Abstract
Purpose
With its high economic benefits, navel orange (Citrus sinensis) has been widely planted and fertilizer has been increasingly applied in the subtropical China in the last 30 years. Comprehensive assessments are needed to explore the long-term fertilization impacts on soil chemical and biological properties in the navel orange orchards.
Materials and methods
Through a large number of soil and leaf samples from the young, middle-aged, and mature navel orange orchards, this study examined the impacts of stand age (corresponding to the fertilization year using compound chemical fertilizer) on seasonal variations in major soil properties and leaf nutrients in the subtropical China.
Results and discussion
Soil total nitrogen (N) and mineral N were significantly higher in the middle-aged and mature orchards than in the young orchard. Total phosphorus (P), available P, labile P, slow P, occluded P, weathered mineral P, total exactable P, and residual P generally increased with fertilization years (P < 0.05), and the increasing percentages for soil P fractions were much higher than those for N variables. The total N and P use efficiencies (plant uptake/soil input) were 20–34 and 10–15 %, respectively. Soil microbial biomass, invertase, urease, and acid phosphatase activities showed significant seasonal variations and decreased with fertilization years. Leaf N concentration significantly decreased with fertilization years, but no difference was found for P.
Conclusions
Soil self-fertilization was impeded, and less fertilizer amount should be applied especially in the older navel orange orchards since N and P accumulations do not increase leaf nutrients but worsen soil biological quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
There is an increasing demand for agricultural products, which not only meet high-quality standards but also are environmentally friendly (Carey et al. 2009). To fulfill this demand, agriculture requires an ecological approach that is largely missing from current managements and research portfolios (Robertson and Swinton 2005). In traditional agricultural production, farmers usually focus on productivities at the expense of environmental costs, such as degradation of soil quality due to inappropriate or incorrect agricultural practices (Springett et al. 1994). Fertilization has been one of the most important ways to improve agricultural productivity around the world. Being the largest fertilizer consumption country, China consumed more than 1/3 total chemical fertilizer of the world, approximately 52.0 × 106 tons/year (Li and Jin 2011). Clearly, it is a challenge for agriculturists and ecologists to achieve a balance between high food production and environmental protection (Lemaire et al. 2014), since over fertilization has brought lots of problems, such as soil structure degradation and environmental pollution (Kumazawa 2002; Fernández-Escobar et al. 2009; Jiao et al. 2012).
Over fertilization is defined as “the application of fertilizer in excess of the tree or crop capacity to use it for optimum productivity” (Weinbaum et al. 1992), which has been widely found in intensive agricultural areas (Conley et al. 2009; Ma et al. 2012). Vitousek et al. (2009) stressed that fertilizer used in developing countries like China far exceed those in the USA and Northern Europe, and much of the excess fertilizer (>200 kg N ha−1 year−1) is lost to the environment. Hatano et al. (2002) found crop nitrogen (N) uptake ranged from 52 to 148 kg N ha−1 year−1, which only accounted for 14.2–83.4 % of total N input. Even in the poor Karst region, southwest China, 16.6–85.8 % of total input N was lost from the fields in farm systems (Hatano et al. 2002). Soil fertility, especially N and phosphorus (P) availability, is a key concern in sustainable agriculture and a central emphasis of projects attempting to monitor soil quality (Schloter et al. 2003). Both N and P are generally the key limiting nutrients in terrestrial ecosystems, but in cases of excessive application they could become pollutants in surrounding areas (Carey et al. 2009). Meanwhile, soil microbes are considered as a sensitive indicator of soil fertility (Joergensen and Emmerling 2006), because they are involved in nutrient cycling, maintenance of soil structure, and organic matter decomposition (Wardle et al. 2004). Additionally, soil enzymes and microbe-based processes are considered particularly important because they usually respond more rapidly than chemical and physical variables to environmental changes and stresses such as N and P pollutions (Schloter et al. 2003). Recently, leaf nutrients and N/P ratio have been used to indicate soil N and/or P limitation to plant growth (Koerselman and Meuleman 1996; Aerts and Chapin 2000; Güsewell 2004). To sum up, studies on soil and leaf nutrients and the budget between input and output were widely carried out for agricultural ecosystem (Pathak et al. 2010) or orchard ecosystem in many countries in order to assess and guide fertilization management (Roccuzzo et al. 2012).
Navel orange (Citrus sinensis) is a common fruit distributed in more than 100 countries and a major horticultural commodity in the world (Hu et al. 2008; Shamseldin et al. 2010; El-Wakeel and Mansour 2014). It was introduced to China from America in 1978 and has been widely cultivated for fresh food and orange juice processing in south China because of its deliciousness, good texture, and evident nutraceutical effects (Hu et al. 2008). Jiangxi Province is the largest navel orange production base in south China with the area of about 100,000 ha in 2009 (Guo 2011). As a major contributor to the local economy, sustainable development of navel orange orchards plays a vital role in farmers’ income (Guo 2011). Local farmers primarily aim to produce high yields of high-quality navel oranges through intensive management practices such as N and P fertilizer application, weed control, and pest management; however, the impacts of these management practices on navel orange soil properties and the surrounding environment have not yet been assessed (Wang et al. 2011).
To evaluate the impacts of long-term fertilization practices on the soil quality and the environment, we attempted to determine the temporal dynamics of soil physical, chemical, biological properties, and leaf nutrients along an age gradient of intensively managed navel orange orchards as well as the nutrient balance between plant uptake and soil input. We also explored the synchronism between belowground and aboveground processes through simultaneously measuring the seasonal dynamics of leaf and soil nutrients in the navel orange orchards of the southern China. Our main hypotheses are (1) soil N and P concentrations increase with increasing stand age under fertilization because the amounts of nutrients annually removed were lower than those input into soil; (2) soil microbial properties including biomass and enzyme activities decrease with increasing stand age due to over fertilization (Paudel et al. 2012); and (3) leaf N and P levels increase but N/P ratio depends on the relative increase in soil N and P availability (Chen et al. 2015).
2 Materials and methods
2.1 Study area
The study area (25° 12′ N, 114° 04′ E) is a typical mid-subtropical region, located in Xinfeng County, Jiangxi Province, China. The mean annual precipitation in this region is 1510 mm and average annual temperature is 19.5 °C. The soil is classified as Ultisol developed from sandstones (Staff 2010), which is a typical soil type in the central subtropics of China. The average elevation is about 50 m. The county is one of the major regions for navel orange production and is well known as “the town of Chinese navel orange” due to its most favorable climate and soil conditions for navel orange growth (Guo 2011).
2.2 Plot establishment
Three different aged navel orange orchards (i.e., young, middle-aged, and mature) were selected at nine sites and each of the orchards includes three sites (replications) in Anxi Town of Xinfeng County in February, 2008. The young orchards were planted in 1999 with a spacing of 2 × 2 m. The average basal diameter and height of navel orange trees were 9.2 cm and 2.0 m, respectively. The middle-aged orchards were planted in 1989 or 1990, with a spacing of 2 × 4 m, the average basal diameter and height were 10.0 cm and 2.3 m, respectively. The mature orchards were planted in 1978 at a space of 3 × 3 m, and average basal diameter and height were 19.11 cm and 2.8 m, respectively. All orchards were converted from natural scrubland and fertilized with compound chemical fertilizer (including 350–530 kg N hm−2 year−1, 80–105 kg P hm−2 year−1, 80–100 kg K hm−2 year−1) using intensive management practice. The fertilizer was seasonally (spring/summer/autumn/winter ≈2:3:3:2) applied by digging a shallow furrow under the canopy of each tree. Meanwhile, irrigation, weed control, and pest management were timely implemented in all orchards according to the unified standard. All the chosen plots were unevenly distributed within a 230-ha farm, with southerly aspects and slopes less than 15°.
2.3 Soil general properties
Each plot (20 × 20 m) was further divided into four subplots (10 × 10 m). In each subplot, five soil cores (5 cm diameter) were sampled at 0–15 cm depth after surface litter was carefully removed, and then composited into one sample. In total, 36 soil samples were transported to the laboratory immediately for analysis of soil texture, pH, organic C, total N, and total P. Soil texture (particle size distribution) was determined by the sieve-pipette method. Soil pH was measured in a 1:2.5 mixture of soil and deionized water using a glass electrode. Soil organic C concentration was determined by dichromate oxidation and titration with ferrous ammonium sulfate. Total N concentration was determined by the microKjeldahl method, and total P concentration was analyzed by a phosphomolybdic acid blue color method (Allen 1989). Additionally, a soil sample was collected within each subplot by using a 5.0-cm-height sampling borer for determining soil bulk density at three depths: 0–5, 5–10, and 10–15 cm. The soil bulk density was determined based on the dry soil weight per unit volume of the soil core at each depth. Topsoil organic C, total N, and total P stocks were calculated based on the concentrations, soil depth, and bulk density.
2.4 Soil N availability
Soil available N was measured through a whole growing season. In each 10 × 10 m subplot, five soil samples at 0–15 cm depth were randomly collected with an auger (inner diameter of 5 cm) and composited into one sample in April, 2008 (representing spring), each composite sample was passed through a 2-mm sieve and divided into two subsamples; one subsample was used for determination of soil NH4 +–N, NO3 −–N, and mineral N (NH4 +–N plus NO3 −–N) concentrations (Allen 1989; Huang et al. 2008), and then air dried for P fractions measurement; the other was stored at −20 °C for analysis of microbial biomass and enzyme activities. The measurements of soil N availability were repeated in July 2008, November 2008, and January 2009. These periods were defined as summer, autumn, and winter, respectively.
2.5 Soil P fractions
The air-dried soil was ground to pass through a 0.5-mm sieve and processed following the soil P fractionation sequential procedure (Hu et al. 2011). The corresponding supernatants, sequentially exacted with anion exchange resin, 0.5 M NaHCO3, 0.1 M NaOH, 0.1 M NaOH with sonication, and 1.0 M HCl, then collected by centrifuging samples at 1.7 × 104 m s−2 (3200 rpm) for 5 min, followed by filtering samples through a 0.45-μm micropore filter. P concentration in each supernatant was determined by the phosphomolybdic acid blue color method (Allen 1989). Resin-P, NaHCO3-P, NaOH-P, sonication-P, and HCl-P were defined as available P, labile P, slow P, occluded P, and weathered mineral P, respectively (Hu et al. 2011). Bio-available P is the sum of available P and labile P, extractable P includes available P, labile P, slow P, occluded P, and weathered mineral P. Soil total P was analyzed by a phosphomolybdic acid blue color method after acid digestion (Allen 1989), and the residual P was the difference between total soil P and the extractable P. The soil samples from different seasons were measured to study the seasonal dynamics of soil P supply.
2.6 Soil microbial biomass and enzyme activities
Subsample soils stored at −20 °C were used for measuring microbial biomass and enzyme activities. Soil microbial biomass C and N (MBC and MBN) were measured by the fumigation-extraction procedure. The field moist soils were sieved (2 mm), adjusted to 40 % of water holding capacity, incubated for 7 days at room temperature and then stored at 4 °C for analysis. Then a subsample of 25 g was taken from incubated soil, saturated with purified liquid CHCl3 for 24 h as a fumigation treatment; meanwhile, another 25 g subsample taken from the incubated soil was used as an unfumigated control. Both pretreated soil samples were separately extracted with 100 mL 0.5 mol L−1 K2SO4. Organic C (OC) and total N were measured by dichromate oxidation and the microKjeldahl digestion procedure (Allen 1989), respectively. MBC and MBN (mg kg−1 OC) were estimated from the ratio of difference in C and N concentrations between the fumigated and unfumigated samples using a correction factor of 0.38 and 0.54, respectively (Brookes et al. 1985). Soil invertase activity was measured by 3,5-dinitrosalicylic acid colorimetry and used as glucose indicator (g glucose kg−1 OC day−1). The activities of soil urease (g ammonia kg−1 OC day−1) and acid phosphatase (g phenol kg−1 OC day−1) were measured by the nesslerization and disodium phenyl phosphate colorimetric method (Guan and Shen 1984), respectively. All above indicators were measured in spring, summer, autumn, and winter, respectively.
2.7 Leaf nutrients
Leaves were seasonally collected from April 2008 to January 2009 at the same dates with soil sampling. Three representative trees (with DBH and heights close to the average) were selected for sampling in each plot. Then for each selected tree, the leaves from one first-order branch were collected from each of the four cardinal directions and combined to provide one sample. The leaf samples were washed by deionized water to remove dust, then oven dried at 70 °C for 48 h, ground in a mill and screened with a 0.25-mm sieve. Total N and total P were measured by using the microKjeldahl method and the phosphomolybdic acid blue color method, respectively. Organic C was determined by dichromate oxidation and titration with ferrous ammonium sulfate (Allen 1989). Leaf C/N, C/P, and N/P were calculated using the concentrations of C, N, and P in leaves.
2.8 Nutrient balance between input and demand
In autumn, randomly selected 18 orange trees of different ages were cut and used to set up the biomass models of trunk, branches, twigs, leaves, and fruits. The yearly increases in woody organs (total trunk, branches, and twigs) and leaves were estimated according to the allometric formulas (Roccuzzo et al. 2012). Leaf litter-fall productivity was defined as half fresh leaf biomass since the average lifespan is about 2 years.
All fresh samples of different organs were washed in tap water, rinsed in deionized water, oven dried at 70 °C, milled, and sieved through a 0.25-mm mesh sieve for nutrient analysis. Total N and total P were measured by using the microKjeldahl method and the phosphomolybdic acid blue color method, respectively (Allen 1989).
Nutrient balance budget was prepared following a general conceptual model (Vitousek et al. 2009; Roccuzzo et al. 2012). Fertilizer applications were tabulated as external inputs to the orchard soils and the nutrient turnover of litter-fall and pruned twigs were tabulated as internal inputs. In contrast, nutrient output was brought out from orchard soils by orange fruits, branches for fire woods, and tree itself growth. Finally, the nutrient budget in orchard soil of each stand age per year was calculated as the total input amount by fertilizers and residual decomposition minus the average output amount with orange fruits, branches removal, and tree growth. Annual nutrient input data (2006–2008) were provided by the local agro-technical station.
2.9 Statistical analysis
Kolomgorov-Smirnov test was used to examine whether the data within and between groups followed the normal distribution patterns. All data were analyzed based on plot-level average (soil nutrient variables were averaged from four subplots within a plot; leaf variables were averaged from three sampling trees within a plot). Data on soil general physical and chemical properties were analyzed by using one-way analysis of variance (ANOVA) with stand age as the fixed main effect. The Tukey’s multiple comparisons method was used to evaluate significance of differences among stand ages as well as seasonal differences of variables within a stand age. These statistical analyses were conducted using SPSS 16.0 (SPSS, Chicago, IL). The differences reported in the text were tested and considered significant at a confidence level of 95 % (P = 0.05) unless specifically stated.
3 Results
3.1 Soil physical and chemical properties
Soil texture (the relative ratios of sand, silt, and clay) and C/N ratio did not vary significantly (P > 0.05) with stand age (Table 1). Bulk density was significantly higher in the young and mature navel orange orchards than in the middle-aged orchard. Organic C, total N, and P concentrations showed an increasing tendency with stand age, while soil pH, C/P, and N/P decreased with stand age (Table 1).
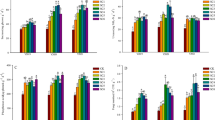
Soil N availability was influenced by both stand age and season (Fig. 1a–c; Table S1, Electronic supplementary material). Annual average of soil mineral N (including both NH4 +–N and NO3 −–N) concentration was significantly lower (P < 0.05) in the young orchard than in older orchards (Fig. 1c). Soil NH4 +–N, NO3 −–N, and mineral N concentrations were generally higher in spring and summer than in autumn and winter for all orchards (Fig. 1a–c). Except residual P, soil extractable P fractions (i.e., available P, slow P, labile P, weathered mineral P, and occluded P) were significantly different among stand ages (Table S1, Electronic supplementary material), with significantly higher P availability in the older orchards (Fig. 1d–j). Soil total extractable P showed a significantly increasing tendency with stand age (Fig. 1i). Soil residual P in the young orchard was significantly lower than that in the older orchards, while not significantly different between middle-aged and mature orchards (Fig. 1j). Soil available, labile P concentration, and mineral P concentration were generally lower in summer than in other seasons (Fig. 1d, e, h).
Seasonal dynamics of topsoil (0–15 cm) N supply and P fractions in the navel orange orchards along an age gradient in mid-subtropical China. Error bars show the standard error of the mean. Different lowercase letters in the same season indicate significant differences (P < 0.05) of mean values among three different aged orchards
3.2 Nutrient balance and use efficiency
Nutrient inputs from fertilizers were far more than the tree demands (Fig. 2). N inputs in the young, middle-aged, and mature orchards from fertilizers were 523.5, 422.5, and 354.0 kg N hm−2 year−1, with 120.0, 105.0, and 84.0 kg P hm−2 year−1, respectively, while the amounts of N demands for these orchards were 104.1, 150.0, and 118.0 kg N hm−2 year−1, and those of P were 11.5, 15.6, and 12.4 kg P hm−2 year−1, respectively. N and P use efficiencies (NUE and PUE, plant uptake/soil input) were 19.89–33.90 and 9.80–14.86 %, respectively. The nutrient input deceased with increasing stand age, but the nutrient need was higher in the middle-aged orchard than in the young and mature orchards. Additionally, the yearly surplus N and P amounts were much in excess and almost equaled to the stocks at 0–15 cm soils in the young and middle-aged orchards but were about half the stocks in the mature orchard (Table 1; Fig. 2).
Annual soil nitrogen and phosphorus balances in navel orange orchards of three different stand ages. The up and down arrows represent nutrient output and input (kg hm−2 year−1 in unit), respectively. The numerical value below dotted lines represent the difference between nutrient input and output of navel orange orchard
3.3 Soil microbial biomass and activities
Soil microbial biomass C and N ranged from 1.72 to 82.70 and 0.24–2.45 mg kg−1 OC, respectively, with higher values in the young than in the middle-aged and mature orchards, and lower values in spring than in other seasons (Fig. 3). Microbial biomass C/N ranged from 6.88 to 37.06, with higher values in the young than in the middle-aged and mature orchards, and higher in autumn than in other seasons. The relationships among microbial biomass C and N, and soil mineral N varied with season, and the annual average values negatively correlated with the NO3 −–N concentrations (Fig. S1, Electronic supplementary material). In contrast, soil microbial biomass C and N negatively or insignificantly correlated with soil P variables in each season. Additionally, soil microbial biomass C/N negatively correlated with soil organic C (r = −0.79, n = 9, P < 0.05), total N (r = −0.62, n = 9, P = 0.07), and total P (r = −0.83, n = 9, P < 0.01).
Seasonal dynamics of topsoil (0–15 cm) microbial biomass and major enzyme activities in the navel orange orchards along an age gradient in the mid-subtropical China. Different lowercase letters in the same season indicate significant differences (P < 0.05) of mean values among three different aged orchards
The activities of three enzymes varied with seasons and stand ages (Fig. 3). The average invertase activity was higher in the young and middle-aged orchards than in the mature orchard and higher in spring and summer than in autumn and winter. Urease and acid phosphatase activities significantly decreased with stand age during each season. In general, the enzyme activities negatively correlated with soil N and P variables and varied among seasons. There were significantly positive correlations between urease and acid phosphatase activities (r = 0.73 ∼ 0.78, n = 9, P < 0.05), as well as between microbial biomass C and N (r = 0.75 ∼ 0.82, n = 9, P < 0.05) in each season, but all these did not correlate with the invertase activity (Fig. S1, Electronic supplementary material). Interestingly, annual average invertase activity positively correlated with pH (r = 0.81, n = 9, P < 0.05), negatively correlated with soil bulk density (r = −0.73, n = 9, P < 0.05), organic C (r = −0.79, n = 9, P < 0.05), and most P variables (Fig. S1, Electronic supplementary material).
3.4 Leaf nutrients and their seasonal dynamics
Average leaf N concentration and N/P ratio were significantly higher in the young orchard than the older orchards, with no significant difference between the middle-aged and mature orchards (Fig. 4). Average leaf organic C concentration and C/N ratio increased significantly with stand age, while leaf P concentration and C/P ratio were not significantly different among the different aged orchards. The average leaf N/P ratio for all stands was about 18. The lowest and highest leaf N concentrations were shown in summer and autumn, respectively, for the young orchard, while the lowest leaf N concentration occurred in spring for both middle-aged and mature orchards. In contrast, the lowest leaf P concentration was shown in autumn and the highest in summer (Fig. 4). Leaf N concentration did not significantly correlate with soil N nutrients but negatively correlated with soil available, labile, and total extractable P in some seasons. Leaf P concentration did not significantly correlate with either soil P or N nutrients (see Table S2, Electronic supplementary material).
Seasonal dynamics of leaf nutrients and their stoichiometric ratios in the navel orange orchards along an age gradient in the mid-subtropical China. Error bars show the standard error of the mean. Different lowercase letters in the same season indicate significant differences (P < 0.05) of mean values among three different aged orchards
4 Discussion
4.1 Soil nutrient accumulations due to over fertilization
Long-term fertilization likely leads to accumulations of N and P in soils, and to potential environmental problems, such as soil and water pollutions especially in managed croplands (Zhu and Chen 2002; Fernández-Escobar et al. 2009). In this study, both soil N and P concentrations and stocks in the mature orchard were much higher than in the young and middle-aged orchards. Meanwhile, the mineral N and available P were more than two and six times in the mature than young orchards, respectively. The nutrient budget investigated further testified our results that over fertilization is the key reason to increased N and P concentrations in orchard soils. Similar result was found by Tong et al. (2009) that long-term fertilization caused the accumulation of N in paddy soils in subtropical China. Chen et al. (2008) reported that total P utilization efficiency in China’s agricultural ecosystem was about 46 %, and excessive P fertilizer application in the arable farming system led to the accumulation of soil P and a risk to the riparian water quality. Wang et al. (2010) also found that nutrients loss in runoff was very serious with 2.32–7.41 t ha−1 year−1 NO3 −–N, 0.79–6.97 t ha−1 year−1 NH4 +–N, and 0.10–4.69 t ha−1 year−1 P in the citrus orchards in the Three Gorges Region, China. Clearly, the surplus of N and P easily moved into local groundwater and surface water bodies as well as air with N x O greenhouse gas (Bouwman et al. 2002; Kumazawa 2002; Smol 2009). Thus, it is not surprised that NUE and PUE were only 19.89–33.90 and 9.80–14.86 %, respectively, in our study orchards.
Meanwhile, soil N/P, the ratios of mineral N to available P and the N surplus to P surplus decreased with stand age. In addition, PUE was less than NUE, and the increasing percentage of PUE with stand age was higher than that of NUE. These implied that N fertilizer uses should be decreased in the older orchards, while P fertilizer uses should be reduced for all aged orchards. Further research is needed to quantify the suitable N and P fertilizer use amounts in different aged orchards.
4.2 Soil acidification and microbial activity degradation
Soil is generally a strong buffer for pH; however, pH dropped from about 4.7 in the young and middle-aged orchards to 4.4 in the mature orchard in our study. Our result was in agreement with Ling et al. (2010) who found that average pH in the navel orange orchard soils was 0.46 lower than that of non-orchard soils in southern Jiangxi province of China. Liang et al. (2010) also found that a significant decrease in pH occurred in the first 4 years after the establishment of the new orchards. Additionally, He et al. (1999) reported that soil pH decreased with increasing NH4 +–N fertilizer, and the 4-year application of 112 kg N ha−1 year−1 led to a decrease in pH of 0.7 to 1.7 units, and an increase in leaching of P and K in grape orchards. Thus, soil acidification might be caused by the urea and ammonium sulfate (Treseder 2008).
Decreases in soil pH caused by fertilizer application are likely to reduce soil microbial and enzyme activities (Ajwa et al. 1999; Zhong et al. 2007). Lower soil pH would expedite soil microbial turnover, leading to the reduction in soil microbial biomass (Jia et al. 2010). Moreover, microbes may become magnesium or calcium limited or they may experience aluminum toxicity as the soil pH decreased (Treseder 2008). Consistent with our hypothesis, soil microbial biomass C and N and acid phosphatase activity were significantly higher in the young orchard than in the middle-aged and mature orchards, invertase activity was higher in the young and middle-aged orchards than in the mature orchard, and urease activity decreased with increasing stand age. Pietri and Brookes (2009) found that soil acidification led to alter microbial community structure and decrease microbial biomass. All of these indicated that soil microbial activities and their roles in nutrient transformation were weakened due to long-term fertilizer uses in the older navel orange orchards, which also suggested a potential soil N and/or P saturation in the older orchards of the study region.
We used the RDA biplot method to further quantify the relationships between microbial biomass/activities and nutrient availabilities. In the biplot of RDA (Fig. S1, Electronic supplementary material), the first axis accounted for more than 97 % of the variance in soil properties for all seasons as well as the annual average. This could be due to the close relationships among most soil variables, including positive correlations among microbial biomass C and N, urease and acid phosphatase activities in each season and for the annual average, and negative correlations between invertase activity and various P fractions in each season. However, some relationships between soil microbial activities and nutrient properties varied with season. For example, the correlation between invertase activity and mineral N was positive in spring and winter, but generally negative in summer and autumn, except for a non-significant correlation between invertase and NH4 +–N in summer. Therefore, in addition to soil nutrients, our study suggested that soil microbial variables might be also influenced by seasonal environmental condition (Wardle 1998; Zeller et al. 2001).
Additionally, N fertilization may alter soil microbial biomass and activity via increasing soil N availability resulting in direct immobilization by microbes either by decreasing their C/N ratio or increasing their overall biomass (Wallenstein et al. 2006). According to the enzyme inhibition hypothesis, mineral N addition can inhibit those enzymes involved in soil recalcitrant C decomposition, and this can reduce microbial activity (Gallo et al. 2004). MBC and MBN negatively correlated with mineral N and available P concentrations, indicating excessive N and P suppressed soil microorganisms (Bonanomi et al. 2011). Soil microbial biomass C/N negatively correlated with soil organic C, total N, and total P, which further indicated a decreasing microbial N immobilization rate with organic C and nutrient accumulations in the older orchards. Therefore, our results suggested that the role of microbes in soil nutrient cycling would be decreased in the older orchards as also found by Doran and Zeiss (2000). Soil microbial activities were weakened with long-term fertilization in the older navel orange orchards, which indicated that soil self-fertilization capacity might be gradually lost and the tree nutrients would increasingly rely on the additional fertilizers. Thus, the conflictions among higher productivity, less fertilization, and better environment could be intensified if the management practice was not altered.
4.3 Leaf nutrients and the implications
According to criteria reported by Menino (2012), the optimum levels of leaf N and P were 24–27 and 1.2–1.6 g kg−1, respectively. The leaf N (>28 g kg−1) and P concentrations (>1.7 g kg−1) in our study excess the criteria, which indicated that tree growth might not be limited by nutrients. Meanwhile, leaf N concentration decreased with stand age and correlated with soil mineral N in spring and autumn. We deduced that leaf N level would be regulated by soil N supply in fast-growing season (summer), while the N supply in other seasons, especially in spring, could exceed the plant demands and thus lead to potential N leaching (Chen et al. 2009). Leaf P concentration kept relatively stable among the three stand ages and did not significantly correlate with any soil P fractions in each season, which might imply an excessive P supply to navel orange even during periods of maximum demand (Hu et al. 2011). In contrast, soil available P, labile P, slow P, and occluded P negatively correlated with leaf N concentrations in spring, summer, and autumn, but not in winter. Therefore, our results supported the viewpoint that the response of plant leaf to soil nutrient supply may manifest physiological adaptation and seasonal feedback (Schoenau and Davis 2006; Chen et al. 2012, 2015). Meanwhile, these relationships indicated that soil P supply might exceed the plant demands in growing seasons. In addition, annual average leaf N/P ratio (∼20) was higher in the young orchard than in the middle-aged and mature orchards (∼17), further indicating that soil P supply is richer than N availability in the older orchards.
5 Conclusions
Seasonal variations in soil N mineralization, P fractions, microbial biomass, enzyme activities, and leaf nutrients were examined in the young, middle-aged, and mature navel orange orchards. Long-term fertilization led to N and P accumulations in soils, which not only deteriorated soil quality by decreasing microbial biomass and enzymatic activities, but acidified the soil with the degradation of soil self-fertilization capacity. Decreasing fertilizer application should be taken into consideration to accomplish the sustainable development of the orchard ecosystems. We also found that leaf N/P ratio could reflect the relative richness of N and P in soils since they usually respond more rapidly than soil physical and chemical variables to environmental presses.
References
Aerts R, Chapin F III (2000) The mineral nutrition of wild plants revisited. Adv Ecol Res 30:1–67
Ajwa HA, Dell CJ, Rice CW (1999) Changes in enzyme activities and microbial biomass of tallgrass prairie soil as related to burning and nitrogen fertilization. Soil Biol Biochem 31:769–777
Allen SE (1989) Chemical analysis of ecological materials, 2nd edn. Blackwell Scientific Publications, Oxford
Bonanomi G, D’Ascoli R, Antignani V, Capodilupo M, Cozzolino L, Marzaioli R, Puopolo G, Rutigliano FA, Scelza R, Scotti R, Rao MA, Zoina A (2011) Assessing soil quality under intensive cultivation and tree orchards in Southern Italy. Appl Soil Ecol 47:184–194
Bouwman A, Boumans L, Batjes N (2002) Emissions of N2O and NO from fertilized fields: summary of available measurement data. Glob Biogeochem Cycles 16:1–13
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen—a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Carey PL, Benge JR, Haynes RJ (2009) Comparison of soil quality and nutrient budgets between organic and conventional kiwifruit orchards. Agric Ecosyst Environ 132:7–15
Chen M, Chen J, Sun F (2008) Agricultural phosphorus flow and its environmental impacts in China. Sci Total Environ 405:140–152
Chen FS, Zeng DH, Fahey TJ (2009) Changes in soil nitrogen availability due to stand development and management practices on semi-arid sandy lands, in northern China. Land Degrad Dev 20:481–491
Chen FS, Niklas KJ, Chen GS, Guo D (2012) Leaf traits and relationships differ with season as well as among species groupings in a managed Southeastern China forest landscape. Plant Ecol 213:1489–1502
Chen FS, Niklas KJ, Liu Y, Fang XM, Wan SZ, Wang HM (2015) Nitrogen and phosphorus additions alter nutrient dynamics but not resorption efficiencies of Chinese fir leaves and twigs differing in age. Tree Physiol 35:1106–1117
Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Havens KE, Lancelot C, Likens GE (2009) Controlling eutrophication: nitrogen and phosphorus. Science 323:1014–1015
Doran JW, Zeiss MR (2000) Soil health and sustainability: managing the biotic component of soil quality. Appl Soil Ecol 15:3–11
El-Wakeel H, Mansour N (2014) Fertilizing young navel orange trees with sulfur and wood ash as a source of sustainable agriculture. J Hortic Sci Biotechnol 6:50–58
Fernández-Escobar R, Marin L, Sánchez-Zamora M, García-Novelo J, Molina-Soria C, Parra M (2009) Long-term effects of N fertilization on cropping and growth of olive trees and on N accumulation in soil profile. Eur J Agron 31:223–232
Gallo M, Amonette R, Lauber C, Sinsabaugh RL, Zak DR (2004) Microbial community structure and oxidative enzyme activity in nitrogen-amended north temperate forest soils. Microb Ecol 8:218–229
Guan SY, Shen GQ (1984) Enzyme activities in main soil in China. Acta Pedol Sin 21:368–381 (in Chinese)
Guo HC (2011) Rhizosphere effect of soil nutrient availability in navel orange orchards of south Jiangxi. Master dissertation, Nanchang University, Nanchang, pp 1–82 (in Chinese)
Güsewell S (2004) N: P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Hatano R, Shinano T, Taigen Z, Okubo M, Zuowei L (2002) Nitrogen budgets and environmental capacity in farm systems in a large-scale karst region, southern China. Nutr Cycl Agroecosyst 63:139–149
He Z, Alva A, Calvert D, Li Y, Banks D (1999) Effects of nitrogen fertilization of grapefruit trees on soil acidification and nutrient availability in a Riviera fine sand. Plant Soil 206:11–19
Hu J, Li P, Wang W, Wu Y, Yi K (2008) The produce and preserve situation of Navel Orange in China. Jiangsu Agric Sci 3:10–13 (in Chinese)
Hu XF, Chen FS, Nagle G, Fang YT, Yu MQ (2011) Soil phosphorus fractions and tree phosphorus resorption in pine forests along an urban-to-rural gradient in Nanchang, China. Plant Soil 346:97–106
Huang ZQ, Xu ZH, Chen CR (2008) Effect of mulching on soil labile soil organic matter pools, microbial functional diversity and nitrogen transformations in two hardwood plantations. Appl Soil Ecol 40:229–239
Jia S, Wang Z, Li X, Sun Y, Zhang X, Liang A (2010) N fertilization affects on soil respiration, microbial biomass and root respiration in Larix gmelinii and Fraxinus mandshurica plantations in China. Plant Soil 333:325–336
Jiao W, Chen W, Chang AC, Page AL (2012) Environmental risks of trace elements associated with long-term phosphate fertilizers applications: a review. Environ Pollut 168:44–53
Joergensen RG, Emmerling C (2006) Methods for evaluating human impact on soil microorganisms based on their activity, biomass, and diversity in agricultural soils. J Plant Nutr Soil Sci 169:295–309
Koerselman W, Meuleman AF (1996) The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450
Kumazawa K (2002) Nitrogen fertilization and nitrate pollution in groundwater in Japan: present status and measures for sustainable agriculture. Nutr Cycl Agroecosyst 63:129–137
Lemaire G, Franzluebbers A, Carvalho PCDF, Dedieu B (2014) Integrated crop-livestock systems: Strategies to achieve synergy between agricultural production and environmental quality. Agric Ecosyst Environ 190:4–8
Li S, Jin J (2011) Characteristics of nutrient input/output and nutrient balance in different regions of China. Sci Agric Sin 44:4207–4229
Liang MQ, Xue J, Fan YL, Li X, Peng LZ (2010) Studies on soil acidification of navel orange orchards in Ganzhou city, Jiangxi Province. South Chin Fruits 4:6–8
Ling LL, Peng LZ, Chun CP, Cao L, Jiang CL, Lei T (2010) Relationship between leaf yellowing degree and nutrimental elements levels in navel orange (Citrus sinensis Osbeck) leaves in southern Jiangxi province of China. Sci Agric Sin 17:3602–3607
Ma L, Velthof GL, Wang FH, Qin W, Zhang WF, Liu Z, Zhang Y, Wei J, Lesschen JP, Ma WQ, Oenema O, Zhang FS (2012) Nitrogen and phosphorus use efficiencies and losses in the food chain in China at regional scales in 1980 and 2005. Sci Total Environ 434:51–61
Menino R (2012) Leaf analysis in citrus: interpretation tools. Adv Citrus Nutri. Springer, pp 59–79
Pathak H, Mohanty S, Jain N, Bhatia A (2010) Nitrogen, phosphorus, and potassium budgets in Indian agriculture. Nutr Cycl Agroecosyst 86:287–299
Paudel BR, Udawatta RP, Kremer RJ, Anderson SH (2012) Soil quality indicator responses to row crop, grazed pasture, and agroforestry buffer management. Agroforest Syst 84:311–323
Pietri JCA, Brookes PC (2009) Substrate inputs and pH as factors controlling microbial biomass, activity and community structure in an arable soil. Soil Biol Biochem 41:1396–1405
Robertson GP, Swinton SM (2005) Reconciling agricultural productivity and environmental integrity: a grand challenge for agriculture. Front Ecol Environ 3:38–46
Roccuzzo G, Zanotelli D, Allegra M, Giuffrida A, Torrisi BF, Leonardi A, Quiñones A, Intrigliolo F, Tagliavini M (2012) Assessing nutrient uptake by field-grown orange trees. Eur J Agr 41:73–80
Schloter M, Dilly O, Munch JC (2003) Indicators for evaluating soil quality. Agr Ecosyst Environ 98:255–262
Schoenau JJ, Davis JG (2006) Optimizing soil and plant responses to land-applied manure nutrients in the Great Plains of North America. Can J Soil Sci 86:587–595
Shamseldin A, El-Sheikh MH, Hassan H, Kabeil S (2010) Microbial bio-fertilization approaches to improve yield and quality of Washington navel orange and reducing the survival of nematode in the soil. J Am Sci 6:264–271
Smol JP (2009) Pollution of lakes and rivers: a paleoenvironmental perspective. John Wiley & Sons
Springett JA, Gray R, Reid JB, Petrie R (1994) Deterioration in soil biological and physical properties associated with kiwifruit (Actinidia deliciosa). Appl Soil Ecol 1:231–241
Staff SS (2010) Keys to soil Taxonomy Eleventh Edition. United States Department of Agriculture Natural Resources Conservation Service
Tong C, Xiao H, Tang G, Wang H, Huang T, Xia H, Keith SJ, Li Y, Liu S, Wu J (2009) Long-term fertilizer effects on organic carbon and total nitrogen and coupling relationships of C and N in paddy soils in subtropical China. J Am Sci 106:8–14
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
Vitousek PM, Naylor R, Crews T, David M, Drinkwater L, Holland E, Johnes P, Katzenberger J, Martinelli L, Matson P (2009) Nutrient imbalances in agricultural development. Science 324:1519
Wallenstein MD, McNulty S, Fernandez IJ, Boggs J, Schlesinger WH (2006) Nitrogen fertilization decreases forest soil fungal and bacterial biomass in three long-term experiments. For Ecol Manag 222:459–468
Wang L, Tang L, Wang X, Chen F (2010) Effects of alley crop planting on soil and nutrient losses in the citrus orchards of the Three Gorges Region. Soil Till Res 110:243–250
Wang P, Liu J-H, Xia R-X, Wu Q-S, Wang M-Y, Dong T (2011) Arbuscular mycorrhizal development, glomalin-related soil protein (GRSP) content, and rhizospheric phosphatase activity in citrus orchards under different types of soil management. J Plant Nutr Soil Sci 174:65–72
Wardle DA (1998) Controls of temporal variability of the soil microbial biomass: a global-scale synthesis. Soil Biol Biochem 30:1627–1637
Wardle DA, Bardgett RD, Klironomos JN, Setala H, van der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633
Weinbaum SA, Johnson RS, DeJong TM (1992) Causes and consequences of overfertilization in orchards. Hort Technol 2:112–121
Zeller V, Bardgett RD, Tappeiner U (2001) Site and management effects on soil microbial properties of subalpine meadows: a study of land abandonment along a north–south gradient in the European Alps. Soil Biol Biochem 33:639–649
Zhong WH, Cai ZC, Yin LC, Zhang H (2007) Effects of the long-term application of inorganic fertilizers on microbial community diversity in rice-planting red soil as studied by using PCR-DGGE. Acta Ecol Sin 27:4011–4018
Zhu Z, Chen D (2002) Nitrogen fertilizer use in China—contributions to food production, impacts on the environment and best management strategies. Nutr Cycl Agroecosyst 63:117–127
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (31160107 & 31260199) and Jiangxi Provincial Department of Science and Technology (2012BAB204005 and 20153BCB22008). We thank Dr. De-Hui Zeng and Guang-Sheng Chen for manuscript improvement, Peng-Fei Liao, Yi-Zhen Liu, and Zhe-Xia Zhao for their field work and soil analyses, and David Duncan for English improvement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhiqun Huang
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 250 kb)
Rights and permissions
About this article
Cite this article
Wan, SZ., Gu, HJ., Yang, QP. et al. Long-term fertilization increases soil nutrient accumulations but decreases biological activity in navel orange orchards of subtropical China. J Soils Sediments 17, 2346–2356 (2017). https://doi.org/10.1007/s11368-016-1439-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-016-1439-9