Abstract
Purpose
Vegetables are major economic crops in China. Their cultivation usually involves high fertilizer application rates leading to significant losses of N and P to the wider environment, resulting in water contamination and low nutrient use efficiency. Hence, it is a matter of urgency to understand the mechanisms and factors that affect N and P losses in vegetable production systems in order to develop optimum fertilization regimes.
Materials and methods
Different fertilization regimes were applied in a long-term chili (Capsicum spp. L.) production soil to study the effects on nitrogen (N) and phosphorus (P) runoff losses, microbial biomass, microbial community, and crop yields. Three fertilization regimes were implemented: control (no fertilizer; CK), farmer’s fertilization practice (FFP), and site-specific nutrient management (SSNM). A fixed collection device was used to quantify the total volume of water output after each precipitation event. All water samples were analyzed for total nitrogen, ammonium nitrogen (NH4+-N), nitrate nitrogen (NO3−-N), total phosphorus (TP), and available phosphorus (AP). Soil samples were collected for analysis of the physicochemical properties and for DNA extraction after chili harvest. High-throughput sequencing was used to further investigate the relationship between the microbial community and nutrient losses.
Results and discussion
The SSNM fertilizer regime resulted in a 23.3% yield increase and enhanced agronomic N use efficiency from 11.87 to 15.67% compared with the FFP treatment. Soil available nutrients (i.e., AN and AP) and ATP content increased significantly after SSNM implementation. Under the SSNM regime, N losses decreased by 25.8% compared with FFP but did not lead to significantly different P losses. High-throughput sequencing results showed that each treatment formed a unique microbial community structure. VPA results revealed that the microbial community structure was mainly (50.56%) affected by the interactions between N and P. Mantel results indicated that the soil properties that significantly affected soil microbial community structure followed the order: AP, AK, and salinity.
Conclusions
Our study has demonstrated that SSNM not only generates lower N losses but also provides higher contents of soil available nutrients and plant yield, which were mainly attributed to the multiple top dressings and meeting of the plants’ demand with adequate nutrient supplies. The combined data showed that the microbial community differentiation between the different fertilizer regimes was mainly linked to the interactions between N and P in the soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Agricultural activities have altered nutrient cycling in farmland ecosystems, accelerating the circulation of elements in various ecosystems and leading to increased losses of nutrients such as N and P. The N and P lost have major detrimental environmental effects, including surface water eutrophication (Carpenter et al. 1998). In addition, inappropriate use of fertilizers (i.e., excessive fertilization and inappropriate fertilizer application timing) may also lead to soil quality deterioration, such as soil acidification and soil structural degradation (Verhoeven et al. 1996). Over the past three decades, the area devoted to vegetable crops has risen by a factor of more than seven, from 3.3 million ha to 24.8 million ha, accounting for 14.5% of the total farmland in China (FAO 2011).
An intensive vegetable production system involves the use of high rates of fertilizers and pesticides, multiple crops per year, and the use of irrigation (Zhu et al. 2013). Fertilizer application rates for intensive vegetable systems have reached about 1500 kg N ha−1 year−1 in China (Ju et al. 2006), which are more than three times those for non-vegetable production systems (Zheng et al. 2004). In order to pursue high yields, N fertilizer use for agricultural production has been increasing for decades in China, but N utilization efficiency has been decreasing due to the excessive N application rates (Lin et al. 2007). It has been estimated that global N fertilizer input to agricultural production outpaces human N demand by a factor of more than seven (Deluca 2009). As a consequence, less than one fifth of fertilizer N and P contribute to food production, and the rest is wasted (Albiac 2009). Most of the unused portions of the N in agricultural production will eventually end up in lakes and rivers as a pollutant (Zhu and Chen 2002). Therefore, it is important to develop and verify an optimal fertilization regime for vegetable crop soil, to improve fertilizer N use efficiency and minimize the negative environmental impacts.
Site-specific nutrient management (SSNM) was suggested by the International Rice Research Institute (IRRI). In SSNM, fertilizer application is based on the timing and quantity of plant needs (Liu et al. 2010). This approach has been shown to be an effective means of reducing fertilizer application rates and increasing nutrient use efficiency (Dobermann et al. 2003). SSNM practices are designed to take into account the soil nutrient supplying capacity, crop yield targets, the previous local nutrient management situation, and corresponding crop nutrient requirements, as well as socioeconomic factors (Liang et al. 2013). During the growing season, a few crop nutrition status indicators are measured to ensure that N, P, and K are applied at the times required by crops. This approach was based on scientific principles developed through nearly a decade of field research and advocates reasonable use of indigenous nutrients originating from soil and plant residues. Fertilizers are applied at appropriate times to overcome nutrient deficiency and make up the difference between the total crop nutrient demand and the supply from indigenous sources to achieve a yield target (Pampolino et al. 2007).
The effects of agricultural management, including alternate wetting and drying irrigation and rice-wheat rotational cropping, on surface runoff after adopting the SSNM system have been studied (Liang et al. 2013; Pampolino et al. 2007). It is increasingly accepted that SSNM can mitigate N and P losses via surface runoff from different field conditions while maintaining yields. Microorganisms play a vital role in agroecosystems through participation in recycling soil nutrients, maintaining soil structure, and promoting plant growth (Nacke et al. 2011). Microbes also respond quickly to environmental changes (e.g., tillage, water management, application of fertilizer), resulting in dynamic changes in microbial biomass, abundance, and diversity (Zhao et al. 2014; Zhao et al. 2016). An appropriate microbial community structure and activity are important factors in maintaining the sustainability and productivity of ecosystems (Cardinale et al. 2006; Chaer et al. 2009). Hence, understanding the shifts of microorganisms following different fertilization regimes is important for selecting suitable management strategies to improve ecosystem services (Acosta-Martinez et al. 2008).
Previous research on the effects of SSNM has mostly focused on paddy soils, bamboo forests, or wheat cropping systems (Pampolino et al. 2007; Khurana et al. 2008; Zhang et al. 2013). Little information is available on the effects of implementing SSNM on surface runoff of nutrients and on soil microbial community structure in vegetable production soils. Hence, studies on vegetable soil under SSNM are urgently needed to bridge this knowledge gap. We hypothesized that (1) N and P losses via surface runoff would be decreased under the SSNM system compared with traditional fertilization practices and (2) different fertilization regimes would alter soil microbial community structure, which would subsequently affect soil nutrient cycling. Specifically, the objectives of this study were to compare the effects of SSNM on runoff losses of N and P, plant growth, and the microbial community, with those of traditional farmer’s fertilization practices.
2 Materials and methods
2.1 Site description
The study site was located in Yuhang County, in northern Zhejiang Province, China (30° 35′ N, 119° 88′ E), which is hill country in subtropical southern China. Much of the area is used for vegetable production. The study site is characterized as a typical subtropical monsoon climate and has a mean temperature of 15.7 °C. Average rainfall is 1454 mm, about 70% of which occurs between March and August. Before the establishment of the experiment, the land was used for rotational production of vegetables for a long time. The soil was classified as yellow brown soil (alfisol) according to the soil classification system of China. The soil properties were pH (H2O) 5.73, organic matter 31.62 g kg−1, total N 1.98 g kg−1, total P 0.57 g kg−1, available N 192.40 mg kg−1, Olsen P 62.76 mg kg−1, and available K 0.25 g kg−1.
2.2 Field experiment design
The experimental field was divided into nine plots, with each plot being 15 m2 (3 m wide × 5 m long). Each plot was surrounded by low cement walls (6 cm wide × 50 cm high), with 30 cm buried into the soil, leaving 20 cm above the soil surface for separation. Cement devices (1 m tall, 1 m wide, and 1 m long) for runoff collection were installed at the lower end of each plot. Watch-Dog 2700 Weather Stations (Spectrum Technologies, Aurora, IL, USA) were also installed near the experimental area to record daily meteorological data during the study period.
The treatment plots were arranged in a randomized block design with three replicates. The fertilizer treatments were a non-fertilizer control (CK), farmers’ fertilizer practices (FFP) and improved site-specific nutrient management. The sources of N, P, and K in inorganic fertilizers were urea, superphosphate, and potassium sulfate, respectively. The sources of N, P, and K in inorganic fertilizers were urea, superphosphate, and potassium sulfate, respectively. Organic fertilizer comprised of pig manure and a suitable amount of spent mushroom compost. All the organic fertilizers were applied as basal fertilizer by broadcasting before planting, whereas the inorganic fertilizers were used both as basal fertilizers and were side-dressed three times (Table S1, Electronic Supplementary Material). For the FFP treatment, according to local farmer practices, the element rates for N, P, and K were 200.0, 80.0, and 133.0 kg ha−1, respectively. The fertilizer rates for SSNM were 208.5, 68.0, and 72.5 kg ha−1 for N, P, and K, respectively. The fertilizers were applied in May (60%), August (15%), and September (25%), 2016 in accordance with the requirements of chili’s different growth stages (Table S1, Electronic Supplementary Material).
2.3 Water, plant, and soil sampling and chemical analysis
Water samples were taken from each treatment following each rainfall event, which occurred at 41, 49, 78, 82, 88, and 119 days after the start of the experiment. Depths of runoff water in devices were measured to calculate the runoff volume, and runoff samples were immediately collected from each of the nine plots in polypropylene bottles. Once collected, runoff samples were shipped in ice to the laboratory for processing. Water samples were preserved with several drops of H2SO4 and subjected to chemical analysis within 24 h after sampling. Water samples were filtrated with a 0.22-μm filter prior to analysis. Concentrations of NH4+-N, NO3−-N, total P (TP), and total N (TN) were determined using a continuous flow analyzer (SAN++, Skalar, Holland). The available nitrogen (AN) and available P (AP) were determined using the alkaline hydrolysis diffusion and molybdenum blue colorimetric methods, respectively. At the end of the growing season, plant yields were determined after harvesting from each plot. Total N, P, and potassium (K) in plant fruits were determined after H2SO4-H2O2 reduction, and using the methods of diffusion, molybdenum blue colorimetry, and flame photometry, respectively. Five soil core samples (top 20 cm soil depth) from each plot were collected on Oct. 4, after chili harvest. Within 2–6 h of collection, the pooled soil samples were transported on ice to the laboratory. One subsample was stored at a temperature of − 80 °C for DNA extraction as well as molecular analysis, and the remaining soil was processed by means of a 2.0-mm sieve in order to analyze soil physicochemical properties.
2.4 Soil microbial biomass C and ATP analysis
Soil microbial biomass C (MBC) was measured using the chloroform fumigation-extraction method (Vance et al. 1987). Soils were extracted after 24-h fumigation using 0.50 M K2SO4 with a 1:4 ratio for 60 min on a rotary shaker, and C was determined with a LiquiTOC element analyzer (Elementer Analysen-system GmbH, Hanau, Germany). Soil adenosine triphosphate (ATP) was extracted by the TIP reagent, which was composed of Na2HPO4, trichloroacetic acid (TCA), and imidazole (Redmile-Gordon et al. 2011). Two different soil extractants were used for extraction: extractant A contained 0.6 M imidazole, 1.10 M TCA, and 0.25 M sodium hydrogen phosphate, while extractant B was prepared as extractant A except that 25 pmol ATP uL−1 was added. Finally, ATP was analyzed using a luminometer to measure light (Jenkinson and Oades 1979).
2.5 Bacterial 16S rRNA gene amplification, Illumina sequencing, and data processing
Total DNA was extracted from 0.5 g soil using a Fast DNA Spin Kit (MP Biomedical, France) according to the manufacturer’s protocol. A Nanodrop-2000 Spectrophotometer (Thermo Scientific, Waltham, MA, USA) was used to measure the purities and concentrations of extracted DNA, and the V3 and V4 regions of the bacterial 16S rRNA gene in the extracted DNA were amplified. The forward primer was 338F (5′-ACT CCT ACG GGA GGC AGC AG-3′), and the reverse primer consisted of a 7-bp barcode and 806R (5′-GGA CTA CHV GGG TWT CTA AT-3′). The purified amplicons were then sent for sequencing on an Illumina Miseq sequencing platform (Illumina Inc., San Diego, CA, USA) at Majorbio Bio-Pharm Tech, Shanghai, China. Sequences were processed and analyzed using Quantitative Insights Into Microbial Ecology (QIIME), and open-reference operational taxonomic unit (OTU) selection was performed after filtering out low-quality or ambiguous reads according to the online instructions for QIIME. The quality sequences were binned into OTUs that were defined at the 97% similarity level by UCLUST (Broadhurst et al. 2012). Differentially abundant features were identified using the approach of the linear discriminant analysis (LDA) effect size (LEfSe) at http://huttenhower.sph.harvard.edu/lefse/ (Segata et al. 2011), and an LDA score > 3.0 was considered as the responding microorganism. Redundancy analysis (RDA), the Mantel test, and variation partitioning analysis (VPA) were carried out using vegan and R (Dixon 2003). The dataset of 16S rRNA gene sequences was deposited in NCBI’s Sequence Read Archive (SRA) with accession number SRP125808.
2.6 Statistical analysis
Statistical tests, which included analysis of variance (ANOVA) and Pearson correlation analysis, were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA) and Origin 8.1. Statistical significance was determined by Duncan’s multiple range test and denoted at p < 0.05.
3 Results
3.1 Crop yields and soil fertility
The yield of chili in the SSNM plots was 4691 kg ha−1, which was 227 and 23.3% higher than those in the CK and FFP treatments, respectively (Table 1). An important characteristic of the soil physicochemical properties under the different fertilization regimes was that soil available nutrients changed significantly, while total soil nutrients (i.e., TN and SOM) were not significantly affected. Soil available nutrients, such as AN and AP, in the SSNM treatment were significantly increased compared with the FFP and CK treatments (Table 1). Meanwhile, SOM and pH were the highest in the FFP treatment, at 40.29 g kg−1 and 6.57, respectively (Table 1). The contents of AK in the FFP and SSNM treatments were 286 and 291% higher than that in the CK treatment (Table 1). Although yields and soil K content from each treatment were positively correlated (R2 = 0.76, p < 0.005), these correlations were much weaker than those between yields and K content in fruits (R2 = 0.97, p < 0.001) (Fig. S1, Electronic Supplementary Material).
3.2 Nitrogen and phosphorus losses
A total of six rainfall events occurred during the plant growth period: four in the basal dressing period (totalling 190.2 mm), one in the first top dressing period (totalling 39.6 mm), and one in the second top dressing period (totalling 155.7 mm) (Fig. 1a). TN losses in the runoff from the different fertilization regimes were 5.90–15.52 kg N ha−1, and the FFP treatment led to the highest (15.52 kg N ha−1) during the experimental period (Table 2). This was 22.6% more than that from the SSNM treatment. TN losses in the study period equalled 7.8% of the total N input in the FFP treatment, while only 5.8% of the applied N was lost in the SSNM treatment (Table 2). The average concentration of N in the runoff samples from the different treatments was generally in the range of 0.21–13.17 mg N L−1 (Fig. 1b), following the order of FFP > SSNM > CK. NO3−-N lost from the FFP treatment was 4.01 kg N ha−1 during the study period, which was significantly higher (by 15.6–65.0%) than that in the other treatments. NO3−-N concentrations in the runoff varied from 1.67 to 3.46 and 3.15 mg N L−1 in the CK, FFP, and SSNM treatments, respectively (Fig. 1b). The NO3−-N concentrations in the runoff stayed high in the early stages of the experiment, and then decreased (Fig. 1b). NH4+-N in the runoff followed the opposite trends to NO3−-N over the experimental period. No significant difference was found in NH4+-N losses between the FFP and SSNM treatments (Table 2).
Nutrient losses as affected by different fertilization regimes: accumulated TN losses (a), NH4+-N and NO3−-N concentrations (b), accumulated TP and AP losses (c), and relationship between TP and AP concentrations in surface runoff (d). NH4+-N and NO3−-N (b) correspond to the left and right Y-axis, and TP and AP (d) correspond to the left and right Y-axis, respectively. Adjusted R2 value with associated P value (d) are shown
The loss pattern of TP in CK was similar to that in the SSNM and FFP treatments (Fig. 1c). TP and AP losses in the runoff varied from 0.125 to 0.534 kg ha−1 and 0.210 to 0.684 kg ha−1 during the study period (Fig. 1c). The AP and TP losses tended to follow similar trends, and correlation analysis indicated that the AP concentrations were significantly correlated with the TP concentrations in the runoff (Fig. 1d).
3.3 Soil microbial biomass C and community structure
The soil MBC significantly increased in the SSNM treatment and reached 495.8 μg g−1, which was 35.1 and 4.3% higher than those in the CK and FFP treatments, respectively (Table 3). The soil ATP content was the highest in the SSNM treatment (10.38 nmol ATP g−1 soil), and this was 31.7 and 20.0% higher than those in the CK and FFP treatments, respectively (Table 3). No statistically significant differences (p < 0.05) in alpha diversity were observed between the FFP and SSNM treatments (Table 3).
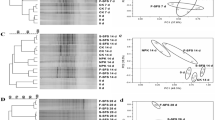
The cluster dendrogram showed the Bray-Curtis dissimilarity of the soil samples from the three fertilization regimes, and the bacterial communities in the SSNM treatment were far apart from those in the FFP and CK treatments (Fig. 3a). The bacterial genetic sequences revealed that Proteobacteria, Acidobacteria, Chloroflexi, Nitrospirae, Actinobacteria, and Bacteroidetes (> 5%) were the dominant phyla of microorganisms in all of the samples (Fig. 2). Abundances of Proteobacteria, Actinobacteria, and Bacteroidetes were significantly higher after the implementation of the SSNM treatment when compared with FFP, whereas Chloroflexi was most abundant in the FFP treatment, where it was significantly more abundant than in the SSNM treatment (Fig. 2). Nitrospirae, one of the important phyla involved in N cycling, was not affected by the different fertilization regimes. LEfSe analysis was conducted to examine which taxa (phylum to genus) were affected by different fertilization treatments (Fig. 4). Those with an LDA score > 3.0 were selected to identify bacterial taxa with statistically significant differences in abundance between fertilization treatments. At the order level, a total of 14 clades were detected, with 7 clades from the CK treatment, 2 clades from FFP, and 5 clades from SSNM (Fig. 4). Among these orders, Corynebacteriales, Pseudonocardiales, and Streptosporangiales, belonging to the Actinobacteria, were significantly increased by the implementation of SSNM. Meanwhile, Anaerolineales, belonging to Chloroflexi, changed significantly in the FFP treatment (Fig. 4).
3.4 Soil physicochemical properties and bacterial community
RDA of Bray-Curtis distances was conducted to examine the associations between environmental properties and bacterial population distribution (Fig. 3b). The effects of soil properties on the bacterial community were shown by the direction and length of the vectors. The soil properties, including pH, TN, AN, TP, AP, AK, SOM, and salinity explained 78.95% of the total bacterial variations. AN and AP were significantly related to the first axis (explaining 55.88% of the total variance), and SOM was significantly related to the second axis (explaining 23.07% of the total variance). A VPA was constructed by defining the explanatory impact of N and P on the bacterial communities (Fig. 3c). A total of 81.28% of the variance of bacteria communities could be explained by N and P contents in water, soil, and plant samples (Fig. 3c). N and P could explain 18.08 and 12.64% of the variations in the bacterial community, respectively. The variation was mostly explained by the interaction between N and P, accounting for 50.56%. The Mantel result (Fig. 3c) indicated that the vital properties that markedly affected bacterial population distribution were AP (r = 0.544, p < 0.01), AK (r = 0.537, p < 0.05), and salinity (r = 0.472, p < 0.05) (Fig. 3c).
The cluster dendrogram (a) of Bray-Curtis dissimilarity of samples based on relative abundance. Redundancy analysis (b) of Bray-Curtis distances of 16S rRNA of samples. Variation partitioning analysis (c) shows the effects of nitrogen (N), phosphorus (P), and the interactions between them on the bacterial community. Circles show the percentage of variation explained by each factor individually and the “r” in Mantel test represents the correlation coefficient between environmental and microbial matrices
4 Discussion
4.1 Effects of different fertilization regimes on N and P losses
Rainfall and fertilization have been considered the most important factors determining soil nutrient runoff losses (Shan et al. 2015). The heaviest rainfall (on Sept. 11, 119 days after fertilization) resulted in 39.19 and 34.14% of the TN losses in the FFP and SSNM treatments, respectively (Fig. 1). Hence, environmental conditions (i.e., rainfall intensity) around the time of fertilizer application should be taken as a critical factor for controlling N runoff losses (Peng et al. 2011). Furthermore, the relatively poor growth of plants in the FFP treatment may also have increased the risk of N losses in surface runoff. The FFP treatment caused the highest TN runoff losses in the present study (15.52 kg N ha−1), whereas SSNM resulted in a much lower 3.50 kg N ha−1 loss. These observed results were consistent with those reported in other studies (8.8–18.5 kg ha−1 year−1) (Liang et al. 2013, Shan et al. 2015). The N in the runoff consisted predominantly of soluble inorganic N, mainly NO3−-N (Di and Cameron 2002) (Fig. 1b). NH4+-N is easily adsorbed onto soil mineral and organic matter surfaces (Rosenfeld 1979); hence, it is usually not the dominant form of inorganic N in runoff. While NH4+-N losses in our experiment accounted for 28.1–36.5% of the N in the runoff (Table 2), this may be attributable to the large amount of organic fertilizer applied. Organic fertilizers have been reported to contain some ammonium, which can increase the average concentration of NH4+-N in surface runoff (Shan et al. 2015). The loss ratio of P in the FFP and SSNM treatments was lower than 1% (Table 2), similar to those reported in other studies (0.03–1.09 kg ha−1 year−1) (Djodjic et al. 2004). The TP and AP concentrations in runoff had a strong correlation (R2 = 0.933), mainly because the P in the runoff water was mainly in the form of available P (Pote et al. 1996). Therefore, the SSNM practice decreased overall fertilizer application rates through better synchronization of nutrient supply to meet the demands of the growing vegetable crops, thereby lowering nutrient concentrations in floodwater.
4.2 Response of soil microbial biomass and community structure
MBC can be altered by fertilization, and combined applications of organic and inorganic fertilizers may provide the biggest benefits (Liu et al. 2010) (Table 3). ATP, as an indicator of active bacterial biomass, was the highest in the SSNM treatment, indicating that the SSNM system was conducive to microbial activity.
Previous studies have reported that fertilization can impact soil bacterial community structure (Li et al. 2014; Zhao et al. 2014). The relative abundance variation at the phylum level and LEfSe at each taxonomic level (from phylum to genus) (Figs. 2 and 4) was conducted to detect population distribution variations among the three fertilization regimes. Firstly, hierarchical cluster analysis (Fig. 3a) of the similarity of the bacterial communities confirmed that each treatment led to a unique community structure. We observed that Proteobacteria, Actinobacteria, and Bacteroidetes were all both dominant and responding phyla in the SSNM treatment, whereas only Chloroflexi was dominant at the phylum level in the FFP treatment. A greater abundance of Actinobacteria was found in aerobic environments with a full supply of N (Li et al. 2014). Bacteroidetes was associated with decomposition of recalcitrant C compounds, especially following N additions (Nemergut et al. 2008). Similar results were found in the N and organic carbon treatments in our experiment. Acidobacteria, a ubiquitous and abundant member of the soil bacterial community, have been suggested to be closely associated with pH (Jones et al. 2009). The highest Acidobacteria abundance in our study was in the CK treatment, probably because of the relatively acidic soil (pH 5.27). Secondly, the responding microbes selected under LEfSe analysis further demonstrated that the bacterial community was altered by the different fertilization treatments. Although Chloroflexi was not a responding bacterium in the FFP treatment, Anaerolineae, a class within this phylum, showed a remarkable abundance increase (Fig. 4), a finding consistent with previous studies. The large amount of organic fertilizer applied and the high oxygen consumption in the decomposition process might have significantly affected this facultative anaerobic order (Zhao et al. 2014).
LEfSe cladogram indicating the phylogenetic distribution of bacterial lineages under different fertilization regimes. Colored circles show the differentially abundant taxa, and the color is corresponding to the different fertilization regimes in which the taxon is the highest abundance (red for CK, green for FFP, blue for SSNM). Six rings of the cladogram stand for domain (innermost), phylum, class, order, family, and genus, and lineages with LDA values higher than 3.0 are displayed
4.3 Microbial community associations with environmental characteristics
Multiple studies have demonstrated that environmental factors such as acidity, available nitrogen and potassium, soil organic carbon, and soil salinity are prevailing environmental factors that can shape soil bacterial population distribution with fertilizer application (Li et al. 2014; Zhao et al. 2014). Significant impacts of fertilizer on the soil microbial community composition have been reported, whereby fertilizer applications can enrich soil nutrients and improve soil properties (Fierer et al. 2007, Li et al. 2014). In this study, results showed that the microbial population distribution was highly related to the soil physicochemical properties, which were mediated by different fertilization regimes. The RDA and Mantel test showed that microbial community structure was significantly related to AP. Phosphorus is an essential element for all forms of life and a constituent of nucleic acids (Adeola 1999); hence, the content and availability of P can influence the composition and diversity of the microbial community. As is shown in our study, the interactions between N and P were major factors (50.56%) that impacted the microbial community. These results indicated that the soil microorganisms were impacted by N and P migration and transformation in the soil, plants, and water. Runoff losses and plant uptake were two important pathways for nutrient migration after fertilizer application. Additionally, the VPA and Mantel test showed that AK (r = 0.537, p = 0.013) and salinity (r = 0.472, p = 0.012) contributed less than AP to microbial community variation. Potassium is an essential element for plant growth, and salinity can influence microbial growth and activity through osmotic stress limits and the metabolic quotient (Rietz and Haynes 2003). Therefore, fertilization affected the microbial structure by changing the microbial living environment, mainly through the cycle of soil nitrogen and phosphorus, followed by altering the soil properties and plant growth conditions, which also indirectly affected the microorganisms.
5 Conclusions
In summary, for the first time, we provided direct evidence of economic and environmental benefits of adopting the SSNM system in vegetable production systems. Our results have clearly demonstrated that fertilizer regime not only affects plant yields but also N losses, soil physicochemical properties, microbial biomass, and bacterial community structure. The SSNM system decreased TN and TP losses by 22.6 and 8.8%, respectively, and increased the crop yields by 23.3% due, in part, to multiple topdressings and matching the plant demand with nutrient supplies. The SSNM system was shown to be a more cost-effective and environmentally friendly fertilization regime than FFP. Positive correlations were found between bacteria community and certain soil physiochemical properties, such as AP, AK, and salinity, and the relative abundance of Proteobacteria, Actinobacteria, and Bacteroidetes changed significantly following the SSNM application. Our results also showed that microbial biomass responded differently to the different fertilization regimes and that this also has major implications for the sustainability of the fertilization regimes in the long term.
References
Acosta-Martinez V, Dowd S, Sun Y, Allen V (2008) Tag-encoded pyrosequencing analysis of bacterial diversity in a single soil type as affected by management and land use. Soil Boil Biochem 40:2762–2770
Adeola O (1999) Nutrient management procedures to enhance environmental conditions: an introduction. J Anim Sci 77:427–429
Albiac J (2009) Nutrient imbalances: pollution remains. Science 326:665–665
Broadhurst MJ, Ardeshir A, Kanwar B, Mirpuri J, Gundra UM, Leung JM, Wiens KE, Vujkovic-Cvijin I, Kim CC, Yarovinsky F, Lerche NW, McCune JM, Loke P (2012) Therapeutic helminth infection of macaques with idiopathic chronic diarrhea alters the inflammatory signature and mucosal microbiota of the colon. PLoS Pathog 8(11):e1003000
Cardinale BJ, Srivastava DS, Duffy JE, Wright JP, Downing AL, Sankaran M, Jouseau C (2006) Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443:989–992
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8:559–568
Chaer G, Fernandes M, Myrold D, Bottomley P (2009) Comparative resistance and resilience of soil microbial communities and enzyme activities in adjacent native forest and agricultural soils. Microb Ecol 58:414–424
Deluca TH (2009) Nutrient imbalances: follow the waste. Science 326:665–665 665; author reply 666
Di HJ, Cameron KC (2002) Nitrate leaching in temperate agroecosystems: sources, factors and mitigating strategies. Nutr Cycl Agroecosys 64:237–256
Dixon P (2003) VEGAN, a package of R functions for community ecology. J Veg Sci 14:927–930
Djodjic F, Borling K, Bergstrom L (2004) Phosphorus leaching in relation to soil type and soil phosphorus content. J Environ Qual 33:678–684
Dobermann A, Witt C, Abdulrachman S, Gines HC, Nagarajan R, Son TT, Tan PS, Wang GH, Chien NV, Thoa V, Phung CV, Stalin P, Muthukrishnan P, Ravi V, Babu M, Simbahan GC, Adviento M (2003) Soil fertility and indigenous nutrient supply in irrigated rice domains of Asia. Agron J 95:913–923
Food and Agriculture Organization (FAO). United Nations (2011) FAO statistical databases.
Fierer N, Bradford MA, Jackson RB (2007) Toward an ecological classification of soil bacteria. Ecology 88:1354–1364
Jenkinson DS, Oades JM (1979) Method for measuring adenosine-triphosphate in soil. Soil Boil Biochemist 11:193–199
Jones RT, Robeson MS, Lauber CL, Hamady M, Knight R, Fierer N (2009) A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J 3:442–453
Ju XT, Kou CL, Zhang FS, Christie P (2006) Nitrogen balance and groundwater nitrate contamination: comparison among three intensive cropping systems on the North China Plain. Environ Pollut 143:117–125
Khurana HS, Phillips SB, Singh B, Alley MM, Dobermann A, Sidhu AS, Singh Y, Peng S (2008) Agronomic and economic evaluation of site-specific nutrient management for irrigated wheat in Northwest India. Nutr Cycl Agroecosys 82:15–31
Li C, Yan K, Tang L, Jia Z, Li Y (2014) Change in deep soil microbial communities due to long-term fertilization. Soil Biol Biochem 75:264–272
Liang XQ, Chen YX, Nie ZY, Ye YS, Liu J, Tian GM, Wang GH, Tuong TP (2013) Mitigation of nutrient losses via surface runoff from rice cropping systems with alternate wetting and drying irrigation and site-specific nutrient management practices. Environ Sci Pollut Res 20:6980–6991
Lin D, Fan X, Hu F, Zhao H, Luo J (2007) Ammonia volatilization and nitrogen utilization efficiency in response to urea application in rice fields of the Taihu Lake region, China. Pedosphere 17:639–645
Liu E, Yan C, Mei X, He W, Bing SH, Ding L, Liu Q, Liu S, Fan T (2010) Long-term effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in Northwest China. Geoderma 158:173–180
Nacke H, Thuermer A, Wollherr A, Will C, Hodac L, Herold N, Schoening I, Schrumpf M, Daniel R (2011) Pyrosequencing-based assessment of bacterial community structure along different management types in German forest and grassland soils. PLoS One 6(2):e17000
Nemergut DR, Townsend AR, Sattin SR, Freeman KR, Fierer N, Neff JC, Bowman WD, Schadt CW, Weintraub MN, Schmidt SK (2008) The effects of chronic nitrogen fertilization on alpine tundra soil microbial communities: implications for carbon and nitrogen cycling. Environ Microbiol 10:3093–3105
Pampolino MF, Manguiat IJ, Ramanathan S, Gines HC, Tan PS, Chi TTN, Rajendran R, Buresh RJ (2007) Environmental impact and economic benefits of site-specific nutrient management (SSNM) in irrigated rice systems. Agric Syst 93:1–24
Peng S, Yang S, Xu J, Luo Y, Hou H (2011) Nitrogen and phosphorus leaching losses from paddy fields with different water and nitrogen managements. Paddy Water Environ 9:333–342
Pote DH, Daniel TC, Sharpley AN, Moore PA, Edwards DR, Nichols DJ (1996) Relating extractable soil phosphorus to phosphorus losses in runoff. Soil Sci Soc Am J 60:855–859
Redmile-Gordon M, White RP, Brookes PC (2011) Evaluation of substitutes for paraquat in soil microbial ATP determinations using the trichloroacetic acid based reagent of Jenkinson and Oades (1979). Soil Biol Biochem 43:1098–1100
Rietz DN, Haynes RJ (2003) Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol Biochem 35:845–854
Rosenfeld JK (1979) Ammonium adsorption in nearshore anoxic sediments. Limnol Oceanogr 24:356–364
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12(6):R60
Shan L, He Y, Chen J, Huang Q, Lian X, Wang H, Liu Y (2015) Nitrogen surface runoff losses from a Chinese cabbage field under different nitrogen treatments in the Taihu Lake Basin, China. Agr Water Manage 159:255–263
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass-C. Soil Biol Biolchem 19:703–707
Verhoeven J, Koerselman W, Meuleman A (1996) Nitrogen- or phosphorus-limited growth in herbaceous, wet vegetation: relations with atmospheric inputs and management regimes. Trends Ecol Evol 11:494–497
Zhang Q, Shamsi IH, Wang J, Song Q, Xue Q, Yu Y, Lin X, Hussain S (2013) Surface runoff and nitrogen (N) loss in a bamboo (Phyllostachys pubescens) forest under different fertilization regimes. Environ Sci Pollut Res 20:4681–4688
Zhao J, Ni T, Li Y, Xiong W, Ran W, Shen B, Shen Q, Zhang R (2014) Responses of bacterial communities in arable soils in a rice-wheat cropping system to different fertilizer regimes and sampling times. PLoS One 9(1):e85301
Zhao J, Ni T, Li J, Lu Q, Fang Z, Huang Q, Zhang R, Li R, Shen B, Shen Q (2016) Effects of organic-inorganic compound fertilizer with reduced chemical fertilizer application on crop yields, soil biological activity and bacterial community structure in a rice-wheat cropping system. Appl Soil Ecol 99:1–12
Zheng XH, Han SH, Huang Y, Wang YS, Wang MX (2004) Re-quantifying the emission factors based on field measurements and estimating the direct N(2)O emission from Chinese croplands. Global Biogeochem Cy 18(2). https://doi.org/10.1029/2003GB002167
Zhu ZL, Chen DL (2002) Nitrogen fertilizer use in China—contributions to food production, impacts on the environment and best management strategies. Nutr Cycl Agroecosys 63:117–127
Zhu T, Zhang J, Yang W, Cai Z (2013) Effects of organic material amendment and water content on NO, N2O, and N2 emissions in a nitrate-rich vegetable soil. Biol Fert Soils 49:153–163
Funding
The authors gratefully acknowledge the financial support from the National Key Science and Technology Project: Water Pollution Control and Treatment (2014ZX07101-012), the National Key Research and Development Program of China (2016YFD0801103), and Science & Research Program of Zhejiang Province (Grant No. 2016C32084).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zucong Cai
Electronic supplementary material
ESM 1
(DOCX 193 kb)
Rights and permissions
About this article
Cite this article
Yi, B., Zhang, Q., Gu, C. et al. Effects of different fertilization regimes on nitrogen and phosphorus losses by surface runoff and bacterial community in a vegetable soil. J Soils Sediments 18, 3186–3196 (2018). https://doi.org/10.1007/s11368-018-1991-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-018-1991-6