Abstract
Ten flavonoids were isolated from the ethyl acetate-soluble fraction of the ethanolic extract of the seeds of Trigonella foenum-graecum and their structures were elucidated on the basis of spectroscopic methods to be 5,7,3′-trihydroxy-5′-methoxylisoflavone (1), biochanin A (2), formononetin (3), irilone (4), tricin (5), daidzein (6), calycosin (7), orientin-2″-O-p-trans-coumarate (8), vitexin-2″-O-p-trans-coumarate (9), and tricin-7-O-β-d-glucopyranoside (10). Compounds 1 and 8 are new flavonoids, and 8 and 9 strongly promoted 2BS cell proliferation induced by H2O2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trigonella foenum-graecum is a plant of Trigonella Linn in Leguminosae whose seeds are used in China as a carminative and tonic herbal medicine. Saponins, flavones, and flavonoids were isolated from the seeds in previous phytochemical research [1–3]. Pharmacological studies showed that the extract of this herbal medicine exhibited antihyperglycemia and antihyperlipidemia effects. In order to develop traditional medicine and find new active compounds, we investigated the constituents of the seeds of this plant. In this paper, we report two new compounds—an isoflavone, 5,7,3′-trihydroxy-5′-methoxylisoflavone (1), and a new flavonoid, orientin-2″-O-p-trans-coumarate (8)—along with eight known constituents, biochanin A (2) [4], formononetin (3) [4], irilone (4) [5], tricin (5) [3], daidzein (6) [4], calycosin (7) [6], vitexin-2″-O-p-trans-coumarate (9) [7], and tricin-7-O-β-d-glucopyranoside (10) [3]. In addition, the antioxidant effects of all compounds were evaluated, and 8 and 9 strongly promoted 2BS cell proliferation induced by H2O2 with viability rates of 29.85 and 25.44%, respectively.
Results and discussion
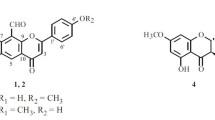
Compound 1 was isolated as a yellow amorphous powder (in CH3OH) with the molecular formula C16H12O6 determined by the parent ions in the positive high-resolution electrospray ionization mass spectrometry (HRESIMS) at m/z 301.1845 [M + H]+ and 323.1048 [M + Na]+. Compound 1 gave a positive result in the Mg–HCl test and a negative result in the Molisch reaction. The UV spectrum showed absorptions at 262 and 292 nm (sh), which are characteristic of an isoflavone skeleton whose presence was confirmed by the singlet at δ H 8.09 (1H, s, H-2) in the 1H-NMR spectrum of 1. In addition, the 1H- and 13C-NMR data showed a methoxyl group at δ H 3.91 (3H, s) and δ C 56.4. In the 1H-NMR spectrum of 1, a set of aromatic signals due to the 3′,5′-disubstituted ring B were clearly visible at δ H 7.06 (1H, t, J = 1.8 Hz, H-4′), 6.99 (1H, br s, H-2′), and 6.97 (1H, br s, H-6′). Two doublets at δ H 6.36 (1H, d, J = 2.0 Hz) and 6.24 (1H, d, J = 2.0 Hz) were characteristic protons of H-6 and H-8 of ring A in the isoflavone skeleton. The methoxyl group (δ H 3.91) was attached to C-5′ (δ C 149.3), as confirmed by their long-range correlation in the HMBC experiment. All the 1H- and 13C-NMR data of 1 in CD3OD were assigned by HMQC and HMBC experiments. Thus, the structure of compound 1 was elucidated to be 5,7,3′-trihydroxy-5′-methoxylisoflavone.
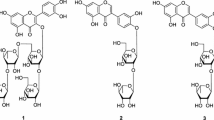
Compound 8 was obtained as a yellow amorphous powder (in CH3OH). Its molecular formula, C30H26O13, was determined by the parent ions in the positive HRESIMS at m/z 595.1258 [M + H]+ and 617.2015 [M + Na]+. Positive results were displayed both in Mg–HCl and Molisch tests and characteristic absorptions at 272 and 317 nm were shown in its UV spectrum, which indicated compound 8 was a flavonoid glycoside. After acid hydrolysis of 8, d-glucose was obtained and identified by coelution with an authentic sample on TLC. The 1H- and 13C-NMR spectra indicated the presence of a p-coumaroyl group (see Table 1) with trans configuration because of the large coupling constant between two proton signals at δ H 7.28 (1H, d, J = 16.4 Hz, H-7″′) and 6.09 (1H, d, J = 16.4 Hz, H-8″′). The ring B in the flavonoid skeleton was 3′,4′-disubstituted as evidenced by the set of aromatic signals at δ H 7.51 (1H, br s, H-2′), 6.87 (1H, d, J = 8.0 Hz, H-5′), and 7.56 (1H, dd, J = 8.0, 2.0 Hz, H-6′). Two singlets at δ H 6.53 (1H, s) and 6.06 (1H, s) were characteristic of protons H-3 and H-6 of ring C and A, respectively, which could be certified by HMQC experiments. In addition to the NMR signals for the p-coumaroyl group and the flavone aglycone moiety, the other resonances were due to a glucopyranosyl unit (see Table 1). Furthermore, a proton signal at δ H 4.98 (1H, d, J = 8.4 Hz, H-1″) was assigned as the glucopyranosyl anomeric proton whose large coupling constant indicated the glucosyl unit was in the β-configuration. HMBC correlations from H-1″ to C-7 (δ C 166.7), C-8 (δ C 102.7), and C-9 (δ C 156.8), and the NMR data of the glucopyranosyl unit confirmed that this sugar was linked to C-8 of the aglycone by a C–C glycosidic bond. In addition, the long-range correlation from H-2″ (δ H 5.46, 1H, dd, J = 10.0, 8.6 Hz) to C-9′″ (δ C 165.7) indicated the p-coumaroyl group was attached to C-2″ of the glucopyranosyl unit. All the 1H- and 13C-NMR data of 8 in CD3OD were assigned by HMQC and HMBC experiments. Thus, the structure of compound 8 was elucidated to be orientin-2″-O-p-trans-coumarate.
Compounds 1–10, isolated from the seeds of Trigonella foenum-graecum L., were tested for their ability to promote 2BS cell proliferation induced by H2O2. Viability rates determined for each compound at a concentration of 1.0 μM are listed in Table 2. The results indicated that the new flavonoid (8) had a strong antioxidant effect with a viability rate (28.85%) comparable to that of NAC (N-acetyl-l-cysteine), the reference substance (28.38%). Furthermore, the 7-OH and 4′-OH groups were important regarding the structure–activity relationship. If they were not present or were substituted, such as in compounds 1–4 and 10, the activity decreased. In addition, the p-coumaroyl was an effective substituent group, and the ortho-diphenolic hydroxyl group in the B ring was very important, such as in compounds 8 and 9 (Fig. 1).
Experimental
General
Melting points (mp) were measured on an X-4 light micromelting apparatus and are uncorrected. The UV spectra were recorded in MeOH on a spectrophotometer. The IR (KBr) spectra were recorded on a Nicolet 670 infrared spectrophotometer. 1D and 2D NMR spectra were recorded on a Bruker Avance 600 instrument with tetramethylsilane (TMS) as the internal standard. The MS spectra were recorded on a Agilent Trap VL spectrometer. Sephadex LH-20 was produced by Amersham Pharmacia Biotech AB. The silica gel for column chromatography and GF254 for TLC were produced by Qingdao Marine Chemical Company. NAC (N-acetyl-l-cysteine) and MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] were purchased from Sigma. All other chemical reagent were made in China are were of analytical grade.
Plant material
The seeds of Trigonella foenum-graecum L. were collected in Yuexi county, Anhui Province, China, in August 2004 and were identified by Prof. Yongjun Liu, Institute of Materia Medica, Shandong Academy of Medical Sciences. A voucher specimen (No 200408016) was deposited in the herbarium of the Institute of Materia Medica, Shandong Academy of Medical Sciences.
Extraction and isolation
The air-dried seeds of Trigonella foenum-graecum L. (10.0 kg) were powdered and percolated with ethanol at room temperature. The ethanolic extract (270 g) was obtained and suspended in water, then partitioned successively with petroleum ether, chloroform, ethyl acetate, and n-butanol. The chloroform extract (70 g) was chromatographed on silica gel (200–300 mesh, 1.0 kg) and eluted with a chloroform–methanol gradient (100:0 → 60:40) to give five fractions (C1–C5). Fraction C2 (10.0 g) was chromatographed repeatedly on silica gel (300–400 mesh, 200.0 g) and eluted with petroleum–ethyl acetate (20:1 and 10:1) to yield compounds 3 (16 mg) and 4 (28 mg). Fraction C3 (15.0 g) was chromatographed repeatedly on silica gel (300–400 mesh, 300.0 g) and eluted with petroleum–ethyl acetate (10:1 and 5:1) to yield compounds 2 (42 mg), 6 (50 mg), and 7 (14 mg). Fraction C4 (12.0 g) was chromatographed repeatedly on silica gel (300–400 mesh, 250.0 g) and eluted with petroleum–ethyl acetate (10:1 and 5:1) to yield compounds 1 (26 mg) and 5 (15 mg). The ethyl acetate extract (20 g) was chromatographed on silica gel (200–300 mesh, 0.5 kg) and eluted with a chloroform–methanol gradient (90:10 → 60:40) to give five fractions (E1–E5). Fraction E3 (8.0 g) was chromatographed repeatedly on silica gel (300–400 mesh, 200.0 g) and eluted with chloroform–methanol (10:1 and 5:1) and purified by Sephadex LH-20 to yield compounds 8 (49 mg), 9 (43 mg), and 10 (52 mg).
2BS cell proliferation assay
Cells and materials
The human embryonic lung diploid fibroblasts cells (2BS cells) were previously isolated from female fetal lung fibroblast tissue and had been fully characterized [8, 9]. The 2BS cell line was originally established at the National Institute of Biological Products (Beijing, China). The 2BS cells were considered to be young at PD30 or below and cultured in Dulbecco’s modified Eagle medium (DMEM, GIBCO) supplemented with 10% fetal bovine serum (FBS, Hyclone) and 1% penicillin G/streptomycin sulfate (GIBCO).
Method
Two groups of young 2BS cells were seeded on 96-well plates at a density of 2 × 103 cells/well. One group was incubated in DMEM culture at 37°C for 20 h as the blank group; the other (damaged) group was incubated with 100 μM H2O2 in DMEM culture otherwise under the same conditions. The numbers of 2BS cells for the two groups were then counted under an optical microscope. The viability rate of 2BS cells in the damaged group decreased about 20% after incubation with H2O2 [8].
Compounds 1–10 and NAC were stored as 10 mM solutions in DMSO, then diluted to 0.05, 0.10, 1.0, 5.0, 10.0, 20.0, and 50.0 μM working solutions as necessary. Young 2BS cells were seeded on 96-well plates at a density of 2 × 103 cells/well. After treatments with different chemicals for 2 h, the cells were incubated with 100 μM H2O2 in DMEM culture for 20 h, then 1 mg/ml MTT solution for 2 h. The medium was aspirated, and the resulting formazan product was solubilized with 100 μl DMSO. Viability rate was assessed by measuring absorbance (optical density, OD) at 570 nm with a BioRad microplate reader and calculated from the following formula: \({{\left( {{\text{OD}}_{{\text{sample}}} - {\text{OD}}_{{\text{H}}_{\text{2}} {\text{O}}_{\text{2}} } } \right)} \mathord{\left/ {\vphantom {{\left( {{\text{OD}}_{{\text{sample}}} - {\text{OD}}_{{\text{H}}_{\text{2}} {\text{O}}_{\text{2}} } } \right)} {{\text{OD}}_{{\text{H}}_{\text{2}} {\text{O}}_{\text{2}} } }}} \right. \kern-\nulldelimiterspace} {{\text{OD}}_{{\text{H}}_{\text{2}} {\text{O}}_{\text{2}} } }} \times 100\%\) [9].
Identification
5,7,3′-Trihydroxy-5′-methoxyl-isoflavone (1): C16H12O6, yellow amorphous powder (in CH3OH), mp 279–281°C. UV λmax (MeOH) nm: 262. IR νmax (KBr): 3444, 3311, 3095, 2983, 2380, 1661, 1621, 1578, 1508, 1449, 1346, 1289, 1198, 1177, 1134, 1022, 826, and 851 cm−1. ESI-MS m/z: 301.1 [M + H]+, 323.1 [M + Na]+; positive HRESIMS m/z 323.1048 [M + Na]+, (calc. 323.1025). 1H- and 13C-NMR, see Table 1.
Orientin-2″-O-p-trans-coumarate (8): yellow amorphous powder (in CH3OH), mp 252–255°C, UV λmax (MeOH) nm: 272, 317. IR νmax(KBr): 3355, 1655, 1605, 1513, 1437, 1361, 1259, 1169, 1108, 1083, 1044, and 831 cm−1. ESI-MS m/z: 595.1 [M + H]+, 617.1 [M + Na]+; positive HRESIMS m/z 617.2015 [M + Na]+, (calc. 617.1997). 1H- and 13C-NMR, see Table 1.
References
Yoshikawa M (1997) Medicinal foodstuffs IV. fenugreek seed (1): Structures of trigoneosides Ia, Ib, IIa, IIb, IIIa and IIIb, new furostanol saponins from the seeds of Indian Trigonella foenum-graecum L. Chem Pharm Bull 45:81–87
Yoshikawa M (1998) Medicinal foodstuffs VIII. fenugreek seed (2): Structures of six new furostanol saponins trigoneosides IVa, Va, Vb, VI, VIIb, VIIIb from the seeds of Indian Trigonella foenum-graecum L. Heterocycles 47:397–405
Shang MY, Cai SQ, Han J, Li J, Zhao YY, Zheng JH (1998) Studies on flavonoids from common Fenugreek (Trigonella foenum-graecum L.). Chin J Chin Mater Med 23:614–616 (in Chinese)
Ma Q, Lei HM, Zhou YX, Wang CH (2005) Study on chemical constituents of Trifolium pratense. Chin Pharm J 40:1057–1059 (in Chinese)
Ji WL, Qin MJ, Wang ZT (2001) Studies on the constituents of Belamcanda chinensis (I). J Chin Pharm Univ 32:197–199 (in Chinese)
Ding L, wang MS, Guo GC, Wang HQ (2004) Crystal structure of 7,3′-dihydroxy-4′-methoxyisoflavone. Chin J Struct Chem 23:723–726
Sood AR, Boutard B (1976) A new flavone C-glycoside from Trigonell foenum-graecum. Phytochemistry 15:351–352
Tang ZQ, Zhang ZY, Zheng Y, Corbley MJ, Tong TJ (1994) Cell aging of human diploid fibroblasts is associated with changes in responsiveness to epidermal growth factor and changes in HER-2 expression. Mech Ageing Dev 73:57–67
Li JH, Zhang ZY, Tong TJ (1995) The proliferative response and anti-oncogene expression in old 2BS cells after growth factor stimulation. Mech Ageing Dev 80:25–34
Acknowledgments
The authors are grateful to the Department of Medicinal Analysis, Pharmacy College, Shandong University, for performing NMR, and the Department of Pharmacology, Institute of Materia Medica, Shandong Academy of Medical Sciences, for measuring the compound activities in the 2BS cell proliferation assay.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, GR., Tang, WZ., Yao, QQ. et al. New flavonoids with 2BS cell proliferation promoting effect from the seeds of Trigonella foenum-graecum L.. J Nat Med 64, 358–361 (2010). https://doi.org/10.1007/s11418-010-0407-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-010-0407-8