Abstract
Purpose
The aim of this study was to compare organic and inorganic amendments namely citric acid, ammonium nitrate, compost, and titanium dioxide nanoparticles (TiO2 NPs) with ethylenediaminetetraacetic acid (EDTA) in enhancing the bioavailability of cadmium (Cd) in assisted phytoextraction.
Materials and methods
Uncontaminated soil was spiked with different levels of Cd (25–150 mg kg−1) by using CdSO4 salt. Different levels of five amendments used were as follows: EDTA (0–5 mmol kg−1), citric acid (0–10 mmol kg−1), ammonium nitrate (0–10 mmol kg−1), TiO2 NPs (0–100 mg kg−1), and compost (0–10%). Pelargonium hortorum plants were grown on amended soils for a period of 6 months and different parameter were considered to evaluate bioavailability of Cd upon application of amendments.
Results and discussion
The bioavailability of Cd in soil was increased by 1.2-, 0.8-, 0.4-, and 0.2-fold in EDTA, citric acid, ammonium nitrate, and TiO2 NPs, respectively. However, Cd bioavailability was decreased by 0.5-fold in compost-amended soils. The efficiency of amendments for mobilizing Cd followed the order: EDTA > citric acid > ammonium nitrate > TiO2 NPs > compost. The maximum accumulations of Cd in root (350 mg Cd kg−1) and shoot (943 mg Cd kg−1) were observed upon EDTA application and minimum concentrations in root (125 mg Cd kg−1) and shoot (104 mg Cd kg−1) were observed in compost-amended soils. The maximum (2.7 g) and minimum (1.5 g) plant biomass was observed upon compost and EDTA application, respectively. The maximum Cd uptake per plant (1.44 mg) and metal extraction ratio (4.4%) were observed in citric acid-amended soils due to better biomass produced.
Conclusions
Among all amendments, citric acid can be recommended as an environmentally friendly and effective substitute to EDTA for assisted phytoextraction of Cd to decontaminate polluted soil as Cd uptake per plant and metal extraction ratio were the highest upon application of citric acid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cadmium (Cd) is naturally found trace element and is being introduced in environment through various natural and anthropogenic activities (Khan et al. 2015; Gul et al. 2018a). Only 10% of Cd contamination is through natural activities and the remaining 90% is contributed by human activities (Khan et al. 2017). Among anthropogenic activities, fossil fuel combustion, phosphate fertilizers, industrial and municipal waste, metal smelting, and zinc mining (Guo et al. 2017; Abbas et al. 2018) have been the main sources of Cd contamination. Unlike organics, Cd is persistent, toxic, and non-biodegradable (Chhajro et al. 2016; Guo et al. 2017; Gul et al. 2018b), and high concentrations affect soil microorganisms, plant, and ultimately human through food chain (Li et al. 2016). Keeping in view the adverse effects of Cd on environment and human health, the remediation of Cd contaminated soil is required.
Various soil remediation techniques have been developed, but phytoremediation is accepted by public due to its aesthetic values and is also a cost-effective, environment-friendly technique (Souza et al. 2017). The success and efficiency of phytoremediation mainly depend on (a) the ability of plant to uptake metals from soil and translocate to shoot and (b) availability of metals in soil for plant uptake (Dede and Ozdemir 2016; Gul et al. 2018a). Various Pelargonium cultivars have inherent ability to accumulate high amount of lead (Pb) (Arshad et al. 2008, 2016; Manzoor et al. 2018) and Cd (Gul et al. 2018a, b) in shoot. Among all cultivars, Pelargonium hortorum is considered as Pb and Cd hyperaccumulator (Gul et al. 2018b). P. hortorum is scented geranium belonging to family Geraniaceae. It normally grows in areas with low precipitation and humidity and requires well-drained soil. P. hortorum has the capability to uptake soluble Cd through roots and translocate to shoots (Gul et al. 2018a, b). Therefore, bioavailability of metals is a serious limitation of phytoremediation. Metals are in insoluble form and they are not readily available for plant uptake. Chelating agents such as ethylenediaminetetraacetic acid (EDTA), nitrilotriacetate (NTA), diethylenetriaminepentaacetic acid (DTPA), N-hydroxy ethylenediaminetriacetic acid (HEDTA), ethylenediaminedisuccinate (EDDS), oxalic acid, and citric acid have been used to enhance the mobility of metals (Sarkar et al. 2008; Freitas et al. 2014; Shahid et al. 2014; Pedron et al. 2014; Gul et al. 2018a, b).
Most studies on chelate-induced phytoextraction have focused on the use of EDTA as a chelating agent. EDTA and formed complexes have low biodegradability and high solubility in soil, resulting in high risk of adverse environmental effects due to metal mobilization (Alkorta et al. 2004). In this respect, the amount of Cd taken up by plants has been reported to be much smaller than the amount of Cd mobilized from the soil during EDTA-induced Cd phytoextraction (Chen et al. 2004). Different researchers have shown that addition of EDTA increases the accumulation of Cd and other heavy metals in plants but the adverse effects of EDTA to soil enzymatic activates, soil microbes, and plants have also been reported (Epelde et al. 2008; Muhlbachova 2011; Johnson et al. 2010). Chen et al. (2004) reported that 3.5 and 20.6% of soil Pb and Cd, respectively, were leached from the soil after the application of 5 mmol kg−1 of EDTA.
EDTA degrades very slowly and remains in soil after 5 to 6 months of application (Neugschwandtner et al. 2012). Therefore, the substitute of EDTA in enhancing the phytoextraction of Cd is required. Various other soil amendments such as citric acid, compost, and ammonium nitrate are commonly used to enhance the crop growth and yield. It is also reported that these amendments are helpful in the uptake of metals from the contaminated sites (Chen et al. 2017; Gul et al. 2018a). Recently, researchers focused on the use of TiO2 NPs to enhance the nutrient uptake and improve crop yield (Zahra et al. 2017; Rizwan et al. 2019). But the impact of TiO2 NPs on the mobility of heavy metals is not studied in comparison to different amendments.
Therefore, the present study was designed to compare different organic and inorganic amendments with the commonly used non-biodegradable chelating agent, i.e., EDTA, for increasing Cd bioavailability and uptake by P. hortorum. The specific objectives were to (a) compare the effectiveness of organic and inorganic amendments on the mobility of Cd in soil and (b) compare soil amendments for enhanced accumulation of Cd by P. hortorum.
2 Materials and methods
2.1 Soil preparation and analysis
Uncontaminated surface soil (0–15 cm) of clay loam texture having pH 7.39, electrical conductivity 0.33 mS/cm, and organic matter 0.38% was collected from the nursery area of National University of Sciences and Technology (NUST; 33.6426° N, 72.9929° E), Islamabad, Pakistan. Detailed characteristics are presented in Table S1 (ESM). The soil did not contain Cd. Area falls in semiarid climatic zone with moderate winters and summer. Soil of the area is derived from wind deposits and sedimentary rocks. Soil samples were air-dried, crushed, and sieved to remove undecomposed organic materials and rocks and stored in containers. All containers were properly labeled and soil was spiked with four levels of Cd (25, 50, 100, 150 mg kg−1) by using cadmium sulfate (CdSO4) solution. Soil was mixed thoroughly at regular intervals for homogenization and after metal stabilization; soil was used for further experiments.
2.2 Soil amendments
Different organic and inorganic soil amendments such as citric acid, compost, ammonium nitrate, and TiO2 NPs were tested and compared with EDTA to increase the bioavailability of Cd. For the experimental work, different doses of EDTA (0–5 mmol kg−1), citric acid (0–10 mmol kg−1), ammonium nitrate (0–10 mmol kg−1), TiO2 NPs (0–100 mg kg−1), and compost (0–10%) were used and different controls (without Cd, without Cd with addition of amendments, with Cd without addition of any amendments) were maintained for the comparisons.
2.3 Titania nanoparticle preparation and characterization
Titania nanoparticles were prepared by using titanium isopropoxide (C12H28O4Ti), ethanol (C2H6O), distilled water, and hydrochloric acid (HCl) in a ratio 1:15:60:0.2 (Mehrizad et al. 2009). All the chemicals were added in water and stirred for 48 h at room temperature on hot plate. After stirring, solutions were kept in an oven at 90 °C for 48 h and golden yellow crystals were obtained. The crystals were powdered and calcinated at 400 °C for 2 h in muffle furnace (NEYO M-525 SERIES II). The crystallite phase and size of the prepared TiO2 NPs were identified using X-ray diffractometer (XRD). The TiO2 NPs were in anatase phase having average crystalline size of 13 nm. Scanning electron microscopy (SEM; JSM 6490 A, Jeol Ltd., Japan) was used to determine the particle size and surface morphology. Average particle size of TiO2 NPs was 26.5 nm and the particles were spherical in shape (Fig. S1, Electronic Supplementary Material (ESM)).
2.4 Incubation experiments and soil extraction
Incubation experiments were carried out to evaluate changes in solubility of Cd in soil by the addition of different organic and inorganic amendments. Briefly, 10 g of spiked soil (60% WHC) was taken in acid-cleaned petri dishes, and specified amounts of EDTA, citric acid, ammonium nitrate, TiO2 NP solution, and solid compost were added separately. For the control, 3 mL of distilled water was added and all treatments were incubated for 7 days at 28 °C. After incubation, samples were dried overnight in the oven at 65 °C (Shazia et al. 2014). The soluble fraction of Cd was extracted by taking 10 g of sample in a flask and 50 mL of 0.01 M CaCl2 solution (1:5) and shaken for 2 h at 200 rpm. After shaking, the samples were filtered through Whatman filter paper no. 42 and filtrate was analyzed for Cd concentration by atomic absorption spectrophotometer (AAS, PerkinElmer 900T).
2.5 Culture experiments for phyto-accumulation of Cd in amended soils
Pot experiments were carried out to investigate the impact of amendments on Cd accumulation in P. hortorum. Pots were filled with 1 kg of Cd spiked and control (without Cd) soils. One-month-old seedlings of P. hortorum of approximately equal size were transferred to each pot. After 1 month of seedling transplantation, EDTA, citric acid, ammonia nitrate, and TiO2 NPs and compost were applied. The experiments were carried out in greenhouse, illuminated with natural light, and pots were watered regularly to maintain ambient moisture level. The average temperature and humidity for the experimental period were 22.6 °C and 63%, respectively. The maximum and minimum temperature was observed for the months of April and January, respectively. Maximum and minimum humidity was observed in the month of February and November, respectively. Plants were grown for the period of 6 months, and after this exposure time, they were carefully harvested.
2.6 Plant harvesting and analysis
Plants were removed, washed with 0.01 M HCl solution, and rinsed several times with distilled water to remove any external Cd. For fresh biomass, plants were divided in root and shoot, and fresh biomass was measured. After that, plants were dried in the oven at 65 °C for 48 h and dry biomass was measured. For the analysis of Cd, the root and shoot were digested in a mixture of HNO3 and HClO4 (3:1) and Cd content was determined through an AAS (PerkinElmer 900T).
2.7 Cd uptake, metal extraction ratio, and translocation factor
Uptake of Cd by plant is the total amount of Cd accumulated in whole plant, and Cd metal extraction ratio (MER) is the accumulation of Cd in shoot to that of soil. Translocation factor (TF) is the transfer of Cd from the root to shoot. These are very important parameters in the phytoextraction. Both parameters were calculated by using following formulae:
where Cdshoot and Cdroot are the concentrations of Cd in shoot and root, respectively, and SDW and RDW are the dry weights of shoot and root, respectively.
where Cdshoot and Cdsoil are the concentrations of Cd in shoot and soil and Wshoot and Wsoil are the dry weights of shoot and soil, respectively (Mertens et al. 2005).
where Cdshoot and Cdroot are the Cd concentrations in shoot and root, respectively (Zahedifar et al. 2016).
2.8 Statistical analysis
Completely randomized design (CRD) with three replicates for each treatment was used for experimental setup. Analysis of variance (ANOVA) was performed by Statistix 10.0 software followed by Tukey’s post hoc test between the means of treatments for significance difference (P < 0.05).
3 Results
3.1 Influence of amendments on bioavailability of Cd in soil
Efficiencies of all the soil amendments for Cd release were different (Table 1). The application of amendments enhanced the bioavailability of Cd in soil except compost which showed a decrease in the bioavailability of Cd as compared to the control. Without application of any amendment, maximum 11.22 mg Cd kg−1 was observed in 150 mg Cd kg¯1 treatment. The application of amendments improved the bioavailability of Cd in soil. The maximum bioavailable portion of Cd was observed upon 5 mmol kg˗1 EDTA application. Bioavailability of Cd in 150 mg Cd kg−1 treatment was significantly (P < 0.05) increased by 1.2-, 0.8-, 0.4-, and 0.2-fold at 5 mmol kg−1 EDTA, 10 mmol kg−1 citric acid, 10 mmol kg−1 ammonium nitrate, and 100 mg kg−1 TiO2 NP application, respectively, as compared to treatment without application of amendments. However, the application of 10% compost significantly reduced Cd bioavailability by 0.5-fold. The efficiency of amendments in increasing the mobility of Cd in soil followed the sequence EDTA > citric acid > ammonium nitrate > TiO2 NPs > compost.
3.2 Phyto-accumulation of Cd upon soil amendment application
3.2.1 Cd concentration in shoot
P. hortorum accumulated 38.1% Cd in shoot at 150 mg Cd kg−1 treatment as compared to 25 mg Cd kg−1 without application of amendments (Fig. 1). The application of amendments increased Cd concentration in shoot except for compost which reduced the accumulation. At 150 mg Cd kg−1 treatment, Cd accumulation in shoot was increased by 3.9-, 2.4-, 1.0-, and 0.2-fold, upon 4 mmol kg−1 EDTA, 10 mmol kg−1 citric acid, 10 mmol kg−1 ammonium nitrate, and 100 mg kg−1 TiO2 NPs, respectively, as compared to 25 mg Cd kg−1 treatment without the application of amendments. However, application of 10% compost significantly (P < 0.05) decreased Cd concentration in shoot by 0.3-fold (Fig. 1). The maximum accumulation of Cd in shoot of P. hortorum was observed at EDTA application followed by citric acid, ammonium nitrate, and TiO2 NPs. In comparison with EDTA, Cd accumulation was significantly reduced in citric acid (31.6%), ammonium nitrate (59.7%), TiO2 NPs (70.3%), and compost (86.7%) -amended soils.
3.2.2 Cd concentration in root
Figure 2 illustrates the concentration of Cd in roots. P. hortorum has the ability to accumulate Cd in roots without addition of any amendment, and concentration of Cd in roots increased with increasing levels of Cd. P. hortorum accumulated 67.6% more Cd in roots at 150 mg Cd kg−1 treatment as compared to 25 mg Cd kg−1 without application of amendments. The increase in Cd accumulation in roots of P. hortorum was observed upon amendment application. P. hortorum showed 2.3-, 1.5-, 1.0-, and 0.9-fold increase in Cd accumulation in root upon application of 4 mmol kg−1 EDTA, 10 mmol kg−1 citric acid, 10 mmol kg−1 ammonium nitrate, and 100 mg kg−1 TiO2 NPs, respectively, at 150 mg Cd kg−1 treatment as compared to plant grown in 25 mg Cd kg−1 treatment without the application of amendments. On the other hand, addition of 10% compost decreased Cd accumulation by 0.92-fold. The efficiency of all amendments for Cd accumulation in roots follows the sequence: EDTA > citric acid > ammonium nitrate > TiO2 NPs > compost.
3.2.3 Plant biomass
Dry plant biomass of P. hortorum decreased with increasing levels of Cd without application of amendments (Table 2). The maximum dry plant biomass was observed in control group. At 150 mg Cd kg−1 treatment, the biomass was decreased by 22.8%. Upon application of soil amendments, the dry plant biomass of P. hortorum decreased with increasing levels of EDTA. However, for the other amendments, the plant dry biomass increased as the levels of amendments increased in control soil (without Cd). Addition of 5 mmol kg−1 EDTA in control group decreased the dry plant biomass by 62%. However, the application of maximum level of citric acid, ammonium nitrate, TiO2 NPs, and compost increased the dry plant biomass by 10, 11, 12, and 14%, respectively. By comparing combined effects of Cd treatments and amendments, the dry plant biomass was significantly decreased by 46.1, 15.1, 11.9, 5.6, and 4.9% upon the application of maximum level of EDTA, citric acid, ammonium nitrate, TiO2 NPs, and compost, respectively, at 150 mg Cd kg−1 as compared to control (without Cd and amendments).
3.2.4 Cadmium uptake per plant
Cd uptake by P. hortorum in amendment-mediated soil is shown in Fig. 3. Without the application of soil amendments, the maximum (0.57 mg) and minimum (0.48 mg) Cd uptake was observed in 100 and 25 mg Cd kg−1 treatment, respectively. The application of amendments increased Cd uptake with increasing levels except compost which showed decreasing trend. Among all amendments, the maximum Cd uptake was observed in citric acid-amended soil followed by EDTA, ammonium nitrate, and TiO2 NPs. The Cd uptake in citric acid-, EDTA-, ammonium nitrate-, and TiO2 NP-amended soils was increased by 2-, 1.9-, 0.9-, and 0.5-fold, respectively, at the 150-mg Cd kg−1 treatment as compared to 25 mg Cd kg−1 level without application of amendments. On the other hand, the addition of compost reduced Cd uptake by 0.3-fold.
3.2.5 Metal extraction ratio
The metal extraction ratio of P. hortorum grown in Cd treatment without the application of amendments decreased with increasing levels of Cd (Fig. 4). The maximum MER of 1.8% was observed in 25 mg Cd kg−1 treatment without the application of amendments. The application of EDTA, citric acid, ammonium nitrate, and TiO2 NPs increased MER as the dose of amendments increased at same Cd treatment level. The maximum MER of 4.4% was observed upon 10 mmol kg−1 citric acid application in 25 mg Cd kg−1 treatment followed by EDTA (4.3%), ammonium nitrate (3.1%), TiO2 NPs (2.2%), and compost (0.8%). The MER of P. hortorum decreased with increasing levels of Cd and amendments. The MER of P. hortorum was decreased by 46, 50, 72, 75, and 88% at 150 mg Cd kg−1 upon application of higher levels of EDTA, citric acid, ammonium nitrate, TiO2 NPs, and compost, respectively.
3.2.6 Translocation factor
Table 3 illustrates the TF of P. hortorum upon amendment application in Cd-spiked soils. TF of P. hortorum was > 1 in Cd-spiked soils without amendment application. In the present study, with increasing levels of Cd (from 25 to 150 mg kg−1), TF decreased, but at all levels, it was > 1. The application of soil amendments increased the TF, and with increasing levels of amendments and Cd, transfer of Cd from root to shoot increased further. The maximum TF (3.05) was observed at 50 mg Cd kg−1 in combination with 4 mmol kg−1 EDTA and minimum TF (1.19) was observed at 150 mg Cd kg−1 in combination with 10% compost. TF values greater than 1 highlighted the translocation of Cd from roots to shoots.
4 Discussion
The current study showed that amendments play an important role in increasing the bioavailability of Cd in soil except compost which reduced the bioavailable portion in soil. Most of the chelate-assisted phytoextraction studies focused on the use of EDTA and considered it as the best chelating agent in enhancing the mobility of Cd (Xie et al. 2012). The present study also showed that EDTA was more efficient in enhancing the bioavailability and accumulation of Cd in shoots of P. hortorum. The Cd solubility in EDTA-amended soil after 7 days of incubation time was significantly increased by 1.2-fold as compared to control. This increase in Cd solubility may involve different mechanisms, such as the adsorption of Cd on free EDTA and then the detachment of Cd ions from minerals, resulting in increasing Cd solubility in soils (Shahid et al. 2014). The addition of citric acid, ammonium nitrate, and TiO2 NPs increased the solubility of Cd in soil by 0.8-, 0.4-, and 0.2-fold, respectively, but this increase was lower than that with EDTA. In contrast to this, the addition of compost significantly decreased the solubility of Cd by 50%. The stabilizing role of Cd in soil has been reported. High organic matter content increased the retention of Cd and decreased the solubility (Khan et al. 2017).
For the successful phytoextraction of Cd, bioavailable portion must be taken by the plants. Pelargonium species have ability to accumulate heavy metals in shoot and are considered as hyperaccumulator of Pb and Cd (Arshad et al. 2008; Manshadi et al. 2013; Arshad et al. 2016; Manzoor et al. 2018). In the present study, P. hortorum accumulated 262.8 and 176.6 mg Cd kg−1 in shoot and root, respectively, at 150 mg Cd kg−1 without application of amendments. Mahdieh et al. (2013) reported that scented geranium, Pelargonium roseum, accumulated 1957 mg Cd kg−1 of shoot dry weight in 14 days of exposure. Pelargonium species have inherent ability to uptake heavy metals by acidifying the rhizosphere soil which is the main factor in the metal availability (Arshad et al. 2016). In this study, EDTA application significantly increased the accumulation of Cd in shoot and root by 3.9- and 2.3-fold, respectively. Our findings were coherent with another study which reported that the application of 4 g EDTA kg−1 increased Cd accumulation in shoot of marigold (Tagetes sp.) by 3.4-fold as compared to the control (Ali et al. 2016). The application of citric acid, ammonium nitrate, and TiO2 NPs increased the accumulation of Cd in shoot and root by 2.4-, 1.0-, and 0.2-fold and 1.5-, 1.0-, and 0.9-fold, respectively. In contrast, the application of compost showed decrease in Cd accumulation by shoots and root by 0.92- and 0.3-fold, respectively, and this decrease was due to possible stabilization of Cd in soil (Khan et al. 2017). The accumulation of Cd in shoot and root was significantly decreased by 31, 60, 70, 87, and 26, 40, 43, and 70%, respectively, upon the application of citric acid, ammonium nitrate, TiO2 NPs, and compost as compared to EDTA. By comparing all amendments in term of Cd accumulation, the EDTA was more efficient.
Another factor involved in phytoextraction is the ability of plant to survive and produce higher biomass. P. hortorum has the ability to survive in heavy metal-contaminated soil but the biomass decreased at higher Cd levels (Gul et al. 2018b). The maximum plant biomass was observed in the control group, and with the increasing levels of Cd from 0 to 150 mg Cd kg−1, the biomass of P. hortorum was decreased by 22.8%. It is well reported that Cd causes phytotoxicity by decreasing the photosynthetic activity and ultimately reduces the plant biomass (Gul et al. 2018b). The addition of amendments showed various responses. Upon addition of EDTA in control groups, the biomass was decreased by 62%. Similar results were also reported by another study where the dry biomass of Echinochloa crus galli L. was decreased by 49.7% in the control group upon 5 mmol kg−1 EDTA application. This decrease in the dry plant biomass was due to the presence of high content of mobilized heavy metals and toxicity of free EDTA (Ebrahimi 2015). The dry plant biomass of P. hortorum in citric acid-, ammonium nitrate-, TiO2 NP-, and compost-amended soils was 2.94, 2.95, 2.96, and 2.98 g per plant, respectively. Among all amendments, 10% addition of compost in control group showed 14% increase in the dry plant biomass. Ahmad et al. (2015) showed 33.2% increase in the dry plant biomass of wheat upon addition of compost. The increase in dry plant biomass in compost-amended soil might be due to improved nutrient level, soil structure, and water holding capacity and reduction in the bioavailable fraction of Cd in soil (Oldari et al. 2011).
Plants having TF > 1 is considered as a hyperaccumulator. In Cd-treated groups without amendment application, TF > 1 (Table 3) indicated that P. hortorum has the ability to transfer Cd from root to shoot. Similarly, Gul et al. (2018b) reported that P. hortorum showed TF > 1 in Cd-spiked soils. The application of soil amendments further increased the TF. The maximum TF was observed upon EDTA application followed by citric acid, TiO2 NPs, ammonium nitrate, and compost.
The maximum Cd uptake per plant (1.44 mg) was observed in citric acid-amended soil, followed by EDTA, ammonium nitrate, and TiO2 NPs and minimum (0.33 g) was observed in compost-amended soil. In the presence of citric acid, the accumulation of Cd in P. hortorum was 30% lower but the plant biomass was 50% higher than EDTA-amended soil. Therefore, Cd uptake by plant was higher in citric acid-amended soil as compared to EDTA-amended soil. The compost showed maximum plant biomass and growth, but the accumulation was reduced with increasing compost levels as it stabilized the Cd in soil instead of mobilization; therefore, the minimum Cd uptake was observed.
In assisted phytoextraction of Cd, the metal extraction ratios play an important role in identifying the efficiency of amendments in cleaning of soil. The citric acid showed maximum MER and minimum was observed for compost as compared to other amendments. By comparing all amendments, citric acid, ammonium nitrate, and TiO2 NPs can be used as substitute to EDTA, as they showed minimum toxicity to plant in term of biomass and other physiological parameters.
5 Conclusions
EDTA, citric acid, ammonium nitrate, and TiO2 NPs increased the mobility of Cd. Efficiency of amendments to increase mobility of Cd followed the sequence: EDTA > citric acid > ammonium nitrate > TiO2 NPs > compost. The maximum accumulation of Cd in shoot and root was observed in EDTA-amended soil. But MER and Cd uptake were higher in citric acid-amended soil in comparison to other amendments. Overall, citric acid can be used as a substitute to EDTA in assisted phytoextraction of Cd.
References
Abbas T, Rizwan M, Ali S, Adrees M, Mahmood A, Rehman MZ, Ibrahim M, Arshad M, Qayyum MF (2018) Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol Environ Saf 148:825–833
Ahmad I, Akhtar MJ, Zahir ZA, Mitter B (2015) Organic amendments: effects on cereals growth and cadmium remediation. Int J Environ Sci Technol 12:2919–2928
Ali SY, Paul M, Chaudhury S (2016) EDTA-enhanced phytoextraction of Cd and Pb in spiked soil with Marigold and associated potential leaching risk. Int J Environ Agric Res 2(5):114–118
Alkorta I, Hernández-Allica J, Becerril JM, Amezaga I, Albizu I, Onaindia M (2004) Chelate-enhanced phytoremediation of soils polluted with heavy metals. Rev Environ Health 3:55–70
Arshad M, Silvestre J, Pinelli E, Kallerhoff J, Kaemmerer M, Tarigo A, Shahid A, Guiresse M, Pradere P, Dumat C (2008) A field study of lead phytoextraction by various scented pelargonium cultivars. Chemosphere 71:2187–2192
Arshad M, Merlina G, Uzu G, Sobanska S, Sarret G, Dumat C, Silvestre J, Pinelli E, Kallerhoff J (2016) Phytoavailability of lead altered by two Pelargonium cultivars grown on contrasting lead-spiked soils. J Soils Sediments 16(2):581–591
Chen YH, Li XD, Shen ZG (2004) Leaching and uptake of heavy metals by ten different species of plant during EDTA-assisted phytoextraction process. Chemosphere 57:187–196
Chen Y, Liu M, Deng Y, Zhong F, Xu B, Hu L, Wang M, Wang G (2017) Comparison of ammonium fertilizers, EDTA, and NTA on enhancing the uptake of cadmium by an energy plant, Napier grass (Pennisetum purpureum Schumach). J Soils Sediments 17:2786–2796
Chhajro MA, Rizwan MS, Guoyong H, Kubra KA, Honging H (2016) Enhanced accumulation of Cd in castor (Ricinus communis L.) by soil applied chelators. Int J Phytorem 18:664–670
Dede G, Ozdemir S (2016) Effect of elemental sulfur on heavy metal uptake by plant growing on municipal sewage sludge. J Environ Manag 166:103–108
Ebrahimi M (2015) Effect of EDTA treatment method on leaching of Pb and Cr by Phragmites australis (Cav.) Trin. Ex Steudel (common reed). Caspian J Environ Sci 13(2):153–166
Epelde L, Allica JH, Becerril JM, Blanco F, Garbius C (2008) Effects of chelates on plants and soil microbial community: comparison of EDTA and EDDA for lead phytoextraction. Sci Total Environ 401:21–28
Freitas EV, Nascimento CW, Silva WM (2014) Citric acid assisted phytoextraction of lead in field: the use of soil amendments. Water Air Soil Pollut 225(1):1796
Gul I, Manzoor M, Hashim N, Kallerhoff J, Arshad M (2018a) Comparison of EDTA, citric acid and TiO2 nanoparticles to support Cd phytoaccumulation in spiked soil. Proceedings of the 2nd International Conference on Recent Trends in Environmental Science and Engineering (RTESE'18) 119. https://doi.org/10.11159/rtese18.119
Gul I, Manzoor M, Rizwan M, Silvestre J, Hina K, Kallerhoff J, Arshad M (2018b) EDTA-assisted phytoextraction of lead and cadmium by Pelargonium cultivars grown on spiked soil. Int J Phytorem. https://doi.org/10.1080/15226514.2018.1474441
Guo JM, Lei M, Yang X, Yang, Wan XM, Chen TB, Zhou XY, Gu SP, Guo GH (2017) Effect of fertilizers on the Cd uptake of two sedum species (Sedum spectabile Boreau and Sedum aizoon L) as potential Cd accumulators. Ecol Eng 106:409–414
Johnson A, Gunawardana B, Singhal N (2010) Amendments for enhancing copper uptake by Brassica juncea and Lolium perenne from solution. Int J Phytorem 11:215–234
Khan A, Khan S, Khan MA, Qamar Z, Waqas M (2015) The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: a review. Environ Sci Pollut Res 22(18):13772–13799
Khan MA, Khan S, Khan A, Alam M (2017) Soil contamination with cadmium, consequences and remediation using organic amendments. Sci Total Environ 601–602:1591–1605
Li Y, Tang H, Hu Y, Wang X, Ai X, Tang L, Matthew C, Cavanagh J, Qiu J (2016) Enrofloxacin at environmentally relevant concentrations enhances uptake and toxicity of cadmium in the earthworm Eisenia fetida in farm soils. J Hazard Mater 308:312–320
Mahdieh M, Yazdani M, Mahdieh S (2013) The potential of Pelargonium roseum plant for phytoremediation of heavy metals. Environ Monit Assess 185(9):7877–7881
Manshadi M, Ziarati P, Ahmadi M, Fekri K (2013) Greenhouse study of cadmium and lead phytoextraction by five Pelargonium species. Int J Farm Alli Sci 2(18):665–669
Manzoor M, Gul I, Silvestre J, Kallerhoff J, Arshad M (2018) Screening of indigenous ornamental species from different plant families for Pb accumulation potential exposed to metal gradient in spiked soils. Soil Sediment Contam 27(5):439–453
Mehrizad A, Gharbani P, tabatabii SM (2009) Synthesis of nanosized TiO2 powder by sol gel method in acidic conditions. J Iran Chem Res 2:145–149
Mertens J, Luyssaert S, Verheyen K (2005) Use and abuse of trace metal concentrations in plant tissue for biomonitoring and phytoextraction. Environ Pollut 138:1–4
Muhlbachova G (2011) Soil microbial activities and heavy metal mobility in long-term contaminated soils after addition of EDTA and EDDS. Ecol Eng 37:1064–1071
Neugschwandtner RW, Tlustos P, Komarek M, Szakova J, Jakoubkova L (2012) Chemically enhanced phytoextraction of risk elements from a contaminated agricultural soil using Zea mays and Triticum aestivum: performance and metal mobilization over a three year period. Int J Phytorem 14:754–771
Oldari M, Arthurson V, Pell M, Svensson K, Nehrenheim E, Abubakar J (2011) Land application of organic waste—effects on the soil ecosystem. Appl Energy 88:2210–2218
Pedron F, Rosellini I, Petruzzelli G, Barbafieri M (2014) Chelant comparison for assisted phytoextraction of lead in two contaminated soils. Resource Environ 4(5):209–214
Rizwan M, Ali S, Ali B, Adrees M, Arshad M, Hussain A, Rehman MZ, Waris AA (2019) Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214:269–277
Sarkar D, Andra SS, Sumathi KM, Saminthan DR (2008) Chelant aided enhancement of lead mobilization in residential soils. Environ Pollut 156(3):1139–1148
Shahid M, Austruy A, Echevarria G, Arshad M, Sanaullah M, Aslam M, Nadeem M, Nasim W, Dumat C (2014) EDTA-enhanced phytoremediation of heavy metals: a review. Soil Sediment Contam 23(11):389–416
Shazia A, Shazia I, Mahmood U (2014) Effects of chelating agents on heavy metal extraction from contaminated soils. Res J Chem Sci 4:70–87
Souza LA, Piotto FA, Dourado MN, Schmidt D, Franco MR, Boaretto LF, tezotto T, Ferreira RR, Azevedo RA (2017) Physiological and biochemical responses of Dolichos lablab L. to cadmium support its potential as a cadmium phytoremediator. J Soils Sediments 17:1413–1426
Xie Z, Wu L, Chen N, Liu C, Zheng Y, Xu S, Li F, Xu Y (2012) Phytoextraction of Pb and Cu contaminated soil with maize and microencapsulated EDTA. Int J Phytorem 14:727–740
Zahedifar M, Moosavi AA, Shafigh M, Zarei Z, Karimian F (2016) Cadmium accumulation and partitioning in Ocimum basilicum as influenced by the application of various potassium fertilizers. Arch Agron Soil Sci 62(5):663–673
Zahra Z, Waseem N, Zahra R, Lee H, Badshah MA, Mehmood A, Choi HK, Arshad M (2017) Growth and metabolic responses of rice (Oryza sativa L.) cultivated in phosphorus-deficient soil amended with TiO2 nanoparticles. J Agric Food Chem 65:5598–5606
Funding
The authors would like to thank the National University of Sciences and Technology (NUST), Islamabad for providing financial support for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare related to reported work here.
Additional information
Responsible editor: Kitae Baek
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 268 kb)
Rights and permissions
About this article
Cite this article
Gul, I., Manzoor, M., Hashim, N. et al. Comparative effectiveness of organic and inorganic amendments on cadmium bioavailability and uptake by Pelargonium hortorum. J Soils Sediments 19, 2346–2356 (2019). https://doi.org/10.1007/s11368-018-2202-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-018-2202-1




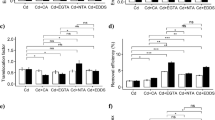

 = 25 mg Cd kg−1;
= 25 mg Cd kg−1;  = 50 mg Cd kg−1;
= 50 mg Cd kg−1;  = 100 mg Cd kg−1;
= 100 mg Cd kg−1;  = 150 mg Cd kg−1)
= 150 mg Cd kg−1)
 = 25 mg Cd kg−1;
= 25 mg Cd kg−1;  = 50 mg Cd kg−1;
= 50 mg Cd kg−1;  = 100 mg Cd kg−1;
= 100 mg Cd kg−1;  = 150 mg Cd kg−1)
= 150 mg Cd kg−1)
