One new phenylglycoside, 1-hydroxy-3,4,5-trimethoxyphenyl-1-O-[6′-O-(4″-carboxy-1″ ,3″ ,5″ - trihydroxy)phenyl]-β-D-glucopyranoside (1), along with eight known ones, isosalicin (2), vanilloloside (3), (4-hydroxy-3,5-dimethoxyphenyl)methyl-β-D-glucopyranoside (4), crenatin (5), hydrageifolin I (6), tachioside (7), isotachioside (8), and koaburside (9), were isolated from the stems of Spiraea prunifolia var. simpliciflora. The chemical structures of these compounds were identified on the basis of spectroscopic data such as NMR, FAB-MS, and IR. All these compounds were isolated from this plant for the first time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Spiraea prunifolia var. simpliciflora (Rosaceae), commonly called “Bridal Wreath,” is a deciduous, latifoliate shrub that is widely distributed in Korea. The leaves are oval shaped, hairless with alternate phyllotaxis, and are glossy dark green and often turn purplish or reddish orange in autumn. The flowers are snowy white, obovoid form, and bloom in early to mid spring. The follicle fruits ripen in September [1]. Previous studies have reported several chemical constituents from the roots of S. prunifolia var. simpliciflora, including triterpenoids (ursolic acid, tormentic acid) [2], sterols (campesterol, β-sitosterol, β-sitosterol-3-O-β-D-glucopyranoside) [1, 2], a phenolic compound (p-hydroxycinnamic acid methyl ester) [3], and a monoterpene glycoside (prunioside) [4]. However, the stems of S. prunifolia var. simpliciflora have not yet been studied. In addition, the methanol extract of the roots of S. prunifolia var. simpliciflora has been reported to suppress the production of nitric oxide (NO) [5]. Therefore, this study was initiated to identify the pharmaceutically active compounds from the stems of S. prunifolia var. simpliciflora. This paper describes the procedure of isolation and identification of one new phenylglycoside, along with eight known ones.
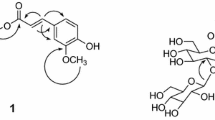
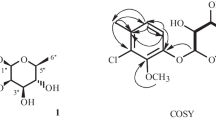
Compound 1, brown amorphous powder, negative FAB-MS m/z 497 [M – H]–, HR-FAB-MS m/z 497.1298 [M – H]–, indicating the molecular formula to be C22H26O13 (calcd for C22H25O13, 497.1295). The IR spectrum showed absorbance bands from a hydroxyl group (3363 cm–1) and an aromatic ring (1506 cm–1). In the PMR spectrum a broad singlet coupling pattern was observed from olefine methine proton signals at δ 7.80 (2H, br.s, H-2″, 6″) and 6.68 (2H, br.s, H-2, 6) due to the presence of two 1,2,3,5-tetrasubstituted benzene rings. The proton signals in the oxygenated region, including a hemiacetal proton signal at δ 5.52 (1H, d, J = 9.2 Hz, H-1′), four oxygenated methines at δ 4.30 (3H, m, H-2′, 3′, 5′) and 4.20 (1H, dd, J = 8.8, 8.8 Hz, H-4′), and oxygenated methylene proton signals at δ 5.19 (1H, br.d, J = 12.0 Hz, H-6′a) and 4.95 (1H, dd, J = 12.0, 6.0 Hz, H-6′b), indicated the presence of a hexose moiety. Three methoxyl proton signals were also observed at δ 3.67 (6H, s, 2 × OCH3) and 3.79 (3H, s, OCH3). Therefore, compound 1 was determined to be a phenylglycoside consisting of two 1,2,3,5-tetrasubstituted benzene rings with three methoxy groups and one hexose moiety. The 13C NMR spectrum also indicated the presence of two 1,2,3,5-tetrasubstituted benzene rings, including eight oxygenated olefine quaternary carbon signals at δ 167.3 (C-1″), 155.2 (C-1), 154.3 (C-3, 5), 147.5 (C-3″, 5″), 141.1 (C-4″), and 135.3 (C-4) and four olefine methine carbon signals at δ 110.2 (C-2″, 6″) and 96.0 (C-2, 6). The carbon signals of the sugar were observed as a hemiacetal carbon at δ 103.3 (C-1′), four oxygenated methine carbons at δ 78.1 (C-3′), 75.8 (C-5′), 74.9 (C-2′), and 71.4 (C-4′), and one oxygenated methylene carbon at δ 64.5 (C-6′), which were of a β-D-glucopyranosyl moiety. The anomer carbon of the sugar was confirmed to be of β-configuration from the coupling constant of the hemiacetal proton signal (J = 9.2 Hz) [6]. In addition, three methoxy groups at δC 60.7 (4-OCH3) and 56.1 (3, 5-OCH3) were observed. To elucidate the structure of compound 1, including the position of the functional group, a heteronuclear multiple bonding connectivity (gHMBC) experiment was performed. In the gHMBC spectrum, cross peaks were observed between δ 155.2 (C-1) and δ 5.52 (H-1′) and between δ 167.3 (C-1″) and δ 5.19 (H-6′a), δH 4.95 (H-6′b), which confirmed that 1-hydroxy-3,4,5-trimethoxyphenyl and 4″-carboxy-1″,3″,5″-trihydroxyphenyl are, respectively, linked to the hydroxyl of C-1′ and C-6′ of the glycopyranosyl moiety. In addition, the oxygenated olefine quaternary carbon signals at δ 154.3 (C-3,5) and 135.3 (C-4) exhibited correlations with the methoxy proton signals at δ 3.79 (4-OCH3) and 3.67 (3, 5-OCH3), respectively. Finally, compound 1 was determined to be 1-hydroxy-3,4,5-trimethoxyphenyl- 1-O-[6′-O-(4″-carboxy-1″,3″,5″-trihydroxy)phenyl]-β-D-glucopyranoside, a new compound.

Compounds 2–9 were identified as isosalicin [7], vanilloloside [8], (4-hydroxy-3,5-dimethoxyphenyl)methyl-β-Dglucopyranoside [9], crenatin [10], hydrageifolin I [11], tachioside [12], isotachioside [12], and koaburside [13], respectively. This identification was confirmed by comparison with spectroscopic data in the literature.
Experimental
General Methods. The silica gel (SiO2) used for column chromatography (CC) was Kiesel gel 60 (Merck, Darmstadt, Germany), and the octadecyl SiO2 (ODS) was Lichroprep RP-18, 40–60 μm (Merck). Thin-layer chromatography (TLC) analysis was carried out using Kieselgel 60 F254 and RP-18 F254S (Merck) plates, and the spots were detected using a Spectroline Model ENF-240 C/F UV lamp (Spectronics Corporation, Westbury, NY, USA) and spraying a 10% H2SO4 solution and heating. Deuterium solvents were purchased from Merck Co. Ltd. and Sigma Aldrich Co. Ltd. (St, Louis, MO, USA). Nuclear magnetic resonance (NMR) spectra were collected with a 400 MHz FT-NMR spectrometer (Varian Inova AS-400, Palo Alto, CA, USA). IR spectra were obtained with a PerkinElmer Spectrum One FT-IR spectrometer (Buckinghamshire, England). Fast atom bombardment ionization mass spectrometry (FAB-MS) were recorded on a JEOL JMS-700. Optical rotations were measured using a JASCO P-1010 digital polarimeter (Tokyo, Japan).
Plant Material. The stems of Spiraea prunifolia var. simpliciflora were provided by GFC Co., Suwon, Korea in August 2011 and identified by Prof. Dae-Keun Kim, Woosuk University, Jeonju, Korea. A voucher specimen (KHU-NPCL-201108) is deposited at the Laboratory of Natural Products Chemistry, Kyung Hee University, Yongin, Korea.
Extraction and Isolation. Dried stems of Spiraea prunifolia var. simpliciflora (8 kg) were extracted with 80% MeOH (60 L × 4) at room temperature for 24 h, filtered, and concentrated in vacuo. The concentrated MeOH extract (691 g) was then poured into H2O (4.2 L) and successively extracted with EtOAc (4.2 L × 3) and n-BuOH (3.2 L × 3) and concentrated to produce residues of the EtOAc fraction (SPE, 92 g), the n-BuOH fraction (SPB, 136 g), and the water fraction (SPW, 403 g). The n-BuOH fraction was then subjected to a Diaion HP-20 CC (11 cm × 40 cm) and eluted as follows: H2O (20.8 L) → MeOH (15 L) → acetone (9 L) yielding five fractions (SPB-1 to SPB-5). SPB-4 [32 g, elution volume/total volume (Ve/Vt) 0.580–0.582] was subjected to a SiO2 CC (11 cm × 11 cm) and eluted with CHCl3–MeOH–H2O (12:3:1 → 10:3:1 → 7:3:1 → 65:35:10, 8 L of each), yielding 21 fractions (SPB-4-1 to SPB-4-21). SPB-4-8 [242 mg, Ve/Vt 0.076–0.111] was subjected to ODS CC (3 cm × 18 cm) and eluted with MeOH–H2O (1:1, 1.2 L), yielding 16 fractions (SPB-4-8-1 to SPB-4-8-16). SPB-4-8-4 [131.8 mg, Ve/Vt 0.133–0.167] was subjected to SiO2 CC (3 cm × 15 cm) and eluted with EtOAc–n-BuOH–H2O (40:5:1, 2.5 L), yielding 16 fractions (SPB-4-8-4-1 to SPB-4-8-4-16), along with a purified compound 9 [SPB-4-8-4-6, 26.8 mg, Ve/Vt 0.177–0.214, TLC (RP-18 F254S) R f 0.60, MeOH–H2O (2:3)]. SPB-4-11 [988 mg, Ve/Vt 0.162–0.247] was subjected to ODS CC (4 cm × 8 cm) and eluted with MeOH–H2O (1:4, 1.6 L), yielding 19 fractions (SPB-4-11-1 to SPB-4-11-19). SPB-4-11-1 [270 mg, Ve/Vt 0.013–0.175] was subjected to SiO2 CC (3.5 cm × 14.5 cm) and eluted with EtOAc–n-BuOH–H2O (40:5:1, 3 L), yielding 16 fractions (SPB-4-11-1-1 to SPB-4-11-1-16) and purified compound 7 [SPB-4-11-1-2, 3.4 mg, Ve/Vt 0.177–0.225, TLC (Kiesel gel 60 F254) R f 0.57, EtOAc–n-BuOH–H2O (10:5:1)] and compound 4 [SPB-4-11-1-13, 31.5 mg, Ve/Vt 0.597–0.808, TLC (Kiesel gel 60 F254) R f 0.30, EtOAc–n-BuOH–H2O (10:5:1)]. SPB-4-11-5 [199.1 mg, Ve/Vt 0.213–0.300] was subjected to SiO2 CC (3 cm × 14.5 cm) and eluted with EtOAc–n-BuOH–H2O (40:5:1, 2.7 L) which ultimately led to isolation of compound 2 [SPB-4-11-5-2, 17 mg, Ve/Vt 0.097–0.119, TLC (RP-18 F254S) R f 0.60, MeOH–H2O (1:1)]. SPB-4-13 [1.46 g, Ve/Vt 0.249–0.520] was subjected to ODS CC (4.5 cm × 9 cm) and eluted with MeOH–H2O (1:4 → 2:3, 3.2 L of each), yielding 25 fractions (SPB-4-13-1 to SPB-4-13-25). SPB-4-13-5 [107.5 mg, Ve/Vt 0.031–0.041] was subjected to SiO2 CC (3 cm × 14 cm) and eluted with EtOAc–n-BuOH–H2O (50:5:1, 5.0 L), which ultimately led to the isolation of compound 8 [SPB-4-13-5-5, 11.5 mg, Ve/Vt 0.074–0.109, TLC (Kiesel gel 60 F254) R f 0.57, EtOAc– n-BuOH–H2O (10:5:1)]. SPB-4-13-6 [88.7 mg, Ve/Vt 0.044–0.059] was subjected to SiO2 CC (2.5 cm × 16 cm) and eluted with EtOAc–n-BuOH–H2O (40:5:1, 2.4 L), yielding 13 fractions (SPB-4-13-6-1 to SPB-4-13-6-13) and producing compound 3 [SPB-4-13-6-8, 21.3 mg, Ve/Vt 0.308–0.429, TLC (Kiesel gel 60 F254) R f 0.38, EtOAc–n-BuOH–H2O (10:5:1)]. SPB-4-18 [3.4 g, Ve/Vt 0.707–0.980] was subjected to ODS CC (4.5 cm × 9 cm) and eluted with MeOH–H2O (1:4, 3.6 L), yielding 16 fractions (SPB-4-18-1 to SPB-4-18-16). SPB-4-18-5 [509.7 mg, Ve/Vt 0.022–0.024] was subjected to SiO2 CC (3.5 cm × 14 cm) and eluted with EtOAc–n-BuOH–H2O (20:5:1, 3.2 L), yielding 17 fractions (SPB-4-18-5-1 to SPB-4-18-5-17) along with compound 5 [SPB-4-18-5-2, 57 mg, Ve/Vt 0.022–0.024, TLC (Kiesel gel 60 F254) R f 0.39, CHCl3–MeOH–H2O (65:35:10)].Fraction SPB-5 [43 g, Ve/Vt 0.600–1.000] was subjected to SiO2 CC (11 cm × 13 cm) and eluted with CHCl3–MeOH–H2O (18:3:1 → 11:3:1 → 9:3:1 → 7:3:1 → 6:4:1, 7 L of each), yielding 22 fractions (SPB-5-1 to SPB-5-22). Fraction SPB-5-14 [3.2 g, Ve/Vt 0.574–0.715] was subjected to SiO2 CC (4 cm × 13.5 cm) and eluted with EtOAc–n-BuOH–H2O (25:5:1, 8 L), yielding 21 fractions (SPB-5-14-1 to SPB-5-14-21). Fraction SPB-5-14-2 [174.3 mg, Ve/Vt 0.039–0.082] was subjected to ODS CC (3 cm × 6.5 cm) and eluted with MeOH–H2O (1:4 → 1:1, 2.1 L of each), yielding 18 fractions (SPB-5-14-2-1 to SPB-5-14-2-18) and ultimately producing compound 1 [SPB-5-14-2-9, 32.1 mg, Ve/Vt 0.323–0.409, TLC (Kiesel gel 60 F254) R f 0.35, CHCl3–MeOH–H2O (7:3:1)]. Fraction SPB-5-14-11 [655.2 mg, Ve/Vt 0.148–0.227] was subjected to ODS CC (4 cm × 6.5 cm) and eluted with MeOH–H2O (1:3 → 1:1, 3.2 L of each) to yield 23 fractions (SPB-5-14-11-1 to SPB-5-14-11-23). Fraction SPB-5-14-11-7 [32.7 mg, Ve/Vt 0.040–0.080] was subjected to Sephadex LH-20 CC (4 cm × 6.5 cm) and eluted with 50% MeOH (0.5 L), yielding 10 fractions (SPB-5-14-11-7-1 to SPB-5-14-11-7-10) and resulting in the isolation of compound 6 [SPB-5-14-11-7-3, 12.4 mg, Ve/Vt 0.680–0.720, TLC (RP-18 F254S) R f 0.50, MeOH–H2O (1:1)].
1-Hydroxy-3,4,5-trimethoxyphenyl-1-O-[6′-O-(4″-carboxy-1″,3″,5″-trihydroxy)phenyl]-β-D-glucopyranoside (1). Brown amorphous powder; [α] 25D +22.7° (c 0.53, MeOH); negative FAB-MS m/z 497 [M – H]–, HR-FAB-MS m/z 497.1298 (calcd for C22H25O13, 497.1295). PMR (400 MHz, C5D5N, ′, ppm, J/Hz): 7.80 (2H, br.s, H-2″, 6″), 6.68 (2H, br.s, H-2, 6), 5.52 (1H, d, J = 9.2, H-1′), 5.19 (1H, br.d, J = 12.0, H-6′a), 4.95 (1H, dd, J = 12.0, 6.0, H-6′b), 4.30 (3H, m, H-2′, 3′, 5′), 4.20 (1H, dd, J = 8.8, 8.8, H-4′), 3.67 (6H, s, 3, 5-OCH3), 3.79 (3H, s, 4-OCH3). 13C NMR (100 MHz, C5D5N, δ, ppm): 167.3 (C-1″), 155.2 (C-1), 154.3 (C-3, 5), 147.5 (C-3″, 5″), 141.1 (C-4″), 135.3 (C-4), 110.2 (C-2″, 6″), 103.3 (C-1′), 96.0 (C-2, 6), 78.1 (C-3′), 75.8 (C-5′), 74.9 (C-2′), 71.4 (C-4′), 64.5 (C-6′), 60.7 (4-OCH3), 56.1 (3, 5-OCH3).
References
J. S. Ro, Kor. J. Pharmacogn., 13, 39 (1982).
M. H. Woo, E. H. Lee, S. O. Chung, and W. C. Kim, Kor. J. Pharmacogn., 27, 389 (1996).
H. S. Youn and B. B. Chung, Kor. J. Pharmacog., 18, 107 (1987).
H. C. Oh, G. S. Oh, W. G. Seo, H. O. Pae, K. Y. Chai, T. O. Kwon, Y. H. Lee, H. T. Chung, and H. S. Lee, J. Nat. Prod., 64, 942 (2001).
H. S. So, R. K. Park, H. M. Oh, H. O. Pae, J. H. Lee, K. Y. Chai, S. Y. Chung, and H. T. Chung, J. Ethnopharmacol., 68, 209 (1999).
S. Shrestha, D. Y. Lee, J. H. Park, J. G. Cho, W. D. Seo, H. C. Kang, Y. J. Jeon, S. W. Yeon, M. H. Bang, and N. I. Baek, J. Korean Soc. Appl. Biol. Chem., 55, 689 (2012).
E. A. Krasnov, V. A. Raldugin, I. V. Shilova, and E. Yu. Avdeeva, Chem. Nat. Compd., 42, 148 (2006).
Y. Koike, M. Fukumura, Y. Hirai, Y. Hori, S. Usui, T. Atsumi, and K. Toriizuka, J. Nat. Med., 64, 245 (2010).
Y. X. Li, X. Yu, S. J. Yu, A. Y. Ma, Z. W. Deng, and W. H. Lin, J. Chin. Pharm. Sci., 19, 256 (2010).
A. Vazquez, M. C. Mato, and J. Mendez, Biol. Plantarum, 36, 629 (1994).
L. Hamerski, M. D. Bomm, D. H. S. Silva, M. C. M. Young, M. Furlan, M. N. Eberlin, I. Castro-Ganboa, A. J. Cavalheiro, and V. S. Bolzani, Phytochemistry, 66, 1927 (2005).
Z. J. Wu, L. Shan, M. Lu, Y. H. Shen, J. Tang, and W. D. Zhang, Chem. Nat. Compd., 46, 298 (2010).
M. Sugiyama and M. Kikuchi, Chem. Pharm. Bull., 40, 325 (1992).
Acknowledgment
This study was supported by a grant from the Next-Generation Bio-Green 21 Program (No. PJ009574012013), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2015, pp. 750–752.
Rights and permissions
About this article
Cite this article
Kim, SY., Song, NY., Cho, JG. et al. Phenylglycosides from the Stems of Spiraea prunifolia var. simpliciflora . Chem Nat Compd 51, 873–876 (2015). https://doi.org/10.1007/s10600-015-1437-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1437-y