Abstract
Purpose
The work aimed to (1) better understand how C rate and type affecting N2O emissions when combined application with different N forms in a strong ammonia oxidation soil, (2) further explore the tradeoff and mechanism of C availability to N transformations, and (3) provide evidence for making targeted N2O mitigation measures in these kinds of agricultural soils.
Materials and methods
The soil was collected from a typical farmer’s field in Quzhou, Hebei province, China, classified as calcareous Cambisols (FAO classification system) and characterized with high pH (7.72), low organic C (9.1 g kg−1), and a strong ammonia oxidation potential. Two microcosm incubations were conducted in laboratory under 20 °C and 70% WFPS (water-filled pore space), and the soils were amended with different glucose rates (0, 0.5, 1.0, 2.0, 4.0 mg C kg−1) in Exp I, and with different C sources (glucose, pectin, starch, cellulose, lignin, straw) in Exp II, meanwhile with NH4+ or NO3−-based fertilizer addition. 480 g (equivalent to oven-dried weight) of pre-incubated soil was placed in a glass jar (1165 mL volume) and adjusted to a field bulk density of 1.34 g cm−3. All the glass jars were placed randomly and incubated for 15 days under aerobic condition.
Results and discussion
Alone application of NH4+ released more N2O (peak at 16.27 μg N kg−1) than alone NO3− application. However, combined application of glucose with NO3− could emit more N2O than when combined with NH4+, and the highest N2O peak value was found in 0.5 g kg−1of glucose. When pectin is combined with NH4+, resulted in higher accumulated N2O emissions than other C sources, while applying straw and cellulose had negligible effects on N2O emissions but enhanced CO2 emissions under the incubation conditions. Glucose and pectin were identified as two labile C sources, significantly enhanced N2O emissions. As expected, C addition could stimulate mineral N immobilization.
Conclusions
Combined application of C and N enhanced N2O and CO2 emissions albeit to different extents. A higher accumulated N2O emission was found when C/N ratio was lower (12.5). The stimulating effect of glucose and pectin as labile C sources with N addition on N2O production should be considered, especially in soils with high pH, low organic C, and a strong ammonia oxidation potential. Therefore, it may have the potential to mitigate N2O emissions from the field when avoiding applying both N and C especially rich in glucose and pectin together.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nitrous oxide (N2O) is a potent greenhouse gas (GHG), significantly contributing to a global warming, with about 300-fold higher global warming potential than carbon dioxide (CO2) (IPCC 2007), and involved in the destruction of the protective ozone layer (Ravishankara et al. 2009). Nearly 65% of the total anthropogenic N2O was emitted from agricultural section globally (IPCC 2007), and in China, up to 75% of N2O was emitted from this section (UNEP 2013). Agricultural soils are the largest source of N2O production due to the large use of synthetic nitrogen (N) fertilizers and manure (IPCC 2013). Several biological processes involved in the soil N cycling contribute to N2O emissions, including autotrophic/heterotrophic nitrification, heterotrophic denitrification, nitrifier denitrification, and dissimilatory nitrate reduction to ammonium (DNRA) and anammox, and nitrification and denitrification are considered as the major N2O sources in soils (Butterbach-Bahi et al. Butterbach-Bahl et al. 2013). Nitrification is the main process of N2O production under aerobic, high ammonia, low nitrate concentration conditions, while heterotrophic denitrification-related N2O mainly occurs under oxygen-limited and high nitrate conditions (Wrage et al. 2001, 2005; Kool et al. 2010; Baggs 2011; Butterbach-Bahl et al. 2013; Pilegaard 2013; Zhu et al. 2013). Heterotrophic nitrification might also play an important role in pasture soils, and readily degradable carbon (C) addition could stimulate heterotrophic nitrification and thereby promote N2O emissions (Lan et al. 2019).
The global consumption of fertilizer N was projected to increase at a rate of 1.4% annually from 2014 to 2018, because of meeting the growing population demand for the greater food production (FAO 2015). The extensive use of fertilizer N would greatly contribute to the apparent elevation in atmospheric N2O concentrations (Shcherbak et al. 2014). An emerging body of research shows that N fertilizer type is the key affecting factor on N2O emissions under certain O2 and moisture condition. Greater N2O emissions were reported from nitrate (NO3−)-based fertilizers than ammonium (NH4+)-based fertilizers with the high moisture and C soils (Dobbie and Smith 2003; Smith et al. 2012). However, the summary of available measurements from fertilized fields showed that (NH4+)-based fertilizers had greater N2O emissions than that of (NO3−)-based fertilizers (Bouwman et al. 2002; Li et al. 2013). It is still not clear of the effects of N forms on N2O emissions in the soils characterized with high pH, low organic C, and a strong ammonia oxidation potential, especially simultaneously combined with different C components.
Previous studies have shown that the application of readily degradable C, such as glucose, could inhibit nitrification-related N2O by reducing NH4+ availability via immobilization (Freppaz et al. 2007; Ma et al. 2016), which also depended on the availability and application rate of external C input (Granli and Bøckman 1994). But N2O production from the soil could also be stimulated by the application of readily degradable C possibly due to increasing denitrification (Wrage et al. 2001). Carbon could provide not only energy for soil microbe but also electron acceptor for denitrification (Yokoyama et al. 1992; Rivett et al. 2008; Zhu et al. 2015). The contrasting effects of readily degradable C on nitrification and denitrification might lead to discrepant results on the effect of C application on N2O emissions from the soil. The effects of different C components (with different degradable extents) on N2O emission and N transformation under aerobic condition, especially simultaneously combined applying with (NH4+) or (NO3−)-based fertilizer, are incompletely understood, and future research is urgently needed. To date, studies that combined examine the effect of C and N forms on N2O emissions are also scarce. How the interaction of C availability and N forms affect N2O emissions is still unclear.
In the present study, different forms and doses of C and N were applied to a strong ammonia oxidation soil in order to investigate the interaction of C availability and N forms affecting N2O emissions and N transformation. We hypothesized that labile C with N addition would enhance N2O emissions, and higher C/N ratio could decrease N2O emissions due to enhancing N immobilization. The findings from this study would help us to develop better C and N managements for mitigating N2O emissions in field conditions.
2 Materials and methods
2.1 Site and properties of soil samples
Soil samples were taken from a typical farmer’s field where carried out winter wheat-summer maize double cropping system in the last many years at Quzhou research station (36.87°N, 115.02°E), Hebei province, China. The site is under warm temperate and semi-arid monsoon climatic conditions. The long-term (from 1980 to 2010) annual average temperature was 13 °C, and the annual average precipitation was 494 mm. Most rainfall occurred during summer (July to September) accounting for 60% of the total rainfall in a year. The soil is a typical calcareous fluvo-aquic soil belonging to calcareous Cambisols (FAO classification system), which has low NH4+ and dissolved C but with high NO3− and pH and is characterized by a high nitrification potential (Wan et al. 2009; Ju et al. 2011; Ju and Zhang 2017). Ten soil cores (0–20 cm) were randomly taken from the field (about half hectare) after summer maize harvest in 2011 to make a composited sample; the sample was stored in an ice box before returning to the laboratory within half a day. The sampled soil was homogenized, sieved (2 mm), air-dried, and stored at room temperature (20 °C) before the incubation commencement.
The soil was light loam containing 39.34% sand, 58.25% silt, and 2.41% clay, with a filed bulk density 1.34 g cm−3, a pH of 7.72 (1:2.5, soil/water), a soil organic carbon concentration of 9.5 g kg−1, a dissolved organic C of 45.0 mg kg−1, a soil total N concentration of 0.7 g kg−1, and NH4+ and NO3− concentrations of 0.38 and 31.82 mg kg−1, respectively. The potential nitrification rate was 204.5 mg NO3−-N kg−1 day−1 and measured by the method of Hart et al. (1994).
2.2 Experimental design
Two laboratory incubation experiments were conducted. Experiment I aimed to test the effects of different C application rates with N forms on N2O emissions and N transformation. It was conducted with two N forms (ammonium sulfate, (NH4)2SO4 and calcium nitrate, Ca(NO3)2) and four levels of C (glucose) addition. Apart from CK treatment, 40 mg N kg−1 as NH4+ or NO3−, was applied with or without glucose for each treatment. The four glucose rates were 0.5, 1.0, 2.0, and 4.0 g C kg−1, respectively, and the corresponding C/N ratios of the applied C and N were 12.5, 25, 50, and 100, respectively. Each treatment had three replicates. Therefore, eight treatments plus three controls (no N or C addition and N addition only) were carried out in the Experiment I as follows:
- 1.
CK (no N and C addition, CK)
- 2.
(NH4)2SO4 (NH4+)
- 3.
(NH4)2SO4 + 0.5 g C kg−1 glucose(NH4+ + Glu.0.5)
- 4.
(NH4)2SO4 + 1.0 g C kg−1 glucose(NH4+ + Glu.1.0)
- 5.
(NH4)2SO4 + 2.0 g C kg−1 glucose (NH4+ + Glu.2.0)
- 6.
(NH4)2SO4 + 4.0 g C kg−1 glucose (NH4+ + Glu.4.0)
- 7.
Ca(NO3)2 (NO3−)
- 8.
Ca(NO3)2 + 0.5 g C kg−1 glucose (NO3−+ Glu.0.5)
- 9.
Ca(NO3)2 + 1.0 g C kg−1 glucose (NO3−+ Glu.1.0)
- 10.
Ca(NO3)2 + 2.0 g C kg−1 glucose (NO3− + Glu.2.0)
- 11.
Ca(NO3)2 + 4.0 g C kg−1 glucose (NO3−+ Glu.4.0)
Experiment II aimed to test the effects of different C types with N addition on N2O emissions and N transformation. Six C sources (glucose, pectin, starch, cellulose, lignin and wheat straw) were used with C rate of 0.5 g C kg−1. N rate was the same as experiment I as 40 mg N kg−1 of NH4+ or NO3−, respectively. Each treatment had three replicates. Therefore, 12 treatments plus three controls (no N or C addition and N addition only) were carried out in experiment II as follows:
- 1.
CK (no N and C addition, CK)
- 2.
(NH4)2SO4 (NH4+)
- 3.
(NH4)2SO4 + 0.5 g C kg−1 glucose (NH4+ + glue)
- 4.
(NH4)2SO4 + 0.5 g C kg−1 pectin (NH4+ + pectin)
- 5.
(NH4)2SO4 + 0.5 g C kg−1 starch (NH4+ + starch)
- 6.
(NH4)2SO4 + 0.5 g C kg−1 cellulose (NH4+ + cellulose)
- 7.
(NH4)2SO4 + 0.5 g C kg−1 lignin (NH4+ + lignin)
- 8.
(NH4)2SO4 + 0.5 g C kg−1 straw (NH4+ + straw)
- 9.
Ca(NO3)2 (NO3−)
- 10.
Ca(NO3)2 + 0.5 g C kg−1 glucose (NO3− + glu)
- 11.
Ca(NO3)2 + 0.5 g C kg−1 pectin (NO3− + pectin)
- 12.
Ca(NO3)2 + 0.5 g C kg−1 starch (NO3− + starch)
- 13.
Ca(NO3)2 + 0.5 g C kg−1 cellulose (NO3− + cellulose)
- 14.
Ca(NO3)2 + 0.5 g C kg−1 lignin (NO3− + lignin)
- 15.
Ca(NO3)2 + 0.5 g C kg−1 straw (NO3− + straw)
2.3 Procedure of the incubation experiments
Fresh soil samples were pre-incubated under 50% WFPS (water-filled pore space) at 20 °C for a week in the dark to stabilize the microbial community and avoid fluctuations of soil respiration in the dry-wet process. A total of 480 g (equivalent to oven-dried weight) of pre-incubated soil was placed in a glass jar (1165 mL volume with 16.3 heights and 9.5 inner diameters) and adjusted to a field bulk density of 1.34 g cm−3. The C sources like pectin, starch, cellulose, lignin, and straw were mixed directly with the soils, and the straw was dried and ground (passed through 1 mm mesh) before addition. The solutions of NH4+ or NO3−for each treatment were applied evenly to the soil in the glass jar using a syringe to achieve the target soil moisture of 70% WFPS, which was the moisture level as maximum N2O emission from the previous studies (Huang et al. 2014). Each glass jar was covered with parafilm, which allowed air exchange but prevented water loss during the incubation. Soil moisture was replenished every 4 days. All the glass jars were placed randomly and incubated for 15 days at 20 °C in the dark. There were 33 glass jars (11 treatments *3 replicates) for experiment I and 45 jars (15 treatments * 3 replicates) for experiment II for gas samplings and the last destructive soil sampling, respectively. Totally, 132 glass jars (11 treatments * 3 replicates * 4 sampling times) for experiment I, and 135 glass jars for experiment II (15 treatments * 3 replicates * 3 sampling times) were incubated for destructive soil samplings and analysis.
2.4 Gas sampling and analysis
Gas samples (20 ml) were collected on days 1, 2, 3, 5, 8, 11, and 15 for N2O and CO2 concentrations analysis. Before gas sampling, parafilm was removed, and the air was allowed to exchange with the ambient air for 5 min. Subsequently, the jars were sealed with rubber seals equipped with a three-way valve, and this time point was defined as the zero point. Gas samples were collected at 0, 10, and 20 min with syringes. Samples were analyzed by a modified gas chromatography (Agilent 6820, Santa Clara, USA) with an ECD detector for N2O and TCD detector for CO2 concentration (Zheng et al. 2008) respectively. The ECD detector was fitted with a 63Ni and operated at 350 °C. High-purity dinitrogen (99.999%) was used as the carrier gas for N2O analysis. Ten percent of CO2 in pure N2 was used as a make-up gas for ECD. The GC was calibrated using known concentrations of mixed gas (0.354 ppm N2O in pure N2). Air conditioning was used in the laboratory during measurements to minimize the tendency of the ECD to drift with changing temperature.
2.5 Soil sampling and analysis
Destructive soil sampling was carried out for soil mineral N analysis on days 0, 3, 5, and 15 for experiment I, and on days 0, 3, and 15 for experiment II. Each soil sample was extracted with 1 M KCl (soil: 1 M KCl = 1:5) to determine the concentrations of NH4+ and NO3− using a continuous flow analyzer (AA3, Seal Analytical, Norderstedt, Germany).
2.6 Calculations and statistical analysis
N2O and CO2 fluxes were calculated according to the following equation:
where F is the N2O (μg N2O-N kg−1 day−1) or CO2 (mg CO2-C kg−1 day−1) emission rate, T (°C) is the air temperature in the incubation glass jar, M is the molecular weight of N in molecular N2O (28 g mol−1) or molecular weight of C in molecular (CO2 g mol−1), 60 (min) is the number of minutes in an hour, 24 is the number of hours in a day, V (L) is the volume of the headspace, c is the concentration of N2O (nL L−1) or CO2 (μL L−1), t (min) is the time from jar sealing, dc/dt is the change in N2O (nL L−1 min−1) or CO2 (μL L−1 min−1) concentration per unit of time, 22.4 (L mol−1) is the molecular volume at 101 kPa and 273 K, and 0.48 (kg) is the weight of oven-dried soil in the jar.
Accumulated N2O and CO2 emissions during the whole incubation were derived from the sums of measurement and no-measurement days estimated by linear interpolation (Mosier et al. 2006).
Differences in accumulated N2O and CO2 emissions were analyzed by one-way analysis of variance (ANOVA) and compared by Duncan’s multiple range test at the 5% level using IBM SPSS Statistics 20; all the figures were made by Sigmaplot 12.5.
3 Results
3.1 Effects of C rates amended with ammonium or nitrate on N2O and CO2 emissions (experiment I)
3.1.1 N2O and CO2 emissions
Glucose addition significantly stimulated N2O emissions regardless of applied N forms (Fig. 1). More N2O produced after glucose addition combined with NO3− than with NH4+. N2O emissions when glucose with NH4+ form reached the peak immediately in the first incubation day but followed by a repaid decline as the incubation proceeded (Fig. 1a). The highest peak value was found in the low rate of glucose treatment (0.5 g C kg−1), while the lowest peak value was under the highest glucose rate (4 g C kg−1). N2O peak value from NH4+ + Glu.0.5 treatment was about 150 times higher than that from only NH4+-N addition treatment. However, a different trend was observed when glucose addition with NO3− form (Fig. 1b). Addition of high rate of glucose (4 g C kg−1) significantly stimulated N2O emissions on the first day and decreased afterward, but such response was not observed in lower rates (0.5, 1, and 2 g kg−1) treatments. N2O fluxes peaked on the second day of the incubation in the NO3− + Glu.0.5 and NO3− + Glu.1.0 treatments, and kept consistent during the first 2 days in NO3− + Glu.2.0 treatment. The N2O peak values followed the order NO3− + Glu.0.5 > NO3− + Glu.1.0 > NO3− + Glu.4.0 > NO3− + Glu.2.0. N2O peak from NO3− + Glu.0.5 treatment was about 235 times higher than that from only NO3−-N addition treatment. N2O flux from NO3− treatment was similar to CK treatment (from 0 to 6.7 μg N kg−1 day−1) during the incubation, suggesting there were little N2O emissions despite the presence of NO3− in the soil.
CO2 fluxes increased with the addition of glucose and NH4+ (Fig. 1c) or NO3−-forms (Fig. 1d). CO2 fluxes of NH4+ + glucose treatments showed an uptrend in the first 5 days of the incubation, then decreasing gradually afterward. CO2 flux was the highest in 2.0 g C kg−1of glucose rate and increased by about 20-fold compared with only NH4+ addition. As for CO2 fluxes in treatments with glucose plus NO3−-based fertilizer addition (Fig. 1d), glucose addition enhanced CO2 fluxes to different degrees, and the order of the CO2 peak value was NO3− + Glu.2.0 > NO3− + Glu.4.0 > NO3− + Glu.0.5 > NO3− + Glu.1.0.
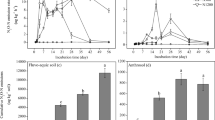
Accumulated N2O and CO2 emissions were regulated by the ratios of external C and N inputs (Fig. 2, Table A1—Electronic Supplementary Material-ESM). The accumulated N2O emission was the highest when C/N ratio was 12.5 (Fig. 2a) and decreased along with increasing C/N ratio under both N forms. The N2O emissions were significantly higher (P < 0.05) when the N source was NO3− than NH4+ with equal glucose addition in most treatments except for Glu.2.0 treatment (Table A1 – ESM). The trends of accumulated CO2 emissions (Fig. 2b) were different from N2O emissions, and it increased along with increasing C/N ratio and reached the peak when C/N was 50, and then decreased when C/N ratio was 100 in both N forms. The accumulated CO2 emissions were higher but not significantly (P > 0.05) when N form was NO3− than NH4+ with equal glucose addition in most treatments except for Glu.2.0 treatment (P < 0.05) (Table A1—ESM).
3.1.2 Dynamics of NH4+ and NO3− concentration and N balance
The concentrations of NH4+ decreased after NH4+ and glucose addition in the first 5 days of incubation, indicating high nitrification or immobilization occurred in the soil (Fig. 3a). The concentrations of NO3− generally increased under NH4+ treatment without glucose addition (Fig. 3c), but it decreased quickly to zero on the third day of the incubation in all the addition of glucose treatments regardless of how much C added. Few NH4+ was detected, and it remained consistently low (below 1 mg N kg−1) during the whole incubation in all NO3− treatments (Fig. 3b). The NO3− concentrations were relatively stable during the whole incubation in CK and NO3− treatments, indicating low processes of N transformations in the soil. Whereas with the application of glucose (Fig. 3d), the NO3− concentrations decreased sharply to zero in the first 3 days of incubation in all glucose treatments, indicating high denitrification or immobilization occurred in the soil. The increased trend of NO3− concentrations in NO3− + Glu.0.5 treatment after 3 days showed mineralization again with low dose of glucose addition.
Nitrogen balance, as seen in Table A2 (ESM), reflects soil N transformations and their relationship with N2O emissions. The positive N balance could be explained by immobilization of mineral N (NH4++NO3−) or other gaseous loss such as NH3 or N2, while the negative N balance could be explained by mineralization of organic N in the soil. Different C/N ratios due to different amount of glucose addition affected soil N mineralization and mobilization in various ways. In control (CK) and NO3− treatments, low negative N balance was observed while a low positive N balance was observed in NH4+ treatment. However, all treatments with glucose addition had relatively large positive values of N balance, demonstrating high mineral N immobilization or other gaseous losses. With the glucose addition, the N balance values of NO3− form were higher than that of NH4+form except NO3− + Glu.0.5 treatment .
3.2 Effects of C types amended with ammonium or nitrate on N2O and CO2 emissions (experiment II)
3.2.1 N2O and CO2 emissions
The highest N2O flux was found when pectin is applied with NH4+ (Fig. 4a, b); however, when N form changed to NO3−, the highest N2O was emitted from glucose treatment (Fig. 4c, d). N2O fluxes from all N treatments reached the peak on the first day of the incubation, and followed by a quick decline afterward. The accumulated N2O emissions are shown in Fig. 5a and Table A3 (ESM). During the whole incubation, total N2O emissions of pectin and glucose treatments were higher than other C treatments combined with either NH4+ or NO3−. The accumulated N2O emissions of other four C treatments followed the order NH4+ + lignin > NH4+ + starch > NH4+ + straw > NH4+ + cellulose, and NO3− + starch > NO3− + straw > NO3− + lignin > NO3− + cellulose, but the differences were not significant (P > 0.05). Accumulated N2O emissions of NO3− + cellulose were the lowest among the different C treatments combined with either NH4+ or NO3−.
N2O and CO2 fluxes of different treatments during the incubation in experiment II. Nitrogen source for (a, b, e, f) was (NH4)2SO4, for (c, d, g, h) was Ca(NO3)2. The insert panel (b, d, f, h) are shown without NH4++Glu. and NH4++Pectin to make the figure more clear; Error bars are the standard error of three replicates
The CO2 fluxes reached the peak at different incubation times after different C addition with NH4+ form (Fig. 4e, f). With the glucose addition, CO2 fluxes peaked on the first incubation day up to 175.8 mg C kg−1 day−1, and followed by a decrease trend. Whereas for other C treatments, the CO2 emissions peak occurred after 2 days and even after 11 days for the straw treatment, indicating that different C types stimulated the microbial respiration to different degrees. Cellulose and straw addition significantly (P < 0.05) increased accumulated CO2 emissions during the whole incubation (Fig. 5b; Table A3), and more CO2 produced when N form was NO3− compared to NH4+.
When applied N as NO3− form (Fig. 4g, h), the dynamic of CO2 fluxes were similar to that of NH4+ treatments. With the addition of glucose and pectin, higher CO2 fluxes were observed than other treatments especially for the first 8 days and peaked on the first day of the incubation. The CO2 emissions reached the peak in the rest of the C treatments lagged behind the treatments of NO3− + Glu and NO3− + pectin and lasted longer during the incubation. NO3− + Glu and NO3− + pectin treatments produced the highest accumulated CO2 during the incubation (Table A3). The lowest CO2 fluxes were observed in lignin treatment combined with either NH4+ or NO3−.
3.2.2 Dynamics of NH4+ and NO3− concentration and N balance
When the N forms as NH4+, the soil NH4+ concentrations decreased (Fig. 6a), and while the NO3− concentrations increased before the third day(Fig. 6c), and then slightly decreased till the end of the incubation. The concentrations of NH4+ in the NH4+ + Glu treatment was the lowest among C addition treatments as glucose might increase the immobilization of NH4+rapidly. At the end of the incubation, there were various amounts of NO3− detected in the treatments with the range of about 10 in NH4+ + Glu treatment to about 40 mg N kg−1 in NH4+ + starch treatment. When applied N as NO3− form, the NH4+ concentrations were low and had a slight uptrend at the end of the incubation probably due to mineralization (Fig. 6b). Apart from cellulose addition, NO3− concentrations decreased to different degrees among other C treatments (Fig. 6d).
When applied N form as NH4+, relatively larger values of N balance were observed with the addition of glucose, cellulose, and straw treatments (Table A4—ESM), indicating higher mineral N immobilization or other gaseous losses in these treatments. When applied N form as NO3−, the values of N balance showed positive in all treatments except the relatively high negative N balance only in NO3− + cellulose treatment, indicating enhanced mineralization with addition of cellulose.
4 Discussion
In the current incubation study, the combined application of C and N significantly enhanced N2O and CO2 emissions, albeit to varying degrees, which was consistent with other field results (Ju et al. 2011; Bao et al. 2012; Cui et al. 2012; Gao et al. 2014). Moreover, different types of C and N had different effects on increasing N2O and CO2 emissions. For example, when applied C source was pectin or glucose, more accumulated N2O were emitted than other C sources regardless of applied N forms. Several previous studies revealed that soil N2O production rate could be stimulated by adding readily degradable C, possibly due to increasing denitrification (Wang et al. 2005; Cheng et al. 2012; Ameloot et al. 2016; Mehnaz et al. 2018). In our study, NO3− concentrations sharply decreased after C and N addition indicating denitrification occurrence or immobilization. C addition could greatly enhance O2 consumption and microorganism respiration in the soil, where generated anaerobic microsites which was favorable for denitrification (Huang et al. 2014; Song et al. 2019). We did not think that DNRA could occur because the NH4+ concentration did not increase at the end of the incubation in both experiments. However, the immobilization of NO3− is actually a possible process of NO3− decrease. A meta-analysis also showed that specific exogenous organic C input at a high rate (> 0.5 g C kg−1) or with a high C/N ratio (> 18) could enhance microbial NO3− immobilization (Cheng et al. 2017).
Our results indicated that the soils treated with high concentrations (> 0.5 g C kg−1) of glucose produced lower N2O than NH4+ + Glu 0.5 during the incubation (Table A1—ESM, Fig. 1). It is possibly because glucose was easy to be used by soil microorganism, and high concentrations might stimulate the growth and activity of heterotrophic microorganisms (Schimel and Bennett 2004; Booth et al. 2005), thus assimilation of mineral N (Burger and Jackson 2003; Myrold and Posavatz 2007), which reduced the NH4+ substrate for nitrification and then reduced related N2O production as well. And it was also reflected by higher CO2 production in this treatment (Table A1). Another reason for lower amounts of N2O emissions from high application rate of glucose (> 0.5 g kg−1) than NH4+ + Glu 0.5 was that it might stimulate the anaerobic conditions which enhanced the activity of the enzyme responsible for reduction of N2O to N2 (Murray et al. 2004; Kool et al. 2010).
NH4+ and NO3−- N forms are two main N fertilizer types in crop production, which are also substrates for nitrification and denitrification respectively, and have various effects on N2O emissions from different soil types. Bouwman et al. (2002) reported that more N2O was emitted when applied NO3− than NH4+-based fertilizers from New Zealand and Germany grassland soil due to fine texture and high clay content of the soil. However, in the present study, we found opposite results that application of NH4+-based fertilizer alone produced more N2O than NO3−-based fertilizer due to high nitrification capacity of our studied soil (Ding et al. 2015; Ju and Zhang 2017); NH4+-based fertilizer could be immediately proceeded by nitrifiers and produce large amount of nitrification-related N2O (Wan et al. 2009; Ju et al. 2011; Ju and Zhang 2017; Zhu et al. 2019a, b).
Different C types had various effects on N2O and CO2 emissions, indicating different interactions between C and N with respect to N2O and CO2 production. In our study, the application of glucose and pectin with NH4+ or NO3− resulted in the highest N2O emissions (Table A3—ESM, Fig. 6). Probably because they were more readily degradable C to stimulate growth of soil microorganisms. In contrast, complex C compounds like straw had low ability of NO3− immobilization and produced lower N2O and CO2 than glucose and pectin addition, which was in line with previous studies (Miller et al. 2008; Huang et al. 2013; Qiu et al. 2013). The soluble C types like glucose and pectin were more efficiently used by soil microorganisms in a soil with pH 7.4 under anaerobic condition (de Catanzaro and Beauchamp 1985). Although our incubation condition was aerobic, the C addition might enhance O2 consumption and respiration in soil matrix, which could create anaerobic condition (Song et al. 2019).
The CO2 fluxes and its duration are mainly related with the structure and composition of various organic materials. In our study, the straw treatment combined with NH4+ or NO3− produced small amount of CO2 in the first few days but showed sustained production over time as the straw contained much lower soil labile C and required a longer decomposition time. The limited available C and aerobic condition might inhibit denitrification, resulting in less N2O emissions (Table A3—ESM) and higher final NO3− (Fig. 6) concentrations in NO3− + straw treatment. Only readily available C sources (e.g., glucose and pectin) together with either NH4+ or NO3− stimulated N2O emissions. Therefore, from our current study under the aerobic conditions, the possible mitigation strategies of N2O emission would be avoiding the combination addition of N fertilizers and easily accessible C.
Soil NH4+ and NO3− were involved in many N transformation pathways, including nitrification, denitrification, N immobilization, and mineralization. Nitrification and denitrification depended on NH4+ and NO3− concentrations and O2 status, and soil N mineralization and immobilization were related to the C/N ratio of added substrate (Larsen and McCartney et al. 2000). Higher C/N ratio (27) led to a strong immobilization of N, while lower C/N ratio (15–17) led to mineralization (Bengtsson et al. 2003), which explained soil NH4+ kinetics in the present study (Fig. 2, Table A2—ESM). Moreover, C types could also affect N transformations, and the organic materials with higher C/N ratio (45.5) could have a slower decomposition rate than that with lower C/N ratio (18.8), which stimulated N immobilization (Trinsoutrot et al. 2000; Moritsuka et al. 2004). The immobilization was greater in soils incubated with glucose amendments than those incubated with more complex C compounds (Magill and Aber 2000). The returned C sources in the field are crop roots, straw, manure or compost, which are composed with different C components. As we did the model experiments in laboratory, we are interested in the effects of different C components on N transformations and N2O emissions. The results would help us understand the interactions of C and N when applying the integrates of complex OM in situ.
Previous studies regarding application of C and N rates and types on N2O emissions were almost all under strict anaerobic condition, but our study was aerobic which would be a better representation of real field conditions in upland soils (Ju and Zhang 2017). We still have no direct evidences to show nitrifier denitrification or denitrification in our current treatments (Yang et al. 2017), but measuring O2 concentration in soil microsites might be helpful to further explain the mechanisms of N2O production and differentiate N2O processes in the soil.
5 Conclusions
We observed from the incubation study that the application of mixed C and N substrates enhanced N2O and CO2 emissions albeit to different extents. A higher accumulated N2O emission was found when C/N ratio was lower (12.5). NO3− together with glucose treatments produced more N2O than NH4+-treated soils. The stimulating effect of glucose and pectin as labile C sources with N addition on N2O production should be considered, especially in soils with high pH, low organic C, and a strong ammonia oxidation potential. Under field conditions, therefore, avoiding applying both N and C especially rich in glucose and pectin together could be the suitable targeted strategy for mitigating N2O emissions.
References
Ameloot N, Maenhout P, De Neve S, Sleutel S (2016) Biochar-induced N2O emission reductions after field incorporation in a loam soil. Geoderma 267:10–16
Baggs EM (2011) Soil microbial sources of nitrous oxide: recent advances in knowledge, emerging challenges and future direction. Curr Opin Env Sust 3:321–327
Bao QL, Ju XT, Gao B, Qu Z, Christie P, Lu YH (2012) Response of nitrous oxide and corresponding bacteria to managements in an agricultural soil. Soil Sci Soc Am J 76:130–141
Bengtsson G, Bengtson P, Mansson KF (2003) Gross nitrogen mineralization-, immobilization-, and nitrification rates as a function of soil C/N ratio and microbial activity. Soil Biol Biochem 35:143–154
Booth MS, Stark JM, Rastetter E (2005) Controls on nitrogen cycling in terrestrial ecosystems: a synthetic analysis of literature data. Ecol Monogr 75:139–157
Bouwman AF, Boumans LJM, Batjes NH (2002) Emissions of N2O and NO from fertilized fields: summary of available measurement data. Glob Biogeochem Cycles 16:1058
Burger M, Jackson LE (2003) Microbial immobilization of ammonium and nitrate in relation to ammonification and nitrification rates in organic and conventional cropping systems. Soil Biol Biochem 35:29–36
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Phil Trans R Soc B 368:20130122
Cheng Y, Cai ZC, Chang SX, Wang J, Zhang JB (2012) Wheat straw and its biochar have contrasting effects on inorganic N retention and N2O production in a cultivated black Chernozem. Biol Fert Soils 48:941–946
Cheng Y, Wang J, Wang JY, Chang SX, Wang SQ (2017) The quality and quantity of exogenous organic carbon input control microbial NO3− immobilization: a meta-analysis. Soil Biol Biochem 115:357–363
Cui F, Yan G, Zhou Z, Zheng X, Deng J (2012) Annual emissions of nitrous oxide and nitric oxide from a wheat–maize cropping system on a silt loam calcareous soil in the North China plain. Soil Biol Biochem 48:10–19
De Catanzaro, Beauchamp EG (1985) The effect of some carbon substrates on denitrification rates and carbon utilization in soil. Biol Fert Soils 1:183–187
Ding WX, Chen ZM, Yu HY, Luo JF, Yoo GY, Xiang J, Zhang HJ, Yuan JJ (2015) Nitrous oxide emission and nitrogen use efficiency in response to nitrophosphate, N-(n-butyl) thiophosphoric triamide and dicyandiamide of a wheat cultivated soil under sub-humid monsoon conditions. Biogeosciences 12:803–815
Dobbie KE, Smith KA (2003) Impact of different forms of N fertilizer on N2O emissions from intensive grassland. Nutr Cycl Agroecosyst 67:37–46
Drawing Down N2O to Protect Climate and the Ozone Layer (2013) A UNEP (United Nations Environment Programme) synthesis report. Nairobi, Kenya
FAO (2015) Save Food: Global Initiative on Food Loss and Waste Reduction. Food and Agriculture Organization of the United Nations (FAO), Vialedelle Terme di Caracalla 00153, Rome, Italy
Freppaz M, Williams BL, Edwards AC, Scalenghe R, Zanini E (2007) Labile nitrogen, carbon, and phosphorus pools and nitrogen mineralization and immobilization rates at low temperatures in seasonally snow-covered soils. Bio Fert Soils 43:519–529
Gao B, Ju X, Su F, Meng Q, Oenema O, Christie P, Chen X, Zhang F (2014) Nitrous oxide and methane emissions from optimized and alternative cereal cropping systems on the North China plain: a two-year field study. Sci Total Environ 472:112–124
Granli T, Bøckman OC (1994) Nitrous oxide from agriculture. Norwegian J of Agr Sci 12:1–128
Hart SC, Stark JM, Davidson EA, Firestone MK (1994) Nitrogen mineralization, immobilization and nitrification. In: Weaver RW et al (eds) Methods of soil analysis, part 2 microbiological and biochemical properties. Soil Science Society of America, Madison, pp 985–1018
Huang T, Gao B, Christie P, Ju XT (2013) Net global warming potential and greenhouse intensity in a double-cropping cereal rotation as affected by nitrogen and straw management. Biogeosciences 10:7897–7911
Huang T, Gao B, Hu XK, Lu X, Well R, Christie P, Bakken LR, Ju XT (2014) Ammonia-oxidation as an engine to generate nitrous oxide in an intensively managed calcareous fluvo-aquic soil. Sci Rep 4:3950
IPCC (2007) Climate change 2007: the physical science basis. In: Solomon S, Qin D, Manning M (eds) Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
IPCC (2013) Climate change 2013: The physical science basis. Stocker T F, Qin D, Plattner G K, Tignor M, Allen S K, Boschung J, Nauels A, Xia Y, Bex V and Midgley P M (eds) Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, United Kingdom and New York
Ju XT, Zhang C (2017) Nitrogen cycling and environmental impacts in upland agricultural soils in North China: a review. J Integr Agr 16:2848–2862
Ju X, Lu X, Gao Z, Chen X, Su F, Kogge M, Romheld V, Christie P, Zhang F (2011) Processes and factors controlling N(2)O production in an intensively managed low carbon calcareous soil under sub-humid monsoon conditions. Environ Pollut 159:1007–1016
Kool DM, Wrage N, Zechmeister-Boltenstern S, Pfeffer M, Brus D, Oenema O, Van Groenigen JW (2010) Nitrifier denitrification can be a source of N2O from soil: a revised approach to the dual-isotope labelling method. Eur J Soil Sci 61:759–772
Lan T, Liu R, Suter H, Deng O, Gao X, Luo L, Yuan S, Wang CQ, Chen DL (2019) Stimulation of heterotrophic nitrification and N2O production, inhibition of autotrophic nitrification in soil by adding readily degradable carbon. J Soils Sediment:1–10. https://doi.org/10.1007/s11368-019-02417-0
Larsen KL, McCartney DM (2000) Effect of C : N ratio on microbial activity and N retention: bench-scale study using pulp and paper biosolids. Compost Sci Util 8:147–159. https://doi.org/10.1080/1065657X.2000.10701760
Li D, Watson CJ, Ming JI, Lalor S, Rafique R, Hyde B, Lanigan G, Richards K, Holden NM, Humphreys J (2013) A review of nitrous oxide mitigation by arm nitrogen management in temperate grassland-based agriculture. J Environ Manag 128:893–903
Ma Q, Wu Z, Pan F, Wang J, Zhou H, Jiang C, Xu YG, Yu WT (2016) Effect of glucose addition on the fate of urea– 15N in fixed ammonium and soil microbial biomass N pools. Eur J Soil Biol 75:168–173
Magill AH, Aber JD (2000) Variation in soil net mineralization rates with dissolved organic carbon additions. Soil Biol Biochem 32:597–601
Mehnaz KR, Keitel C, Dijkstra FA (2018) Effects of carbon and phosphorus addition on microbial respiration, N2O emission, and gross nitrogen mineralization in a phosphorus-limited grassland soil. Bio Fert Soils 54:481–493
Miller MN, Zebarth BJ, Dandie CE, Burton DL, Goyer C, Trevors JT (2008) Crop residue influence on denitrification, N2O emissions and denitrifier community abundance in soil. Soil Bio Biochem 40:2553–2562
Moritsuka N, Yanai J, Mori K, Kosaki T (2004) Biotic and abiotic processes of nitrogen immobilization in the soil-residue interface. Soil Biol Biochem 36:1141–1148
Mosier AR, Halvorson AD, Reule CA, Liu XJ (2006) Net global warming potential and greenhouse gas intensity in irrigated cropping systems in northeastern Colorado. J Environ Qual 35:1584–1598
Murray PJ, Hatch DJ, Dixon ER, Stevens RJ, Laughlin RJ, Jarvis SC (2004) Denitrification potential in a grassland subsoil: effect of carbon substrates. Soil Biol Biochem 36:545–547
Myrold DD, Posavatz NR (2007) Potential importance of bacteria and fungi in nitrate assimilation in soil. Soil Biol Biochem 39:1737–1743
Pilegaard K (2013) Processes regulating nitric oxide emissions from soils. Philos T R Soc B 368:20130126
Qiu SJ, Ju XT, Ingwersen J, Guo ZD, Stange CF, Bisharat R, Streck T, Christie P, Zhang FS (2013) Role of carbon substrates added in the transformation of surplus nitrate to organic nitrogen in a calcareous soil. Pedosphere 23:205–212
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125
Rivett MO, Buss SR, Morgan P (2008) Nitrate attenuation in groundwater: a review of biogeochemical controlling process. Water Res 42:4215–4232
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Shcherbak I, Millar N, Robertson GP (2014) Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc Natl Acad Sci USA 111:9199–9204
Smith KA, Dobbie KE, Thorman R, Watson CJ, Chadwick DR, Yamulki S, Ball BC (2012) The effect of N fertiliser forms on nitrous oxide emissions from UK arable land and grassland. Nutr Cycl Agroecosyst 93:127–149
Song XT, Ju XT, Topp CFE, Rees RM (2019) Oxygen regulates nitrous oxide production directly in agricultural soils. Environ Sci Technol 53:12539–12547
Trinsoutrot I, Recous S, Bentz B, Lineres M, Cheneby D, Nicolardot B (2000) Biochemical quality of crop residues and carbon and nitrogen mineralization kinetics under nonlimiting nitrogen conditions. Soil Sci Soc Am J 64:918–926
Wan Y, Ju X, Ingwersen J, Schwarz U, Stange CF, Zhang F, Streck T (2009) Gross nitrogen transformations and related nitrous oxide emissions in an intensively used calcareous soil. Sci Soc Am J 73:102
Wang LF, Cai ZC, Yang LF, Meng L (2005) Effects of disturbance and glucose addition on nitrousoxide and carbon dioxide emissions from a paddy soil. Soil Till Res 82:185–194
Wrage N, Velthof GL, van Beusichem ML, Oenema O (2001) Role of nitrifier denitrification in the production of nitrous oxide. Soil Bio Biochem 33:1723–1732
Wrage N, van Groenigen JW, Oenema O, Baggs EM (2005) A novel dual-isotope labelling method for distinguishing between soil sources of N2O. Rapid Commun Mass Sp 19:3298–3306
Yang L, Zhang X, Ju X (2017) Linkage between N2O emission and functional gene abundance in an intensively managed calcareous fluvo-aquic soil. Sci Rep 7:43283
Yokoyama K, Kai H, Naklang K (1992) Heterotrophic nitrification by sodium chloride-tolerant fungi in soils added with sodium-chloride. Soil Sci Plant Nutr 38:757–762
Zheng XH, Mei BL, Wang YH, Xie BH, Wang YS, Dong HB, Xu H, Chen GX, Cai ZC, Yue J, Gu JX, Su F, Zou JW, Zhu JG (2008) Quantification of N2O fluxes from soil-plant systems may be biased by the applied gas chromatograph methodology. Plant Soil 311:211–234
Zhu X, Burger M, Doane TA, Horwath WR (2013) Ammonia oxidation pathways and nitrifier denitrification are significant sources of N2O and NO under low oxygen availability. Proc Natl Acad Sci U S A 110:6328–6333
Zhu T, Meng T, Zhang J, Zhong W, Mueller C, Cai Z (2015) Fungi-dominant heterotrophic nitrification in a subtropical forest soil of China. J Soils Sediments 15:705–709
Zhu GD, Zhang JB, Müller C, Rees RM, Thorman RE, Sylvester-Bradley R (2019a) Effects of the nitrification inhibitor DMPP (3,4-dimrthylpyrazole phosphate) on gross N transformation rates and N2O emissions. Biol Fert Soils 55:603–615
Zhu GD, Song XT, Ju XT, Zhang JB, Christoph M, Sylvester-Bradley R, Thorman RE, Bingham I, Rees RM (2019b) Gross N transformation rates and related N2O emissions in Chinese and UK agricultural soils. Sci Total Environ 666:176–186
Funding
This work was supported by the National Natural Science Foundation of China (41830751, 41807036) and the National Key Research and Development Program of China (2017YFD0200105).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zucong Cai
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary material
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Yang, L., Liu, R. & Ju, X. Effect of carbon rate and type amended with ammonium or nitrate on nitrous oxide emissions in a strong ammonia oxidation soil. J Soils Sediments 20, 1253–1263 (2020). https://doi.org/10.1007/s11368-019-02524-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-019-02524-y