Abstract
Purpose
Returning straw to soil improved soil carbon sequestration capacity and increase soil organic matter. However, in different soil depth, especially in subsoil, there were few studies on the effects of straw decomposition on soil carbon sequestration and the properties of humic substances. Therefore, an in-situ incubation study, with six different straw rates and three different soil depths, was carried out to explore the effects of straw decomposition on soil organic carbon and humic substance composition at different soil depths.
Materials and methods
The experiment was composed of six straw rates: 0, 0.44, 0.88, 1.32, 2.64, and 5.28% of soil dry mass. The maize straw was proportionately mixed with soil and put into nylon bags. Then, the nylon bags were buried in soil at three depths (15, 30, and 45 cm) and the straw decomposition trial lasted for 17 consecutive months in-situ. Soil samples were collected after completion of the field trial. Humic substances were quantitatively and qualitatively analyzed using the modification method of humus composition and the methods specified by the International Humus Association. Fourier transform infrared spectroscopy and fluorescence spectroscopy were used in this study.
Results and discussion
Results indicated that CO2 concentration increased with increase in soil depth. Compared with the “zero” straw control, soil organic carbon contents in the treatments amended with 1.32, 2.64, and 5.28% maize straw increased significantly, and most accumulations were at 30–45 cm depths. FTIR and fluorescence emission spectra analyses indicated that the addition of straw enhanced the aliphatic structure and decreased the aromaticity of humic acid (HA), that was to say that HA molecular structure approaches to the development of simplification and younger. The maximum change in HA molecular structure was under the 5.28% treatment in the 30–45 cm depth.
Conclusions
Returning maize straw to the subsoil layers is more conducive to the accumulation of soil organic carbon and improvement of the quality and activity of HA and the organic carbon in the subsoil can be renewed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Returning crop straw to soil becomes one of the important measures to improve soil organic matter and maintain soil fertility. However, as the amount of crop straw remaining on the soil surface diminished, the inputs of organic carbon decreased, which may potentially reduce the content of soil organic matter and influence soil formation (Karlen et al. 2011; Jin et al. 2015; Tormena et al. 2017). Humic substance is one of the most important components of soil organic matter (Vergnoux et al. 2011). Humic substance can be classified into humic acid (HA), fulvic acid (FA), and humin (Hu) (Song et al. 2014). Therefore, carbon sequestration in humic substance is very important for understanding global carbon cycle. Studies showed that HAs extracted from fertilized soils contain more aliphatic and phenolic hydroxyl groups than the HAs extracted from un-fertilized soils (Galantini and Rosell 2006). Aliphatic and aromatic molecules containing hydrophobic carbon are selectively preserved in humic substances (Spaccini et al. 2002), and the hydrophobic components are able to prevent the entry of degrading enzymes (Piccolo et al. 1999).
Lenka and Lal (2013) found that the organic matter content in 0–10 cm soil layer increased by 25 and 50%, respectively, with 8 and 16 t hm−2 straw mulching for 15 years. Bhattacharyya et al. (2012) showed that the application of rice straw together with inorganic nitrogen fertilizers can significantly increase the contents of total carbon in surface soil. Many researchers have showed that straw management, incorporating straw into the soil or mulching on the surface, has a significant effect on the properties of the surface soil (Zhang et al. 2012; Chang et al. 2014; Yang et al. 2015). The fertility status in the subsoil impacts on crop yields (Breulmann et al. 2016; Cao et al. 2003). Fontaine et al. (2007) indicated that the poor permeability of the plow pan below (at 20 cm) limits the relocation of water and organic materials into the subsoil, and this jeopardizes subsoil fertility in the long run. Lützow et al. (2006) showed that the environmental conditions in the subsoil are different from those in the topsoil. For example, the temperature change in the subsoil is small and the nutrient availability is low, which may lead to a decrease in organic matter mineralization associated with a potential increase in organic matter accumulation in the subsoil. And Nkebiwe et al. (2016) indicated that deep fertilizers placement can reduce the negative effects of increasing temperatures and droughts on global food production. In a laboratory experiments, Kuzyakov (2010) studied the effects of substrate addition to different soil depths on dynamics of soil organic matter, but the results applied to field scale may not be fully applicable and more field trials are needed.
The purpose of this paper was to test the following hypotheses: (1) more organic carbon accumulation would occur in deeper than shallow soil when the same amount of maize straw was applied; (2) soil humus compositions and HA structure characteristics vary in soil depth. In this study, a 17-month long field incubation experiment was conducted to study the effects of maize straw decomposition on soil organic carbon and humus in different soil depths under the natural conditions in northeastern China.
2 Materials and methods
2.1 Experimental site
The experiment field was selected from the monoculture corn study at Jilin Agricultural University Experimental Station which was located in Nanguan District of Changchun City, Jinlin province (43° 48′ 38.20″ N, 125° 23′ 16.81″ E). The climate in this site belongs to north temperate continental monsoon type, with distinct four seasons and moderate dry-wet characteristics. The mean annual temperature and precipitation were 4.8 °C and 617 mm, respectively, and most of precipitation falls between July and August. The soil in this site was classified as black soil (FAO 1990).
2.2 Experimental materials
Soil was collected from 0 to 15 cm (S1), 15–30 cm (S2), and 30–45 cm (S3) depth. Selected chemical properties of the soil were shown in Table 1. Experimental crop residues were mature maize straw. Straws were chopped into 3–5 cm size pieces. The straw had the following properties: organic carbon content is 437.6 g kg−1, total nitrogen content is 5.72 g kg−1, and C/N is 76.50.
2.3 Experimental design
Six rates of straw to soil was used in this study, ranging from 0% (B0), 0.44%(B1), 0.88% (B2), 1.32% (B3), 2.64% (B4), and 5.28% (B5) of soil dry mass. The treatment of 0.44% is equivalent to the full amount of straw returned to field. According to the annual straw production in this area, we assumed that the total amount of straw returned to soil (bulk density of 1.2 g cm−3) in 0–20 cm layers was 10 t ha−1. A given amount of straw (varied with treatments) was homogeneously mixed with soil (4 kg for each treatment). The mixture was filled into nylon bags (mesh size, 100 μm). The nylon bags were buried into soil at three depths (15, 30, and 45 cm) with three replicates in May 1, 2014. The nylon bags were dug out from the ground after 17 months. The soil samples were air-dried and the undecomposed straw were removed.
2.4 CO2 measurement methods
Concentrations of CO2 in bags were determined using alkali absorption method. Briefly, one end of the infusion tubes (B1-1, Jiangxi Hongda Medical Devices Group, Ltd., China.) with holes were buried into each nylon bag, and the other end is exposed to the surface. Syringes filled with a given volume of NaOH solution were used to suck in a certain volume of gas from the infusion tube. The CO2 in inhaled gas will be absorbed by the NaOH solution and then the syringes were sealed quickly and transported back to lab. The content of CO2 in solutions was immediately determined using alkali titration method. CO2 concentrations were measured approximately every 10 days and the sampling period was from July 6 to September 30 in 2014. And then the relative amount of CO2 in the soil air was calculated.
2.5 Analysis methods
Total nitrogen was analyzed using semi-micro Kjeldahl method; alkalytic nitrogen was analyzed using alkali diffusion method; available phosphorus was analyzed using sodium bicarbonate–molybdenum antimony colorimetry; available potassium was analyzed using flame photometer, pH was measured by using pH meter (Lu 2000). Soil organic carbon (SOC) and the content of C in humic substance were determined using K2Cr2O7 external heating method (Lao 1988).
Humus composition was analyzed following the method described by Dou (2010). Briefly, soil was extracted by mixed alkali solution (NaOH + Na4P2O7) and then centrifuged. The supernatant was humic extractable substance (HE). Thirty milliliters HE was acidified to pH 1 to separate humic acid (HA) from humic fulvic (FA). The precipitation was HA and FA remained in solution. HA was re-dissolved with 0.05 mol L−1 NaOH. All solutions were used for concentration analysis.
HA isolation and purification were processed using the procedure described by International Humic Substances Society (Kuwatsuka et al. 1992). Briefly, the soil was decalcified with HCl, then residues were extracted by NaOH solution at 24 h extraction, then centrifuged. The supernatant was HE. The HE solution was acidified to pH 1.0. The precipitation was HA. The received HA residue was required to remove ash and was dialysed to eliminate excess salts, and freeze-dried afterwards.
FTIR spectra of HA were obtained in the 4000 to 500 cm−1 wavenumber using a Nicolet spectrophotometer on KBr pellets. Fluorescence spectra in emission were obtained with a Hitachi F4500 fluorescence spectrophotometer on water extracts of HA at a concentration of 100 mg L−1 and the pH was adjusted to 8.0. Emission spectra were recorded over 400–650 nm at a constant excitation wavelength of 360 nm. The spectra semi-quantitative analyses were carried out using the analytical software of the instrument. The peak intensities were compared with the percentage of each peak area.
2.6 Statistical analysis
The experimental date and figures were analyzed and plotted using Microsoft Excel 2013 and OriginPro 8.0. The significant differences between different treatments were statistically analyzed by SPSS 22.0. All experiments were analyzed as randomized complete design with three replications. Straw rates and buried depths were treated as fixed factors and a mixed model procedure was used for analyzing the treatment impacts on CO2, SOC, and HA properties. PQ value and HA-C: FA-C were used as the parameters for the degree of humification (Dias et al. 2010). The PQ value was calculated as follows: PQ = HA-C / (HA-C + FA-C)*100. The △LogK was the hue coefficient (Kumada et al. 1967), which was the logarithm disparity between 400 and 600 nm absorbance of humic substance in solution.
3 Results
3.1 CO2 concentration
The CO2 concentration with different straw incorporation levels was reported in Fig. 1. The CO2 concentration increased with increase in straw application rate in the same layer. With incubation time increase, CO2 concentration showed a tendency to first increase and then decrease. In the same straw application level, the increase in CO2 concentration followed the trend of S1<S2<S3. It was observed that CO2 concentration increased with increase in soil depth.
Changes of soil CO2 concentration at different depths. a Changes of soil CO2 concentration at 0–15 cm depth. b Changes of soil CO2 concentration at 15–30 cm depth. c Changes of soil CO2 concentration at 30–45 cm depth. S1, S2, and S3 represent 0–15, 15–30, and 30–45 cm soil layers, respectively. B0, B1, B2, B3, B4, and B5 represent 0, 0.44, 0.88, 1.32, 2.64, and 5.28% of the straw incorporation levels, respectively
3.2 Soil organic carbon content
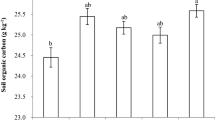
Figure 2 showed the contents of soil total organic carbon after 17 months incubation. The SOC contents increased with increase in straw application level in the same soil layer, and the treatments of B3, B4, and B5 showed significantly increase in SOC contents compared with the control (B0). With the exception of B3 treatment, the SOC contents in the same straw rate treatment followed the trend of S1>S2>S3. In layer S1, S2, and S3, the SOC contents with the treatments from the B1 to B5 increased in the range of 2.11 to 55.18%, 7.86 to 57.11%, and 8.57 to 68.22% than the B0, respectively. Thus, it could be seen that for the treatment with the same straw application rate, the deeper the straw incorporated into soil, the less of C loss from the straw.
Changes of soil organic carbon in different depth. S1, S2, and S3 represent 0–15, 15–30, and 30–45 cm soil layers, respectively. B0, B1, B2, B3, B4, and B5 represent 0, 0.44, 0.88, 1.32, 2.64, and 5.28% of the straw incorporation levels, respectively. Lowercase letters represent significant differences (p < 0.05) between different straw incorporation levels in the same soil layer.
3.3 Composition of soil humus
The concentrations of composition of humus during in-situ incubation were shown in Table 2. The contents of humic extractable substance carbon (HE-C) and humic acid carbon (HA-C) increased with increase in straw application rate in the same layer. As already observed that the increase of HE-C in the treatment with same straw application level followed the trend of S2>S3>S1. The highest relative increase in HA-C occurred in the B5 at the layer S2 with an increase of 85.52% relative to the B0. The highest content of fulvic acid carbon (FA-C) appeared in treatment B5 at the surface layer (S1) and it was 59.72% higher than that in the B0.
Except for the treatments B4 and B5 in layer S1, the addition of straw increased PQ values compared with the B0 (Table 2). HA-C:FA-C ratio was altered with the increasing rates of straw incorporation at all soil layers and the increase followed the trend of S3>S2>S1. These results reflected the transformation of FA to HA and the increase of HA-C fraction with increase in soil depth.
The △LogK values with B0 treatment were decreased with soil depth increased (Table 2). But △LogK values in other treatments showed no consistent changes among straw application and soil depth.
3.4 FTIR spectra analysis
The FTIR spectrum of HA for the treatments with different straw application rates were shown in Fig. 3 and the corresponding absorbing peaks were listed in Table 3. The positions of absorbing peaks were approximately identical, among the treatments. A common and intense broad band at about 3400 cm−1 usually attributed to vibrations of -NH and/or -OH groups of either adsorbed water or silanol moieties in all spectrum; the bands at about 2920 and 2850 cm−1 were associated with the aliphatic C-H group stretching; the absorption peaks at about 1700 cm−1 were assigned to C = O stretching of COOH; the bands at about 1640 cm−1 were mainly due to C = O stretching vibration of amide group (Campitelli and Ceppi 2008; Rashad 2013). The bands at about 2920 and 2850 cm−1 vibration of HA were more intense with straw application than no straw CK and the intensity increased with the rate of straw application, while the changes of vibration at 1640 cm−1 showed an opposite trend.
3.5 Fluorescence emission spectra analysis
The fluorescence emission spectra of HA were shown in Fig. 4. Generally, the fluorescence emission spectra of HA featured a unique broad band with the maximum centered at 500 to 550 nm wavelength, showing similar fluorescent groups of HA in different treatments. In layer S1, the fluorescence peak of HA for treatment B5 was blueshifted (i.e., the characteristic peak shifts in the direction in which the wavelength decreases) in comparison with treatment B0. The similar variations of peaks position in layer S2 and S3 were also observed. The fluorescence peaks of HA were blueshifted for treatment B1, B3, and B5 comparing with treatment B0, and the degree of blueshift in treatment B5 was the largest. The fluorescence intensity of HA was greater for the treatments amended with maize straw than the B0 (Table 4). And observed that the fluorescence intensity of HA also enhanced with increase in straw application rate.
4 Discussion
Straw addition resulted in soil organic carbon increase. Similar results had been reported by Yang et al. (2017). And the increased soil organic carbon was positively related to the straw application rate with significant increase occurred in treatment B3, B4, and B5. These changes in soil organic carbon content reflect the balance between carbon supplement and loss (Sollins et al. 1996). In the short term, high amounts of straw addition to the soil made carbon supplement greater than the loss of soil carbon and resulted in carbon accumulation in the tested soil. We also found that more SOC was accumulated in the 30–45 cm soil than in the 0–15 cm soil layer for the treatments with the same straw rate, which might be attributed to differences in soil aeration (Kisselle et al. 2001) in different soil depths. The soil aeration is higher in the topsoil than in subsoil, so the decomposition of straw in the topsoil was faster than that in subsoil. Lower soil aeration leads to low decomposition of straw which results in more soil organic carbon accumulation (Rottmann et al. 2010; Jiao et al. 2015).
Humic substances are ubiquitous compounds that have a high molecular weight and heterogeneous structure (Wagner et al. 2016). In this study, straw addition increased the contents of HE-C and this result is consistent with previous study (Huang et al. 2008). Song et al. (2014) also showed that humus content was relatively high with the high organic manure addition. This indicates that the degradation of straw will lead to formation of humus (Lhadi et al. 2006). Because humic substances are the main component of soil organic carbon, the accumulation of humic substances has a great influence on soil fertility. The results of humification degree of HA indicate that the proportion of HA increased with increase in soil depths and straw application rates. This was because the carbon in maize straw was preferentially converted to the humic acid rather than to fulvic acid (Song et al. 2017). And it also showed that transformation of FA into HA increased (Ingelmo et al. 2012), which increased the stability of soil organic carbon.
FTIR spectroscopy can be used to measure the transition between molecular vibrational energy levels, which is mainly used to reflect the characteristics of the functional groups of humic substances in soil science (Rodríguez et al. 2016; Ait Baddi et al. 2004; Hung et al. 2013). Straw incorporation compared with non-straw CK increased the aliphatic C-H stretching vibration of HA, which indicates that the application of organic materials made the HA increased in aliphaticity and weakened in aromaticity (Wu et al. 2005). Zhang et al. (2011) considered that the changes of soil humic acid are mainly due to the entry of HA extracted from straw into the soil.
Recently, fluorescence spectroscopy has been using to analyze structure of humic substances in many studies (Valencia et al. 2013; Halim et al. 2013; Horst et al. 2013; Yang et al. 2016; Aftab and Hur 2017). It has been suggested that a reduction in the degree of humification may be revealed by a blueshift of characteristic peak of fluorescence spectrum. On the contrary, the fluorescence spectrum may be redshifted (i.e., the characteristic peak shifts in the direction in which the wavelength increase) if the degree of humification is increased (Senesi et al. 1991; Miano and Senesi 1992). The fluorescence peaks of HA with straw incorporation showed blueshift at different degrees, with the maximum shift in S3B5 treatment. These indicate that the decrease in degree of humification of HA is associated with simplification of the molecular structure of HA. Traversa et al. (2014) showed that the substituent groups are improved with increase in fluorescence intensity, while the lower the fluorescence intensity indicates that the structure of HA is prone to complex. More hydroxyl, methoxy, and amino groups were found in HA from the treatments amended with maize straw. The addition of straw into the soil promotes a large number of metabolizable organic compounds into the soil organic matter (Bayer et al. 2002). The formation of new organic matter is rich in aliphatic compounds and a lower proportion of aromatic carbon, so the structure of HA also changes. Stevenson (1992) considered that the decomposition of straw in subsoil is mainly under the role of anaerobic microorganisms and is not conductive to the increase of HA aromatization. It is favorable for soil organic carbon fractions containing high-aliphatic polymers to remain in the subsoil for a long period of time, thereby increasing the carbon sequestration (Lorenz and Lal 2005).
5 Conclusions
In this paper, the results are consistent with our hypothesis that the soil organic carbon accumulation varied in soil depth and the maximum organic carbon accumulation appeared in deep soil (30–45 cm). The largest change of molecular structure of humic acid was also found in 30–45 cm soil depth. Therefore, straw addition to subsoil was more conducive to carbon sequestration and to quality and quantity improvement of humic acid. These results also could be used as reference for those who are interested in applying crop straw to deep soil. However, future research will need to measure, at the field scales, the influence of straw returning location on soil fertility and crop yields.
References
Aftab B, Hur J (2017) Fast tracking the molecular weight changes of humic substances in coagulation/flocculation processes via fluorescence EEM-PARAFAC. Chemosphere 178:317–324
Ait Baddi G, Hafidi M, Cegarra J, Alburquerque JA, Gonzálvez J, Gilard V, Revel JC (2004) Characterization of fulvic acids by elemental and spectroscopic (FTIR and 13C-NMR) analyses during composting of olive mill wastes plus straw. Bioresour Technol 93:285–290
Bayer C, Martin-Neto L, Mielniczuk J, Saab SDC, Milori DMP, Bangnato VS (2002) Tillage and cropping system effects on soil humic acid characteristics as determined by electron spin resonance and fluorescence spectroscopies. Geoderma 105:81–92
Bhattacharyya P, Roy KS, Neogi S, Adhya TK, Rao KS, Manna MC (2012) Effects of rice straw and nitrogen fertilization on greenhouse gas emissions and carbon storage in tropical flooded soil planted with rice. Soil Till Res 124:9–30
Breulmann M, Boettger T, Buscot F, Gruendling R, Schulz E (2016) Carbon storage potential in size-density fractions from semi-natural grassland ecosystems with different productivities over varying soil depths. Sci Total Environ 545-546:30–39
Campitelli P, Ceppi S (2008) Effects of composting technologies on the chemical and physicochemical properties of humic acids. Geoderma 144:325–333
Cao GJ, Ren J, Wang Y (2003) Study on specific property of high-yielding soil fertility for maize in JiLin province. Journal of JiLin Argricultural University 25:307–310, 314
Chang ZZ, Wang DJ, Yang SJ, Wang C, Zhang SM (2014) Thoughts on returning straw to field. Jiangsu J of Agr Sci 30:304–309
Dias BO, Silva CA, Higashikawa FS, Roig A, Sánchez-Monedero MA (2010) Use of biochar as bulking agent for the composting of poultry manure: effect on organic matter degradation and humification. Bioresour Technol 101:1239–1246
Dou S (2010) Soil organic matter. Science Press, Beijing
FAO-ISRIC (1990) Guidelines for soil description, third ed, revised, food and agricultural organization. Rome
Fontaine S, Barot S, Barré P, Bdioui N, Mary B, Rumpel C (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450:277–280
Galantini J, Rosell R (2006) Long-term fertilization effects on soil organic matter quality and dynamics under different production systems in semiarid Pampean soils. Soil Till Res 87:72–79
Halim M, Spaccini R, Parlanti E, Amezghal A, Piccolo A (2013) Differences in fluorescence properties between humic acid and its size fractions separated by preparative HPSEC. J Geochem Explor 129:23–27
Horst C, Sharma VK, Clayton BJ, Sohn M (2013) Organic matter source discrimination by humic acid characterization: synchronous scan fluorescence spectroscopy and ferrate(VI). Chemosphere 90:2013–2019
Huang HL, Zeng GM, Tang L, Yu HY, Xi XM, Chen ZM, Huang GH (2008) Effect of biodelignification of rice straw on humification and humus quality by Phanerochaete chrysosporium and Streptomyces badius. Int Biodeter Biodegr 61:331–336
Hung PVX, Oh BS, Tung BX, Oh SG, Kim KS, Kim SJ, Moon SH, Kim IS, Jang A (2013) Reflection of the structural distinctions of source-different humic substances on organic fouling behaviors of SWRO membranes. Desalination 318:72–78
Ingelmo F, Molina MJ, Soriano MD, Gallardo A, Lapeña L (2012) Influence of organic matter transformations on the bioavailability of heavy metals in a sludge based compost. J Environ Manag 95:S104–S109
Jiao LN, Li ZH, Yin CC, Wang XF, Xin SY, Yu L (2015) Effect of incorporation of crop straw on composition of soil organic matter and enzyme activity in black soil relative to depth and rate of the incorporation. Acta Pedol Sin 52:198–205
Jin VL, Schmer MR, Wienhold BJ, Stewart CE, Varvel GE, Sindelar AJ, Follet RF, Mitchell RB, Vogel KP (2015) Twelve years of stover removal increases soil erosion potential without impacting yield. Soil Sci Soc Am J 79:1169–1178
Karlen DL, Birell SJ, Hess JR (2011) A five-year assessment of corn stover harvest in central Iowa, USA. Soil Till Res 115-116:47–55
Kisselle KW, Garrett CJ, Fu S, Hendrix PF, Jr DAC, Coleman DC, Potter RL (2001) Budgets for root-derived C and litter-derived C: comparison between conventional tillage and no tillage soils. Soil Bio Biochem 33:1067–1075
Kumada K, Sato O, Ohsumi Y, Ohta S (1967) Humus composition of maintain soil in central Japan with special reference to the distribution of P type humic acid. Soil Sci Plant Nutr 13:151–158
Kuwatsuka S, Watanabe A, Itoh K, Arai S (1992) Comparison of two methods of preparation of humic and fulvic acids, IHSS method and NAGOYA method. Soil Sci Plant Nutr 38:23–30
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Bio Biochem 42:1363–1371
Lao JS (1988) Analytical handbook of soil and agro-chemistry. Agriculture Press, Beijing
Lenka NK, Lal R (2013) Soil aggregation and greenhouse gas flux after 15 years of wheat straw and fertilizer management in a no-till system. Soil Till Res 126:78–89
Lhadi EK, Tazi H, Aylaj M, Genevini PL, Adani F (2006) Organic matter evolution during co-composting of the organic fraction of municipal waste and poultry manure. Bioresour Technol 97:2117–2123
Lorenz K, Lal R (2005) The depth distribution of soil organic carbon in relation to land use and management and the potential of carbon sequestration in subsoil horizons. Adv Agron 88:35–66
Lu RK (2000) Analytical methods for soil and agrochemistry. China Agricultural Science and Technology Press, Beijing
Lützow MV, Kögel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H (2006) Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—a review. Euro J Soil Sci 57:426–445
Miano TM, Senesi N (1992) Synchronous excitation fluorescence spectroscopy applied to soil humic substances chemistry. Sci Total Environ 117:41–51
Nkebiwe PM, Weinmann M, Bar-Tal A, Müller T (2016) Fertilizer placement to improve crop nutrient acquisition and yield: a review and meta-analysis. Field Crop Res 196:389–401
Piccolo AR, Spaccni R, Haberhauer G, Gerzabek MH (1999) Increased sequestration of organic carbon in soil by hydrophobic protection. Naturwissenschaften 86:496–499
Rashad RT (2013) Separation of some rice straw components and studying their effect on some hydro-physical properties of two different soils. J Environ Chem Eng 1:728–735
Rodríguez FJ, Schlenger P, García-Valverde M (2016) Monitoring changes in the structure and properties of humic substances following ozonation using UV-Vis, FTIR and 1H NMR techniques. Sci Total Environ 541:623–637
Rottmann N, Dyckmans J, Georg R (2010) Microbial use and decomposition of maize leaf straw incubated in packed soil columns at different depths. Eur J Soil Biol 46:27–33
Senesi N, Miano TM, Provenzano MR, Brunetti G (1991) Characterization, differentiation and classification of humic substances by fluorescence spectroscopy. Soil Sci 152:248–252
Sollins P, Homann P, Caldwell BA (1996) Stabilization and destabilization of soil organic matter: mechanisms and controls. Geoderma 74:65–105
Song XY, Liu ST, Liu QH, Zhang WJ, Hu CG (2014) Carbon sequestration in soil humic substances under long-term fertilization in a wheat-maize system from north China. J Integr Agr 13:562–569
Song GX, Novotny EH, Mao JD, Hayes MHB (2017) Characterization of transformations of maize residues into soil organic matter. Sci Total Environ 579:1843–1854
Spaccini R, Piccolo A, Conte P, Haberhauer G, Gerzabek MH (2002) Increased soil organic carbon sequestration through hydrophobic protection by humic substances. Soil Biol Biochem 34:1839–1851
Stevenson FJ (1992) Humus chemistry genesis, composition, reactions. China Agricultural University Press, Beijing
Tormena CA, Karlen DL, Logsdon S, Cherubin MR (2017) Corn stover harvest and tillage impacts on near-surface soil physical quality. Soil Till Res 166:122–130
Traversa A, D’Orazio V, Mezzapesa GN, Bonifacio E, Farrag K, Senesi N, Brunetti G (2014) Chemical and spectroscopic characteristics of humic acids and dissolved organic matter along two Alfisol profiles. Chemosphere 111:184–194
Valencia S, Marín JM, Restrepo G, Frimmel FH (2013) Application of excitation-emission fluorescence matrices and UV/Vis absorption to monitoring the photocatalytic degradation of commercial humic acid. Sci Total Environ 442:207–214
Vergnoux A, Guiliano M, Di Rocco R, Domeizel M, Théraulaz F, Doumenq P (2011) Quantitative and mid-infrared changes of humic substances from burned soils. Environ Res 111:205–214
Wagner M, Schmidt W, Imhof L, Grübel A, Jähn C, Georgi D, Petzoldt H (2016) Characterization and quantification of humic substances 2D-fluorescence by usage of extended size exclusion chromatography. Water Res 93:98–109
Wu JG, Wang MH, Jiang YM, Xu Y (2005) Study on humic acids in the soil applied with corn stalk by spectroscopy measurements. Sci Agric Sin 38:1394–1400
Yang HS, Yang B, Dai YJ, Xu MM, Koide RT, Wang XH, Liu J, Bian X (2015) Soil nitrogen retention is increased by ditch-buried straw return in a rice-wheat rotation system. Eur J Agron 69:52–58
Yang Z, Du MC, Jiang J (2016) Reducing capacities and redox potentials of humic substances extracted from sewage sludge. Chemosphere 144:902–908
Yang X, Meng J, Lan Y, Chen WF, Yang TX, Yuan J, Liu SN, Han J (2017) Effects of maize stover and its biochar on soil CO2 emissions and labile organic carbon fractions in Northeast China. Agr Ecosys Environ 240:24–31
Zhang JJ, Hu F, Li HX, Gao Q, Song XY, Ke XK, Wang LC (2011) Effects of earthworm activity on humus composition and humic acid characteristics of soil in a maize residue amended rice–wheat rotation agroecosystem. Appl Soil Ecol 51:1–8
Zhang WJ, Xu MG, Wang XJ, Huang QH, Nie J, Li ZZ, Li SL, Hwang SW, Lee KB (2012) Effects of organic amendments on soil carbon sequestration in paddy fields of subtropical China. J Soils Sediments 12:457–470
Acknowledgements
Financial support for this study was provided by the Jilin Provincial Department of Science and Technology (LFGC14213) and Ministry of Science and Technology Food Project (2012BAD04B02). We also thank three anonymous reviewers for helpful comments to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Francisco Javier González-Vila
Rights and permissions
About this article
Cite this article
Cui, T., Li, Z. & Wang, S. Effects of in-situ straw decomposition on composition of humus and structure of humic acid at different soil depths. J Soils Sediments 17, 2391–2399 (2017). https://doi.org/10.1007/s11368-017-1704-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-017-1704-6