chlorogenic acid [31], methyl 3-O-feruloylquinate [32], sphalleroside [33], (1′S)-(4-hydroxyphenyl) ethane-1′,2′-diol [22], ω-hydroxypropioguaiacone [34], benzyl-β-d-glucopyranoside [33], 3,4,5-trimethoxybenzyl alcohol (SDBS, http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi), p-hydroxybenzaldehyde (Sigma-Aldrich, http://www.sigmaaldrich.com/spectra/fnmr/FNMR001261.PDF), 3,4,5-trimethoxybenzaldehyde (SDBS, http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi), tachioside [35], viridoside [36], salidroside [37], icariside D [38], 2-methoxy-2-(4′-hydroxyphenyl) ethanol [39], tyrosol [40], quercetin [41], hyperin [42], scopolin [43], (+)-syringaresinol [44], lariciresinol-4-O-β-d-glucopyranoside [45].
Experimental
General
Optical rotations were measured with a JASCO DIP-370 digital polarimeter. MS were obtained on a Waters LCT PREMIER 2695. NMR (1H NMR: 400 MHz, 13C NMR: 100 MHz, using TMS as an internal standard) spectra were measured on an AVANCE 400 Fourier transform spectrometer (Bruker). The HMBC spectra were run with the 2,3 J value of 7.7 Hz. Column chromatography: silica gel 60N (63–210 μm, Kanto Chemical), Diaion HP-20 (Mitsubishi Chemical), Sephadex LH-20 (GE Healthcare), Toyopearl HW-40 (TOSOH), MCI-gel: CHP-20P (75–150 μm, Mitsubishi Chemical), YMC-pack ODS-A (YMC). Preparative HPLC: ODS [Mightysil RP-18 GP (250 × 20 mm; 5 μm, Kanto Chemical), CAPCELL PACK C18 SG120 (250 × 20 mm; 5 μm, Shiseido), COSOMOSIL Cholester (250 × 20 mm; 5 μm, Nakarai Tesque), COSMOSIL πNAP (250 × 20 mm; 5 μm, Nakarai Tesque)], gel-permeation chromatography (GPC) [Asahi pack GS-310 2G (Asahi Chemical)]. Preparative HPLC was performed on a JASCO apparatus consisting of a PU-980 prep pump, UV-970UV/VIS (at a wavelength of 280 nm) and RI-930 refractive index detectors at a flow rate of 3.5 ml/min. TLC: silica gel 60 F254 (Merck). Avicel SF cellulose plate (Funakoshi).
Plant material
The aerial parts of M. macrophylla were collected at Tashkent region, Uzbekistan, in July 2002, and were identified by one of authors (O. K. K.). Herbarium specimens were deposited in the botanical garden of the University of Tokushima (specimen No.: UTP040003).
Extraction and isolation
The dried aerial parts of M. macrophylla (3.9 kg) were extracted three times with MeOH at 60°C. After concentration, the MeOH extract (530 g) was successively partitioned with n-hexane, EtOAc, n-BuOH and H2O (2 L each × 3) to give an n-hexane-soluble fraction (188 g), an EtOAc-soluble fraction (24 g), an n-BuOH-soluble fraction (77 g) and an H2O-soluble fraction (241 g). The n-BuOH-soluble fraction (77 g) was subjected to chromatography over Diaion HP-20 [MeOH–H2O (0:1 →1:0)] to give 12 fractions. Fraction 2 was applied to a Sephadex LH-20 column [MeOH–H2O (0:1 → 1:0)] to give chlorogenic acid (133 mg) and fractions 2.1–2.3. Fraction 2.3 was separated by YMC ODS-A column chromatography (CC) [MeOH–H2O (0:1 → 1:0)] to give fractions 2.3.1–2.3.8. Fraction 2.3.4 was purified by GPC (MeOH) to afford tachioside (10 mg). Fraction 2.3.5 was also purified by GPC (MeOH) to give compound 1 (12 mg). Purification of fraction 2.3.8 by GPC (MeOH) gave compound 4 (1.0 mg) and mudanpinoside F (4 mg). Fraction 5 was separated by YMC ODS-A CC [MeOH–H2O (0:1 → 1:0)] to give 8 fractions (5.1–5.8). Fraction 5.5 was purified by GPC (MeOH) to yield scopolin (9 mg). Fraction 5.6 was separated by silica gel CC (CHCl3–MeOH–H2O, 8:2:0.2) and GPC (MeOH) to give benzyl-β-d-glucopyranoside (5 mg). Fraction 5.7 was further applied to silica gel CC (CHCl3–MeOH–H2O, 8:2:0.2) and GPC (MeOH) to afford compound 5 (6 mg). Repeated CC of fraction 5.8 with silica gel CC (CHCl3–MeOH–H2O, 8:2:0.2), GPC (MeOH) followed by ODS HPLC (CAPCELL PAK C18 SG120) (MeOH–H2O, 3:7) gave sphalleroside (18 mg). Hyperin (5 mg) was obtained by crystallization of fraction 6. Fraction 7 was fractionated by Sephadex LH-20 CC [MeOH–H2O (2:3 → 1:0)] to give 14 fractions (7.1–7.14). Fraction 7.4 was further subjected to an MCI CHP-20P column [MeOH–H2O (3:7 → 1:0)] to give 6 fractions (7.4.1–7.4.6). Fraction 7.4.4 was purified by ODS HPLC (CAPCELL PAK C18 SG120) (MeOH–H2O, 2:3) and GPC (MeOH) to yield viridoside (14 mg) and lariciresinol-4-O-β-d-glucopyranoside (2 mg). Fraction 7.4.5 was purified by ODS HPLC (CAPCELL PAK C18 SG120) (MeOH–H2O, 2:3) to give compound 2 (9 mg). Fraction 7.6 was separated by MCI CHP-20P CC [MeOH–H2O (2:3 → 1:0)] and GPC (MeOH) to give methyl 3-O-feruloylquinate (7 mg). Purification of fraction 7.14 by GPC (MeOH) afforded quercetin (21 mg).
The EtOAc-soluble fraction (24 g) was subjected to a silica gel column and eluted with solvents of increasing polarity (n-hexane–EtOAc–MeOH) to give 27 fractions. Fraction 5 was separated by Toyo pearl HW-40 chromatography (CHCl3–H2O, 2:1) to give 5 fractions (5.1–5.5). Fraction 5.2 was subjected to silica gel chromatography [CHCl3–MeOH (98:2 → 0:1)] to give p-hydroxybenzaldehyde (2 mg). Fraction 5.3 was separated by silica gel CC (CHCl3–MeOH, 95:5) and GPC (MeOH) to yield 3,4,5-trimethoxybenzaldehyde (27 mg). Fraction 9 was further fractionated by Sephadex LH-20 chromatography [MeOH–H2O (1:1 → 1:0)] to afford 7 fractions (9.1–9.7). Repeated CC of fraction 9.1 with YMC ODS-A [MeOH–H2O (3:2 → 1:0)], HPLC COSMOSIL πNAP (MeOH–H2O, 3:2) and GPC (MeOH) gave 3,4,5-trimethoxybenzyl alcohol (9 mg). Fraction 9.2 was separated by YMC ODS-A chromatography [MeOH–H2O (1:1 → 1:0)] to give 5 fractions (9.2.1–9.2.5). Fractions 9.2.1, 9.2.3, and 9.2.5 were purified by GPC (MeOH) to afford 3-(3′,4′,5′-trimethoxyphenyl) prop-2-en-1-ol (12 mg), 2-methoxy-2-(4′-hydroxyphenyl) ethanol (38 mg), tyrosol (1 mg), respectively. Fraction 10 was subjected to a Sephadex LH-20 column [MeOH–H2O (7:3 → 1:0)], and then a YMC ODS-A column [MeOH–H2O (3:2 → 1:0)] to give 3 fractions (10.1–10.3). Purification of fraction 10.1 by GPC (MeOH) gave (1′S)-(4-hydroxyphenyl) ethane-1′,2′-diol (17 mg). Fraction 10.2 was purified by GPC (MeOH) to yield ω-hydroxypropioguaiacone (1 mg). Fraction 10.3 was purified by GPC (MeOH) to give (+)-syringaresinol (1 mg). Fraction 14 was repeatedly separated by Sephadex LH-20 [MeOH–H2O (3:2 → 1:0)], YMC ODS-A [MeOH–H2O (3:2 → 1:0)] and GPC (MeOH) to give fraction 14.1–14.8. Fraction 14.3 was purified by silica gel chromatography (CHCl3–MeOH–H2O, 8:2:0.2) to give compound 4 (9 mg) and 1-(3,4,5-trimethoxy-phenyl)-propane-1R,2R-diol (6 mg). Fraction 14.5 was chromatographed over a silica gel column (CHCl3–MeOH–H2O, 8:2:0.2) to yield sphalleroside (13 mg). Fraction 14.6 was purified by silica gel chromatography (CHCl3–MeOH–H2O, 8:2:0.2) to yield compound 2 (19 mg). After removal of the MeOH-insoluble portion of fraction 17, the filtrate was applied on a Sephadex LH-20 column [MeOH–H2O (3:2 → 1:0)] to give 8 fractions (17.1–17.8). Fraction 17.1 was further separated by MCI CHP-20P chromatography [MeOH–H2O (1:1 → 1:0)] and silica gel CC [CHCl3–MeOH–H2O (9:1:0 → 8:2:0.2)] to give fractions 17.1.1–17.1.3. Fraction 17.1.1 was purified by HPLC COSMOSIL πNAP (MeOH–H2O, 3:2) to afford (1R,4R,5S)-5-hydroxyfenchone β-d-glucopyranoside (4 mg), (1R,4S,6R)-6-hydroxyfenchone β-d-glucopyranoside (17 mg), (1R,2R,4S)-1,8-epoxy-2-hydroxy-p-menthan 2-O-β-d-glucopyranoside (7 mg) and (1S,2S,4R)-1,8-epoxy-2-hydroxy-p-menthan 2-O-β-d-glucopyranoside (5 mg). Fraction 17.1.3 was purified by HPLC ODS (Mightysil RP-18 GP) (MeOH–H2O, 3:2) to yield betulalbuside A (5 mg) and 8-hydroxy-6,7-dihydrolinalol 8-O-glucopyranoside (6 mg). Fraction 17.2 was applied to an MCI CHP-20P column [MeOH–H2O (1:1 → 1:0)] to give 5 fractions (17.2.1–17.2.5). Fraction 17.2.1 was purified by GPC (MeOH) to give salidroside (10 mg). Fraction 17.2.2 was separated by GPC (MeOH) to give compound 5 (6 mg) and fractions 17.2.2.1–17.2.2.5. Fraction 17.2.2.4 was purified by HPLC ODS (Mightysil RP-18 GP) (MeOH–H2O, 7:13) to give benzyl-β-d-glucopyranoside (5 mg). Fraction 17.2.3 was purified by GPC (MeOH) and HPLC COSMOSIL πNAP (MeOH–H2O, 1:1) to give viridoside (22 mg). The MeOH-soluble part from fraction 18 was repeatedly fractionated with Sephadex LH-20 [MeOH–H2O (1:1 → 1:0)] and an MCI CHP-20P column [MeOH–H2O (2:3 → 1:0)] to give 9 fractions (18.1–18.9). Fraction 18.3 was applied to GPC (MeOH) and silica gel CC [CHCl3–MeOH–H2O (9:1:0 → 8:2:0.2)] to give icariside D (2 mg). Crystallization of fraction 19 with MeOH gave hyperin (82 mg).
(1′S)-(4-Hydroxyphenyl) ethane-1′,2′-diol 2′-O-β-glucopyranoside (1)
An off-white amorphous powder; [α]D −6.0 (MeOH, c 0.2); 1H NMR (400 MHz, CD3OD): δ 7.24 (2H, d, J = 8.4 Hz, H-2 and 6), 6.79 (2H, d, J = 8.4 Hz, H-3 and 5), 4.37 (1H, d, J = 8.0 Hz, H-1″), 4.24 (1H, dd, J = 9.6, 2.8 Hz, H-1′), 4.01 (1H, dd, J = 9.6, 2.8 Hz, H-2′a), 3.89 (1H, dd, J = 11.2, 2.0 Hz, H-6″a), 3.69 (1H, dd, J = 11.2, 4.8 Hz, H-6″b), 3.57 (1H, t, J = 9.6 Hz, H-2′b), 3.42 (1H, t, J = 8.8 Hz, H-3″), 3.23 (2H, m, H-4″ and 5″), 3.29 (1H, dd, J = 8.8, 8.0 Hz, H-2″); 13C NMR (100 MHz, CD3OD): δ 158.3 (C-4), 132.8 (C-1), 128.7 × 2 (C-2 and 6), 116.1 × 2 (C-3 and 5), 104.8 (C-1″), 78.1 (C-5″), 77.8 (C-3″), 76.6 (C-2′), 75.3 (C-2″), 74.1 (C-1′), 71.6 (C-4″), 62.7 (C-6″); HRESIMS m/z 315.1091 [M–H]− (calcd for C14H19O8, 315.1080).
Enzymatic hydrolysis of 1
A solution of compound 1 (4 mg) in water (1 mL) was treated with β-glucosidase from almonds (4.8 units/mg solid, Sigma) (4 mg) at 37°C for 4 days. The reaction mixture was diluted with MeOH, and the resulting precipitates were filtrated off. The filtrate was purified by silica gel column chromatography [CHCl3–MeOH–H2O (30:1:0 → 7:2:0.2)] to give an aglycone (1a) (1.2 mg), as a white amorphous powder, [α]D +15.0 (MeOH, c 0.15); 1H NMR (400 MHz, pyridine-d 5): δ 7.67 (2H, d, J = 8.4 Hz, H-2 and H-6), 7.22 (2H, d, J = 8.4 Hz, H-3 and H-5), 5.25 (1H, t, J = 5.2 Hz, H-1′), 4.17 (2H, br s, H2-2′); HRESIMS m/z 153.0547 [M–H]− (calcd for C8H9O3, 153.0552), which was identified as (1′S)-(4-hydroxyphenyl) ethane-1′,2′-diol [22], together with sugar. The sugar was identified as glucose by TLC analysis [R f: 0.33, n-BuOH/pyridine/H2O (6:4:3) on Avicel SF cellulose].
3-(4′-Methoxyphenyl)-propanol 1-O-β-glucopyranoside (2)
An off-white amorphous powder; [α]D −25.9 (MeOH, c 0.4); 1H NMR (400 MHz, pyridine-d 5): δ 7.15 (2H, d, J = 8.8 Hz, H-2 and 6), 6.91 (2H, d, J = 8.8 Hz, H-3′ and 5′), 4.83 (1H, d, J = 8.0 Hz, H-1″), 4.55 (1H, dd, J = 11.6, 2.4 Hz, H-6″a), 4.38 (1H, dd, J = 11.6, 5.2 Hz, H-6″b), 4.25 (2H, m, H-3″ and H-4″), 4.11 (1H, dt, J = 9.6, 6.4 Hz, H-1a), 4.07 (1H, m, H-2″), 3.94 (1H, ddd, J = 9.2, 5.2, 2.4 Hz, H-5″), 3.67 (1H, dt, J = 9.6, 6.4 Hz, H-1b), 3.64 (3H, s, –OMe), 2.67 (2H, m, H-3), 1.95 (2H, m, H-2); 13C NMR (100 MHz, pyridine-d 5): δ 158.4 (C-4′), 134.4 (C-1′), 129.8 × 2 (C-2′ and 6′), 114.3 × 2 (C-3′ and 5′), 104.8 (C-1″), 78.6 (C-3″), 78.5 (C-5″), 75.3 (C-2″), 71.7 (C-4″), 68.9 (C-1), 62.9 (C-6″), 55.1 (–OMe), 32.3 (C-2), 31.6 (C-3); HRESIMS m/z 351.1446 [M+Na]+ (calcd for C16H24O7Na, 351.1420).
2-Methoxy-5-((E)-propenyl)-benzene-1,3-diol 1-O-β-glucopyranoside (3)
An off-white amorphous powder; [α]D −60.4 (MeOH, c 1.34); 1H NMR (400 MHz, CD3OD): δ 6.71 (1H, d, J = 1.9 Hz, H-9), 6.53 (1H, d, J = 1.9 Hz, H-6), 6.24 (1H, dd, J = 15.7, 1.3 Hz, H-1′), 6.12 (1H, dq, J = 15.7 Hz, 6.4 Hz, H-2′), 4.91 (1H, d, J = 7.4 Hz, H-1″), 3.88 (1H, dd, J = 12.1, 2.1 Hz, H-6″a), 3.82 (3H, s, –OMe), 3.68 (1H, dd, J = 12.1 Hz, 5.5 Hz, H-6″b), 3.48 (1H, m, H-2″), 3.47 (1H, m, H-3″), 3.41 (1H, m, H-5″), 3.38 (1H, m, H-4″), 1.81 (3H, dd, J = 6.4, 1.3 Hz, Me-3′); 13C-NMR (CD3OD): δ 152.3 (C-3), 151.7 (C-5), 137.6 (C-4), 135.6 (C-1), 131.9 (C-1′), 125.9 (C-2′), 109.0 (C-6), 106.8 (C-2), 102.8 (C-1″), 78.3 (C-5″), 78.1 (C-3″), 75.0 (C-2″), 71.5 (C-4″), 62.6 (C-6″), 61.6 (–OMe), 18.5 (Me-3″); HRESIMS m/z 365.1214 [M+Na]+ (calcd for C16H20O8Na, 365.1212).
1-O-Angeloyl-β-glucopyranose (4)
A white amorphous powder; [α]D −1.3 (MeOH, c 0.1); 1H NMR (400 MHz, CD3OD): δ 6.26 (1H, brq, J = 7.2 Hz, H-3), 5.51 (1H, d, J = 8.0 Hz, H-1′), 3.89 (1H, dd, J = 12.4, 2.0 Hz, H-6′a), 3.72 (1H, dd, J = 12.4, 4.8 Hz, H-6′b), 3.50–3.37 (4H, m, H-2′, 3′, 4′ and 5′), 2.05 (3H, brd, J = 7.2 Hz, H-4), 1.96 (3H, brs, H-5); 13C NMR (100 MHz, CD3OD): δ 167.8 (C-1), 141.1 (C-3), 128.6 (C-2), 95.6 (C-1′), 78.9 (C-3′), 78.4 (C-5′), 74.0 (C-2′), 71.1 (C-4′), 62.4 (C-6′), 20.5 (C-5), 16.1 (C-4); HRESIMS m/z 285.0956 [M+Na]+ (calcd for C16H20O8Na, 285.0950).
2-O-β-Glucopyranosyl-5-methoxy-benzoic acid methyl ester (5)
A white amorphous powder; [α]D −50.8 (MeOH, c 0.1); 1H NMR (400 MHz, CD3OD): δ 7.67 (1H, dd, J = 8.4, 2.0 Hz, H-4), 7.64 (1H, d, J = 2.0 Hz, H-6), 7.26 (1H, d, J = 8.4 Hz, H-3), 5.06 (1H, d, J = 7.2 Hz, H-1′), 3.94 (3H, s, 5-OMe), 3.92 (3H, s, 7-OMe), 3.92 (1H, m, H-6′a), 3.73 (1H, dd, J = 12.0, 5.6 Hz, H-6′b), 3.57 (1H, dd, J = 8.8, 7.2 Hz, H-2′), 3.52 (1H, m, H-3′), 3.51 (1H, m, H-5′), 3.44 (1H, dd, J = 9.6, 8.4 Hz, H-4′); 13C NMR (100 MHz, CD3OD): δ 168.3 (C-7), 152.1 (C-2), 150.4 (C-5), 125.4 (C-1), 124.5 (C-4), 116.4 (C-3), 114.1 (C-6), 101.9 (C-1′), 78.3 (C-5′), 77.8 (C-3′), 74.7 (C-2′), 71.2 (C-4′), 62.4 (C-6′), 56.7 (5-OMe), 52.6 (7-OMe); HRESIMS m/z 367.0992 [M+Na]+ (calcd for C15H20O9Na, 367.1005).
Acid hydrolysis of 2–4
Compounds 2–4 (1 mg each) were separately hydrolyzed with 1 M HCl for 12 h at 80°C. Each reaction mixture was diluted with H2O, and extracted with EtOAc. The H2O layer was neutralized with Amberlite IRA-400 resin and evaporated. The residue was directly analyzed by TLC [R f: 0.33, n-BuOH/pyridine/H2O (6:4:3) on Avicel SF cellulose] to detect glucose in each case.
References
Chen B, Kawazoe K, Takaishi Y, Honda G, Itoh M, Takeda Y, Kodzhimatov OK, Ashurmetov O (2000) Prenylated benzoic acid derivatives from Ferula kuhistanica. J Nat Prod 63:362–365
Duan H, Takaishi Y, Tori M, Takaoka S, Honda G, Ito M, Takeda Y, Kodzhimatov OK, Ashurmetov O (2002) Polysulfide derivatives from Ferula foetida. J Nat Prod 65:1667–1669
Shikishima Y, Takaishi Y, Honda G, Ito M, Takeda Y, Tori M, Takaoka S, Kodzhimatov OK, Ashurmetov O (2002) Sesquiterpenes from Ferula penninervis. J Nat Prod 65:1897–1903
Su BN, Takaishi Y, Honda G, Itoh M, Takeda Y, Kodzhimatov OK, Ashurmetov O (2000) Sesquiterpene phenylpropanoid and sesquiterpene chromone derivatives from Ferula pallida. J Nat Prod 63:520–522
Su BN, Takaishi Y, Honda G, Itoh M, Takeda Y, Kodzhimatov OK, Ashurmetov O (2000) Sesquiterpene coumarins and related derivatives from Ferula pallida. J Nat Prod 63:436–440
Suzuki K, Okasaka M, Kashiwada Y, Takaishi Y, Honda G, Ito M, Takeda Y, Kodzhimatov OK, Ashurmetov O, Sekiya M, Ikeshiro Y (2007) Sesquiterpene lactones from the roots of Ferula varia and their cytotoxic activity. J Nat Prod 70:1915–1918
Tada Y, Shikishima Y, Takaishi Y, Shibata H, Higuti T, Honda G, Ito M, Takeda Y, Kodzhimatov OK, Ashurmetov O, Ohmoto Y (2002) Coumarins and γ-pyrone derivatives from Prangos pabularia: antibacterial activity and inhibition of cytokine release. Phytochemistry 59:649–654
Tamemoto K, Takaishi Y, Chen B, Kawazoe K, Shibata H, Higuti T, Honda G, Ito M, Takeda Y, Kodzhimatov OK, Ashurmetov O (2001) Sesquiterpenoids from the fruits of Ferula kuhistanica and antibacterial activity of the constituents of F. kuhistanica. Phytochemistry 58:763–767
Tamemoto K, Takaishi Y, Kawazoe K, Honda G, Ito M, Kiuchi F, Takeda Y, Kodzhimatov OK, Ashurmetov O, Shimizu K, Nagasawa H, Uto Y, Hori H (2002) An unusual sesquiterpene derivative from Ferula kuhistanica. J Nat Prod 65:1323–1324
Zhou P, Takaishi Y, Duan H, Chen B, Honda G, Itoh M, Takeda Y, Kodzhimatov OK, Lee KH (2000) Coumarins and bicoumarin from Ferula sumbul: anti-HIV activity and inhibition of cytokine release. Phytochemistry 53:689–697
Fu B, Su BN, Takaishi Y, Honda G, Ito M, Takeda Y, Kodzhimatov OK, Ashurmetov O (2001) A bis-sesquiterpene and sesquiterpenolides from Inula macrophylla. Phytochemistry 58:1121–1128
Su BN, Takaishi Y, Tori M, Takaoka S, Honda G, Itoh M, Takeda Y, Kodzhimatov OK, Ashurmetov O (2000) Macrophyllidimers A and B, two novel sesquiterpene dimers from the bark of Inula macrophylla. Tetrahedron Lett 41:1475–1479
Su BN, Takaishi Y, Tori M, Takaoka S, Honda G, Itoh M, Takeda Y, Kodzhimatov OK, Ashurmetov O (2000) Macrophyllols A and B, two unusual novel sesquiterpene and monoterpene dimers from the bark of Inula macrophylla. Org Lett 2:493–496
Su BN, Takaishi Y, Yabuuchi T, Kusumi T, Tori M, Takaoka S, Honda G, Itoh M, Takeda Y, Kodzhimatov OK, Ashurmetov O (2001) Sesquiterpenes and monoterpenes from the bark of Inula macrophylla. J Nat Prod 64:466–471
Matsuhisa M, Shikishima Y, Takaishi Y, Honda G, Ito M, Takeda Y, Shibata H, Higuti T, Kodzhimatov OK, Ashurmetov O (2002) Benzoylphloroglucinol derivatives from Hypericum scabrum. J Nat Prod 65:290–294
Tanaka N, Takaishi Y, Shikishima Y, Nakanishi Y, Bastow K, Lee KH, Honda G, Ito M, Takeda Y, Kodzhimatov OK, Ashurmetov O (2004) Prenylated benzophenones and xanthones from Hypericum scabrum. J Nat Prod 67:1870–1875
Okasaka M, Takaishi Y, Kogure K, Fukuzawa K, Shibata H, Higuti T, Honda G, Ito M, Kodzhimatov OK, Ashurmetov O (2004) New stilbene derivatives from Calligonum leucocladum. J Nat Prod 67:1044–1046
Okasaka M, Takaishi Y, Kashiwada Y, Kodzhimatov OK, Ashurmetov O, Lin AJ, Consentino LM, Lee KH (2006) Terpenoids from Juniperus polycarpus var seravschanica. Phytochemistry 67:2635–2640
Okasaka M, Kashiwada Y, Kodzhimatov OK, Ashurmetov O, Takaishi Y (2008) Monoterpene glycosides from Paeonia hybrida. Phytochemistry 69:1767–1772
Chernenko TV, Glushenkova AI, Nigmatullaev AM (2002) Lipids from Mediasia macrophylla leaves. Chem Nat Compd 38:307–309
Kurimoto S, Okasaka M, Kashiwada Y, Kodzhimatov OK, Takaishi Y (2010) A C14-polyacetylenic glucoside with an α-pyrone moiety and four C10-polyacetylenic glucosides from Mediasia macrophylla. Phytochemistry 71:688–692
Ishikawa T, Kondo K, Kitajima J (2003) Water-soluble constituents of coriander. Chem Pharm Bull 51:32–39
Uaui T, Sakata K, Watanabe S, Ishikami M, Oka N, Okada M, Yamagishi M, Oishi K, Ota T, Tozuka Y (2006) Jpn Kokai Tokkyo Koho JP 3765648 B2
Lin HC, Ding HY, Wu TS, Wu PL (1996) Monoterpene glycosides from Paeonia suffruticosa. Phytochemistry 41:237–242
Ishikawa T, Kitajima J, Tanaka Y (1998) Water-soluble constituents of fennel. III. Fenchane-type monoterpenoid glycosides. Chem Pharm Bull 46:1599–1602
Orihara Y, Furuya T (1994) Biotransformation of 1, 8-cineole by cultured cells of Eucalyptus perriniana. Phytochemistry 35:641–644
Kong LD, Abliz Z, Zhou CX, Li LJ, Cheng CHK, Tan RX (2001) Glycosides and xanthine oxidase inhibitors from Conyza bonariensis. Phytochemistry 58:645–651
Ishikawa T, Tanaka Y, Kitajima J (1998) Water-soluble constituents of fennel VII. Acyclic monoterpenoid glycosides. Chem Pharm Bull 46:1748–1751
Lee AL, Ley SV (2003) The synthesis of the anti-malarial natural product polysphorin and analogues using polymer-supported reagents and scavengers. Org Biomol Chem 1:3957–3966
Matsuura H, Miyazaki H, Asakawa C, Amano M, Yoshihara T, Mizutani J (2004) Isolation of α-glucosidase inhibitors from hyssop (Hyssopus officinalis). Phytochemistry 65:91–97
Cheninat A, Zawatzky R, Becker H, Brouillard R (1988) Caffeoyl conjugates from Echinacea species: structures and biological activity. Phytochemistry 27:2787–2794
Ida Y, Satoh Y, Ohtsuka M, Nagasao M, Shoji J (1994) Phenolic constituents of Phellodendron amurense bark. Phytochemistry 35:209–215
Kitajima J, Ishikawa T, Tanaka Y, Ono M, Ito Y, Nohara T (1998) Water-soluble constituents of fennel V. Glycosides of aromatic compounds. Chem Pharm Bull 46:1587–1590
Achenbach H, Stoecker M, Constenla MA (1988) Flavonoid and other constituents of Bauhinia manca. Phytochemistry 27:1835–1841
Inoshiri S, Sasaki M, Kohda H, Otsuka H, Yamasaki K (1987) Aromatic glycosides from Berchemia racemosa. Phytochemistry 26:2811–2814
Yousef GG, Grace MH, Cheng DM, Belolipov IV, Raskin I, Lila MA (2006) Comparative phytochemical characterization of three Rhodiola species. Phytochemistry 67:2380–2391
Schwab W, Schreier P (1988) Aryl β-d-glucosides from Carica papaya fruit. Phytochemistry 27:1813–1816
Miyase T, Ueno A, Takizawa N, Kobayashi H, Oguchi H (1989) Ionone and lignan glycosides from Epimedium diphyllum. Phytochemistry 28:3483–3485
Matsumura T, Ishikawa T, Kitajima J (2002) Water-soluble constituents of caraway: aromatic compound, aromatic compound glucoside and glucosides. Phytochemistry 61:455–459
Sumarah MW, Puniani E, Blackwell BA, Miller JD (2008) Characterization of polyketide metabolites from foliar endophytes of Picea glauca. J Nat Prod 71:1393–1398
Fujiwara T, Honjo T (1995) Determination of constituents in fruit juice by near infrared spectroscopy Part I. Determination of sugar and acid contents in fruit juice of satsuma mandarin by near infrared spectroscopy. Nippon Shokuhin Kagaku Kogaku Kaishi 42:109–117
Markham KR, Ternai B, Stanley R, Geiger H, Mabry TJ (1978) Carbon-13 NMR studies of flavonoids—III: naturally occurring flavonoid glycosides and their acylated derivatives. Tetrahedron 34:1389–1397
Tsukamoto H, Hisada S, Nishibe S (1985) Coumarin and secoiridoid glucosides from bark of Olea africana and Olea capensis. Chem Pharm Bull 33:396–399
Abe F, Yamauchi T (1988) 9α-Hydroxypinoresinol, 9α-hydroxymedioresinol and related lignans from Allamanda neriifolia. Phytochemistry 27:75–77
Sugiyama M, Kikuchi M (1993) Characterization of lariciresinol glucosides from Osmanthus asiaticus. Heterocycles 36:117–121
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurimoto, Si., Okasaka, M., Kashiwada, Y. et al. Four new glucosides from the aerial parts of Mediasia macrophylla . J Nat Med 65, 180–185 (2011). https://doi.org/10.1007/s11418-010-0444-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-010-0444-3