Abstract
Purpose
Biochar application is deemed to modify soil properties, but current research has been mostly conducted on the degraded land in tropical regions. Using six consecutive years of biochar field trial, we investigated effects of biochar on soil aggregates, structural stability, and soil organic carbon (SOC) and black C (BC) concentrations in aggregate fractions. The findings have important implications in managing soil structure and SOC sequestration in high fertility soils of the temperate areas.
Materials and methods
The study had four treatments: control; biochar rate at 4.5 (B4.5) and biochar rate at 9.0 t ha−1 year−1 (B9.0); and straw return (SR). Soil samples were collected from 0–10-cm layer, and aggregate size distribution was determined with the wet-sieving method. Then, the mean weight diameter (MWD) of aggregates and the aggregate ratio (AR), i.e., the ratio of the >250 μm to the 53–250 μm size were calculated to assess the structural stability. Total SOC and BC concentrations in bulk soil (<2 mm) and separated fractions (i.e., >2000, 250–2000, 53–250, and <53 μm) were measured.
Results and discussion
The B4.5 and B9.0 significantly increased macroaggregate (250–2000 μm) and MWD and AR indices relative to the control. Comparing to the SR, the improvements in soil aggregation under biochar treatments were limited. Additionally, more SOC in larger fractions (>2000, 250–2000, and 53–250 μm) and BC in extracted fractions under biochar soils were observed. These results implied that biochar addition enhanced both native SOC and BC physical protection by aggregation.
Conclusions
Biochar application is effective in mediating soil aggregation, and thus improves both native SOC and BC stabilization in an intensive cropping system of North China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Biochar is produced from waste biomass under the process of thermal degradation with limited oxygen (Lehmann et al. 2009). Using biochar as soil additive is rapidly emerging as a potential means to modify nutrients cycling, reduce soil N2O emissions, and enhance C sequestration (Lehmann 2006; Biederman and Harpole 2013; Singh et al. 2015). There are reports that biochar-amended soil have increased cation exchange capacity (CEC), water retention, liming ability, microbial functions, and thus can enhance plant growth (Sohi et al. 2010; Gul et al. 2015), especially in tropical areas. Thus, biochar amendment is received as a strategy to improve soil fertility to enable economic plus environmental benefits (Biederman and Harpole 2013).
Considerable studies have demonstrated biochar addition could affect various soil properties and processes (Sohi et al. 2010), e.g., enhanced soil physical properties such as soil aggregation, soil aeration, and water retention have been enhanced (Mukherjee and Lal 2013; Zong et al. 2015). Soil aggregation is a key ecosystem process resulting in the formation and stabilization of soil structure, which consists of soil aggregates and the resulting matrix of pore spaces (Lal 1991; Rillig et al. 2015). As such, soil aggregation influences the organization of soil biodiversity and plays a critical role in biogeochemical processes (Gupta and Germida 2015).
The positive role of biochar amendment on soil aggregation has been indicated in both field and laboratory studies on various soil textures from sandy loam to clay (Herath et al. 2013; Ouyang et al. 2013; Gul et al. 2015). Several studies have highlighted the positive interactions between biochar/charcoal and organo-mineral complexes (Brodowski et al. 2005; Brodowski et al. 2006) and between biochar and mycorrhizal fungi (Warnock et al. 2007), all of which could enhance soil aggregate formation and stabilization (Tisdall and Oades 1982; Rillig et al. 2015). Thus, biochar application to soil may not only reduce biochar and native organic matter from decomposition through aggregation, but may also decrease the potential of biochar particles for clogging or cementing soil pores (Cross and Sohi 2011). A better understanding on how biochar particles are entrapped into aggregates may help to reveal the role of biochar amendment on the dynamics of soil structure. Till now, most previous works have been conducted on highly weathered and infertile soils (Glaser et al. 2002; Sohi et al. 2010; Verheijen et al. 2014). However, the benefits of biochar-amended productive, neutral, or alkaline soils in the temperate regions may not be as obvious as for high weathered soils in tropical climate conditions (Atkinson et al. 2010). Moreover, numerous studies on the biochar-amended soils are accompanied by short-term incubation or field studies, and few field data are available (Manyà 2012). Thus, the changes in soil physical properties, such as soil aggregation, induced by biochar application in high fertility soils, particularly in temperate areas are heavily needed.
Burning crop residues in the field is a common practice in North China. Because of high carbon and nutrient contents, crop residues can be used as a source of biochar to improve soil properties. In 2007, a field biochar experiment was established with annual biochar input in a wheat–maize double cropping system in the North China Plain. The effects of biochar application on soil thermal properties (Zhang et al. 2013), enzyme activities (Du et al. 2014), and soil respiration (Lu et al. 2014) have been reported. However, the knowledge regarding the impacts of biochar addition on soil aggregation and the associated soil organic carbon (SOC) stabilization remain unclear. Here, we hypothesized that long-term biochar application improves soil aggregation and alters the associated SOC and black C (BC) distribution in aggregate fractions. The specific objectives of this study were (i) to quantify the effects of biochar on soil aggregation and structural stability, and (ii) to reveal implication of this process on the distribution of SOC and BC within different aggregate fractions.
2 Materials and methods
2.1 Site description
The field experiment was conducted at the Huantai Experimental Station (36o 57’ N, 117o 58’ E) for Ecological and Sustainability and locates in Shandong Province, China. The local cropping system is a winter wheat–maize crop rotation. Since 1990, the average crop yield (wheat and maize) has been over 15 t ha−1 year−1. The experiment site has a typical continental monsoon climate with an average annual temperature of 12.4 °C (Zhang et al. 2015). The soil at the experiment site was a Fluvic Cambisol according to the USDA system.
2.2 Experimental design
The field experiment, established in 2007, was laid out as a randomized block design with four treatments: control (no biochar, CK); low biochar rate at 4.5 t ha−1 year−1 (B4.5), high biochar rate at 9.0 t ha−1 year−1 (B9.0); and straw (wheat and corn) returned to soil (SR). The total amount of crop straws for the two crops was estimated to be about 15 t ha−1 year−1, and about the 30 % of straws was collected and converted to biochar through the pyrolysis procedure (Du et al. 2014). Thus, the biochar input rate under the B4.5 treatment was chosen to 4.5 t ha−1 year−1. Each treatment had three replications, and the plot size was 36 m2. Both biochar and fertilizers were incorporated into the soil with a rotary tillage (about 16 cm depth) before winter wheat crop sowing. The detailed information for the fertilizer and field management was provided by Du et al. (2014).
The biochar product, with diameters of <1 mm, was obtained from a commercial producer (Dongxin Biochar Company, Shandong, with a diameter of <1 mm). The biochar was made by an incomplete combustion of crushed corncob in an open-top concrete tank at 360 °C for 24 h. The density and pH of biochar used were about 0.30 g cm−3 and 8.2, and the H/C and O/C molar ratios were 0.25 and 0.3, respectively. The concentrations of C, H, O, N, available P, and available K were 65.7, 1.4, 0.49, 29.9, 0.91, and 1.60 %, respectively. The ash concentration of the biochar was 72.0 % (dry combustion in a muffle furnace at 550 °C for 2 h). The particle size distribution of the biochar, which was determined with the laser diffraction method (Sympatec GmbH, System-Partikel-Technik, Clausthal-Zellerfeld, Germany), was 16, 34, 34, and 16 % for the <17-, 17–88-, 88–211-, and >211-μm fractions, respectively. The volume mean particle diameter, surface area per volume, and surface area per mass of the biochar were 107 μm, 0.24 m2 cm−3, and 702 cm g−1, respectively.
2.3 Soil sampling and aggregate separation
For determination of water-stable aggregates, soil samples were collected from the upper 0–10-cm layer in March 2012. For each replicate, two intact soil cores were collected and transferred to stainless steel cases. These samples were analyzed independently. In addition, two soil cores were taken from each replication using a stainless steel ring (5 cm high and 5 cm in diameter) for determining soil bulk density.
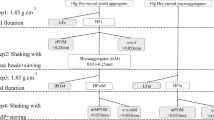
Soil samples were physically fractionated by the wet-sieving method, following a procedure modified by Six et al. (1998). This procedure involves utilizing the disruptive force of slaking and wet-sieving through a series of sieves to obtain four classes: >2000, 250–2000, 53–250, and <53 μm. In brief, about 100-g sample (<8 mm) was transferred to the top 2000-μm sieve and immediately submerged in the water for 5 min. Then, the aggregate size separation was obtained by mechanically oscillating the sieve 7 cm up and down for 50 times within 2 min. After, the water-stable aggregates (>2000 μm) were collected, and the sieving process was continued for the <2000-μm fraction with the next smaller sized sieve. This procedure was repeated for the 250- and 53-μm size sieves. All aggregate fractions were oven-dried (60 °C) for 48 h and weighed.
Mean weight diameter (MWD) of aggregate size distribution was calculated from the following equation (Kemper and Rosenau 1986):
where \( {\overline{x}}_i \) was the mean diameter (mm) of the soil aggregate size fractions and w i was the proportion of each aggregate size with respect to the total sample weight. The aggregate ratio (AR) was defined as:
where the macroaggregate is the soil aggregate size fraction of <250 μm and the microaggregate is the soil aggregate size fraction of 250–53 μm.
2.4 Soil organic C and black C analysis
The SOC concentration in the aggregate fractions was determined by dry combustion of duplicate subsamples using a CNS analyzer (Vario Max CNS, Elementar, Hanau, Germany; Nelson and Sommers 1996). To measure BC in bulk soil (<2 mm) and selected fractions (250–2000, 53–250, and <53 μm), the procedures of Lim and Cachier (1996) were used for soil sample pretreatments and analysis.
2.5 Statistical analyses
The two-way ANOVA was used to determine the treatment, aggregate size, and their interactions on the associated SOC, BC, and BC/SOC ratio. In addition, a one-way ANOVA was used to assess treatment effects on aggregate size distribution and stability indices (MWD and AR), and the associated SOC and BC concentrations, with separation of means tested by the LSD method. Results were given as mean ± standard error (SE) in the figures. The values of P < 0.05 were accepted as statistically significant. All statistical analyses were conducted using the SPSS 17.0 software for Windows (SPSS Chicago, Il).
3 Results
3.1 Physicochemical properties of the bulk soil
Biochar addition significantly changed the soil physicochemical properties chosen in the 0–10-cm layer (Table 1). Overall, the SOC concentration followed the order of B9.0 > B4.5 = SR > CK. The SOC concentrations under B9.0 and B4.5 were 62.0 and 29.7 % higher than that of the CK. The changes in soil bulk density followed the order of CK = B4.5 = SR > B9.0, indicating the soil porosity was increased after higher biochar rate application. Biochar-amended soil showed no difference in available P concentration, but had a higher available K concentration under the B9.0 treatment.
3.2 Water-stable aggregate and structural stability
Biochar changed the water-stable aggregate size and structural stability (Fig. 1A–C). Overall, the silt plus clay fraction (<53 μm) dominated aggregates, accounted for 56 % under SR, and 73 % under CK amendment. Across the treatments, the change in the >2000-μm fraction was not significant (Fig. 1A P > 0.05). The B4.5 and B9.0 treatments enhanced the 250–2000-μm size fraction by 49 and 109 % respectively relative to CK, suggesting that the stability of macroaggregates was increased with increasing of biochar doses. Compared with the SR, however, the magnitude of biochar-induced changes was much less: the 250–2000-μm fraction accounted for 12, 17, and 21 % of the bulk soil under the B4.5, B9.0, and SR treatments. Moreover, the microaggregate fraction (53–250 μm) followed the order of SR = B9.0, and SR > CK = B4.5. Finally, the proportion of the <53-μm fraction was decreased with SR and biochar amendment, with the order of CK = B4.5 > B9.0 > SR (Fig. 1A). These data indicated that biochar-amended soils have larger amounts of macroaggregate (250–2000 μm) as compared with the control, but decreased the fraction of macroaggregate relative to direct straw return to soil.
Water-stable aggregate size distributions (A), mean weight diameter (MWD) of aggregates (B), and aggregate ratio (AR, C), i.e., ratio of macroaggregate (>250 μm) to microaggregate (53–250 μm) as affected by biochar amendments (CK, no biochar amendment; B4.5 or B9.0, biochar rate at 4.5 or 9.0 t ha−1 year−1; SR, straw returned to soil). Data (means ±SE, n = 3) followed by different letters indicate significant differences between treatments for the same soil aggregate fraction or structural stability indices
The MWD and AR of the soil aggregates are indices that varied significantly in response to the treatments (Fig. 1B, C). Overall, biochar significantly (P < 0.05) improved the values of MWD, which changed from 0.284 to 0.414 mm across the treatments. The values of MWD under B4.5, B9.0, and SR were 10.5, 40.6, and 45.6 % higher than that of the control. In contrast, the value of MWD was 24.1 % lower than that of the SR, but the difference in MWD values between SR and B9.0 was not significant (P > 0.05). Likewise, the AR index under B9.0 was 49.9 % higher than that of the control, whereas the differences among SR, B9.0, and B4.5 were insignificant (Fig. 1C). These results demonstrated that biochar had a positive role on soil structural stability, though the effect was less significant in comparison to direct incorporation of crop residue into the soil.
3.3 Total SOC concentrations within aggregate fractions
Two-factor ANOVA showed that treatment and aggregate size had significant effect on SOC concentrations within aggregate fractions (Fig. 2), either independently or interactively. Across the aggregate fractions, the 250–2000-μm fraction under B9.0 had the highest SOC concentration, and the <53-μm fraction under CK had the lowest SOC concentration. For the SOC occluded in the >2000-μm fraction, the B9.0, B4.5, and SR treatments were 56.8, 92.4, and 23.1 % higher than that of the control. In the 250–2000-μm fraction, SOC concentration was improved by 92.7 % under B4.5, and by 120.7 % under B9.0 relative to CK, but no significant difference was observed between the SR and control (P > 0.05). For the 53–250-μm fraction, the associated SOC followed the order of B9.0 = B4.5 > SR > CK. No apparent SOC concentration differences were observed among the four treatments for the <53-μm fraction. Since SOC is closely related to the formation of stable soil aggregates, the aggregate-associated SOC may provide an indication for assessing the stabilization mechanism of SOC.
Aggregate-associated soil organic C (SOC) as influenced by biochar amendments (CK, no biochar amendment; B4.5 or B9.0, biochar rate at 4.5 or 9.0 t ha−1 year−1; SR, straw returned to soil). Data (means ±SE, n = 3) followed by different letters indicate significant differences between treatments for the same soil aggregate fraction (*P < 0.05; **P < 0.01; ***P < 0.001)
3.4 Black C concentrations within aggregate fractions
The changes in BC concentrations were significant among treatments, aggregate sizes, which also showed interactive effects (Fig. 3). Overall, biochar amendment significantly increased BC accumulation in all aggregate fractions determined. The 250–2000-μm fraction had the largest BC concentration under B9.0, whereas the <53-μm fraction had the lowest BC concentration under the CK or SR (Fig. 3). Specifically, the BC occluded within the 250–2000-μm fraction was 85.9 and 182.7 % higher under B4.5 and under B9.0 than that under the CK, whereas less difference was found between CK and SR treatments. A similar trend was observed in the 53–250-μm fraction, where the associated BC concentrations under B4.5 and B9.0 were 1.73 and 2.54 times that of the control. Finally, for the <53-μm fraction, the BC concentration under the B4.5 and B9.0 was higher by 1.24 and 1.94 times than that of CK. These data indicate that biochar application to soil significantly enhanced BC sequestration in soil aggregate, which could potentially protect the biochar itself from decomposition.
Aggregate-associated black C (BC) as influenced by biochar amendments (CK, no biochar amendment; B4.5 or B9.0, biochar rate at 4.5 or 9.0 t ha−1 year−1; SR, straw returned to soil). Data (means ±SE, n = 3) followed by different letters indicate significant differences between treatments for the same soil aggregate fraction (*P < 0.05; **P < 0.01; ***P < 0.001)
4 Discussion
4.1 Effect of biochar on soil aggregate size distribution and stability
In recent years, biochar as an amendment has become the subject of many studies due to its ability to modify soil physical, chemical, biological, and mechanical properties (Sohi et al. 2010; Mukherjee and Lal 2014). Our results clearly demonstrate that biochar-amended soil, in particular at higher rate, significantly enhance the proportion of macroaggregates (>250 μm, Fig. 2a). This is in accordance with the observations of Brodowski et al. (2006) who found that black carbon in the soil acted as a binding agent between aggregate fractions and increased aggregate stability. Similarly, studies from fields (Khademalrasoul et al. 2014; Liu et al. 2014) or incubation conditions (Awad et al. 2013; Herath et al. 2013; Zong et al. 2015) have shown the positive role in soil aggregation for sandy loam to clayey soils. It has been suggested that biochar particles interact with clay minerals through surface hydrophobic–hydrophilic interactions, which may be responsible for soil macroaggregate formation (Joseph et al. 2010).
Although SOC and clay content are the primary agents of aggregation in biochar-amended soil (Khademalrasoul et al. 2014), biochar properties such as surface area and O/C ratio are important to bind the biochar to organo-mineral complexes, which is a preliminary step in the aggregate formation and stabilization process (Glaser et al. 2002; Joseph et al. 2010; Gul et al. 2015). Moreover, the positive interactions between biochar amendment and soil biota (e.g., mycorrhizal fungi and earthworms) might benefit for accentuating soil aggregation (Warnock et al. 2007; Weyers and Spokas 2011). On the other hand, we noticed that biochar was ineffective to induce micro-aggregation (as seen in Fig. 1A), unless combined with a more labile C source (Khademalrasoul et al. 2014). In a word, our study suggests that biochar amendment improve soil macroaggregate formation, but the relevant mechanisms remain to be further investigated.
Biochar effects on soil aggregation are also reflected in the MWD and AR. In the present study, biochar amendment had a higher MWD and AR indices than the control (Fig. 1B, C), suggesting that biochar could increase soil structural stability. More importantly, we observed less difference in the MWD and AR indices between the B9.0 and SR treatments (Fig. 1B, C). These contrasting evidences might imply that crop residues-derived biochars display similar functions on structural stability with uncharred plant litter under investigated soil. We further analyzed the relationships between the MWD and SOC and BC concentrations (Fig. 4a, b). The results showed a significant correlation between MWD and bulk SOC concentration (r = 0.750; P < 0.01; Fig. 4a) and a weak linear relation between MWD and bulk BC concentration (r = 0.317; P = 0.107; Fig. 4b). These results highlight that the bulk SOC rather than the BC plays an overriding role in soil aggregation, probably due to more inert C within the black C fraction.
Soil aggregates are dynamic entities that profoundly influence soil biological, chemical, and physical properties (Gupta and Germida 2015). Our results differ from the findings of some studies (Busscher et al. 2010; Peng et al. 2011), who failed to observe positive effects of biochar (pecan shells at 700 °C or rice straw at 250–450 °C) application on aggregate stability from incubation in the laboratory for 11 or 70 days. A recent study by Zhang et al. (2015) showed that 1-year biochar application did not enhance soil aggregation. Thus, changes in soil properties (e.g., soil aggregation) in response to biochar addition may vary with charring temperature, application rate, soil texture, duration, and others factors. Furthermore, the changes in soil aggregation and pore size distribution responses in biochar-amended soils that promote soil structure modifications (Baiamonte et al. 2015), thereby leading to enhancement of many physicochemical and microbial properties such as CEC, pH, water retention capacity, and soil enzyme activities (Liang et al. 2006; Lehmann et al. 2011; Baronti et al. 2014; Paz-Ferreiro et al. 2014). Biochar-induced soil aggregation and reduction of soil bulk density may help root growth and retain more available water (Abiven et al. 2015; Obia et al. 2016), which probably enhances crop growth. The higher root activity, coupled with the direct role of biochar acting as a binding agent of soil particles (Brodowski et al. 2006), may be responsible for the increase in aggregate stability relative to the control. On the other hand, the improved soil aggregation is regarded as one of the strategies to reduce SOC turnover and enhance C sequestration in the soil.
4.2 Soil organic C occluded within aggregate fractions
In this study, the associated SOC concentration was increased generally with fraction sizes (except for the >2000-μm fraction) (Fig. 2). This pattern is in line with the aggregate hierarchy model proposed by Tisdall and Oades (1982). According to this model, the smallest aggregates are composed of organo-mineral associations, which are bound together with bacterial and fungal debris to form microaggregates (<250 μm). Then, the presence of decomposing roots and hyphae could cluster these small aggregates together to form macroaggregates (>250 μm). The positive interactions between biochar and mycorrhizal fungi for soil aggregation and sequestering C in soils are supported by Warnock et al. (2007) and Rillig et al. (2015). Our data indicate higher C in larger fractions (i.e., >53 μm) than that in the silt plus clay fraction (<53 μm, Fig. 2), particularly in the 250–2000-μm fraction where the associated C was 92.7 % higher under B4.5 and 120.7 % higher under B9.0 than CK. These data further demonstrate that biochar addition significantly increased organic C sequestered in the macroaggregates, despite these larger fractions containing mostly labile C.
Higher SOC in bulk soil (<2 mm) and aggregate fractions were found under biochar-amendment soils (Table 1; Fig. 2). We consider that biochar additions may aid in stabilizing SOC by reducing the rates of mineralization. Biochar particles may have an important role in C sequestration, as indicated by the increasing concentrations of the occluded total SOC with increasing aggregate size (except for the >2000-μm fraction; Fig. 2). The biotic and abiotic decomposition processes may be inhibited by stable aggregates, thereby providing protection to SOC (Six et al. 1998). In the present study, sorption to biochar and occlusion within aggregates are the main possible reasons for the stabilization of organic carbon. A 500-day incubation study revealed that biochar (<0.5 mm, 450–500 °C) amendment improved manure-C stabilization (Rogovska et al. 2011). The research result of Kuzyakov et al. (2009) suggested that adding biochar to soil reduces the decomposition of SOC. Thus, biochar amendment might stabilize the existing organic C in the soil. On the other hand, some previous studies showed that biochar addition could accelerate the decomposition of maize and switchgrass residues in laboratory incubation experiments (e.g., Novak et al. 2010; Awad et al. 2013). Further long-term field researches are required to understand the degradation mechanism of native soil organic matter and exogenous residues inputs causing by biochar addition.
4.3 Black C occluded within aggregate fractions
The stability of black C may be attributed not only to its refractory structure, but also to the poor accessibility when it interacts with the mineral matrix physically (Czimczik and Masiello 2007). In this study, soils amended with biochar generally displayed higher BC concentrations in the 250–2000-, 53–250-, and <53-μm fractions, especially in the 250–2000-μm fraction (Fig. 3), where the associated BC was higher by 85.9 % under B4.5 and 182.7 % under B9.0 than that under CK. Similarly, Brodowski et al. (2006) observed that in grassland and cropland soils, the highest BC concentrations occurred in the small macroaggregates (1–2 mm) fraction, while the lowest BC concentrations were found in the <53-μm fraction. Photo-oxidation of organic compounds may be the mechanism causing the increased biochar concentration in aggregates (Clough and Skjemstad 2000). These researchers further showed that the organic compounds undergoing photo-oxidation were mainly the free light fractions, C attached to the outside of soil aggregates, and the C that was not chemically protected. The analysis using NMR indicated that soil samples after photo-oxidation and hydrofluoric acid treatment had 59 to 84 % of the non-degraded organic C, which was probably char (Skjemstad et al. 1996; Clough and Skjemstad 2000). Thus, black C may be physically protected from enzymatic decomposition by sequestration in water-stable soil aggregates or micropores (<1 micron) (Lehmann et al. 2008). Our data indicate that biochar-amended soils improved biochar itself protection by holding within the larger aggregate fractions.
To better elucidate the distribution of BC among soil aggregates, we normalized the BC concentrations by total organic C concentrations in different fractions. The BC/SOC ratios are strongly influenced by treatments, fraction size, and the between these two factors (Fig. 5). The highest BC proportion under B9.0 soil was in the <53-μm fraction, while the minimum BC proportion occurred in the 53–250-μm fraction. In addition, the highest BC/SOC ratios appeared in B9.0, followed by B4.5, and the lowest was in the 53–250- and <53-μm fractions of the CK and SR treatments. The pattern of BC/SOC ratios generally varied across fractions, which implies that the stabilization mechanism of BC may be different among treatments. A study by Liang et al. (2008) showed that the BC-rich Anthrosols had higher proportions (72–90 %) of C in the more stable organo-mineral fraction than in the BC-poor adjacent soils, indicating some degree of physical stabilization. In the present study, we also observed a higher proportion of BC in the microaggregate and clay plus silt fractions (Fig. 5) in the biochar-amended soils. This confirms earlier reports by Brodowski et al. (2006) that BC is preferentially protected within aggregates, and by Glaser et al. (2000) that BC binds to clay minerals to be stabilized by organo-mineral complexes.
The ratio of black C (BC) concentration to soil organic C (SOC) as influenced by biochar amendments (CK, no biochar amendment; B4.5 or B9.0, biochar rate at 4.5 or 9.0 t ha−1 year−1; SR, straw returned to soil). Data (means ± SE, n = 3) followed by different letters indicate significant differences between treatments for the same soil aggregate fraction (*P < 0.05; **P < 0.01; ***P < 0.001)
5 Conclusions
Our study demonstrated that the 6-year biochar addition significantly improved soil macroaggregate formation and structural stability (as estimated MWD and AR), and altered total SOC and BC distribution within aggregate fractions in an intensive cropping system of the North China Plain. Higher biochar rate showed similar functions on structural stability with crop residue direct return to soil. In biochar-amended soils, higher total SOC accumulation was observed in the larger fractions (>2000, 250–2000, and 53–250 μm), indicating the positive function of biochar on SOC protection through soil aggregation. A positive correlation between SOC concentration and MWD (r = 0.750; P < 0.01), and an insignificant correlation between BC and MWD (r = 0.317; P = 0.107) imply that the positive role of SOC in soil aggregation overrode that of BC. Meanwhile, more BC occluded in the separated fractions (250–2000, 53–250, and <53 μm) under the biochar treatments. These data demonstrated that biochar particle may interact with the mineral matrix to enhance the formation of soil aggregation and organo-mineral complexes, which probably contributed to the stability of BC in soil.
References
Abiven S, Hund A, Martinsen V, Cornelissen G (2015) Biochar amendment increases maize root surface areas and branching: a shovelomics study in Zambia. Plant Soil 395:45–55
Atkinson CJ, Fitzgerald JD, Na H (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337:1–18
Awad YM, Blagodatskaya E, Ok YS, Kuzyakov Y (2013) Effects of polyacrylamide, biopolymer and biochar on the decomposition of 14C-labelled maize residues and on their stabilization in soil aggregates. Eur J Soil Sci 64:488–499
Baiamonte G, De Pasquale C, Marsala V, Cimò G, Alonzo G, Crescimanno G, Conte P (2015) Structure alteration of a sandy-clay soil by biochar amendments. J Soils Sediments 15:816–824
Baronti S, Vaccari FP, Miglietta F, Calzolari C, Lugato E, Orlandini S, Pini R, Zulian C, Genesio L (2014) Impact of biochar application on plant water relations in Vitis vinifera (L.). Eur J Agron 53:38–44
Biederman LA, Harpole WS (2013) Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioenergy 5:202–214
Brodowski S, Amelung W, Haumaier L, Abetz C, Zech W (2005) Morphological and chemical properties of black carbon in physical soil fractions as revealed by scanning electron microscopy and energy-dispersive X-ray spectroscopy. Geoderma 128:116–129
Brodowski S, John B, Flessa H, Amelung W (2006) Aggregate-occluded black carbon in soil. Eur J Soil Sci 57:539–546
Busscher WJ, Novak JM, Evans DE, Watts DW, Niandou MAS, Ahmedna M (2010) Influence of pecan biochar on physical properties of a Norfolk loamy sand. Soil Sci 175:10–14
Clough A, Skjemstad JO (2000) Physical and chemical protection of soil organic carbon in three agricultural soils with different contents of calcium carbonate. Soil Res 38:1005–1016
Cross A, Sohi SP (2011) The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol Biochem 43:2127–2134
Czimczik CI, Masiello CA (2007) Controls on black carbon storage in soils. Glob Biogeochem Cycl 21:GB3005
Du Z, Wang Y, Huang J, Lu N, Liu X, Lou Y, Zhang Q (2014) Consecutive biochar application alters soil enzyme activities in the winter wheat-growing season. Soil Sci 179:75–83
Glaser B, Balashov E, Haumaier L, Guggenberger G, Zech W (2000) Black carbon in density fractions of anthropogenic soils of the Brazilian Amazon region. Org Geochem 31:669–678
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—a review. Biol Fertil Soils 35:219–230
Gul S, Whalen JK, Thomas BW, Sachdeva V, Deng H (2015) Physico-chemical properties and microbial responses in biochar-amended soils: mechanisms and future directions. Agric Ecosyst Environ 206:46–59
Gupta VVSR, Germida JJ (2015) Soil aggregation: influence on microbial biomass and implications for biological processes. Soil Biol Biochem 80:A3–A9
Herath HMSK, Camps-Arbestain M, Hedley M (2013) Effect of biochar on soil physical properties in two contrasting soils: an Alfisol and an Andisol. Geoderma 209–210:188–197
Joseph SD, Camps-Arbestain M, Lin Y, Munroe P, Chia CH, Hook J, van Zwieten L, Kimber S, Cowie A, Singh BP, Lehmann J, Foidl N, Smernik RJ, Amonette JE (2010) An investigation into the reactions of biochar in soil. Soil Res 48:501–515
Kemper W, Rosenau R (1986) Aggregate stability and size distribution. In: Klute A (ed) Methods of soil analysis: part 1. Physical and mineralogical methods, 2nd edn. SSSA, Madison, Wisconsin, pp 425–442
Khademalrasoul A, Naveed M, Heckrath G, Kumari KGID, de Jonge LW, Elsgaard L, Vogel H-J, Iversen BV (2014) Biochar effects on soil aggregate properties under no-till maize. Soil Sci 179:273–283
Kuzyakov Y, Subbotina I, Chen H, Bogomolova I, Xu X (2009) Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol Biochem 41:210–219
Lal R (1991) Soil structure and sustainability. J Sustain Agr 1:67–92
Lehmann J (2006) Black is the new green. Nature 442:624–626
Lehmann J, Solomon D, Kinyangi J, Dathe L, Wirick S, Jacobsen C (2008) Spatial complexity of soil organic matter forms at nanometre scales. Nat Geosci 1:238–242
Lehmann J, Czimczik C, Laird D, Sohi S (2009) Stability of biochar in soil. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science and technology. Earthscan, London, UK, pp 183–206
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43:1812–1836
Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O’Neill B, Skjemstad JO, Thies J, Luizão FJ, Petersen J, Neves EG (2006) Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70:1719–1730
Liang B, Lehmann J, Solomon D, Sohi S, Thies JE, Skjemstad JO, Luizao FJ, Engelhard MH, Neves EG, Wirick S (2008) Stability of biomass-derived black carbon in soils. Geochim Cosmochim Acta 72:6069–6078
Lim B, Cachier H (1996) Determination of black carbon by chemical oxidation and thermal treatment in recent marine and lake sediments and Cretaceous-Tertiary clays. Chem Geol 131:143–154
Liu Z, Chen X, Jing Y, Li Q, Zhang J, Huang Q (2014) Effects of biochar amendment on rapeseed and sweet potato yields and water stable aggregate in upland red soil. Catena 123:45–51
Lu N, Liu X, Du Z, Wang Y, Zhang Q (2014) Effect of biochar on soil respiration in the maize growing season after 5 years of consecutive application. Soil Res 52:505–512
Manyà JJ (2012) Pyrolysis for biochar purposes: a review to establish current knowledge gaps and research needs. Environ Sci Technol 46:7939–7954
Mukherjee A, Lal R (2013) Biochar impacts on soil physical properties and greenhouse gas emissions. Agronomy 3:313–339
Mukherjee A, Lal R (2014) The biochar dilemma. Soil Res 52:217–230
Nelson D, Sommers L (1996) Total carbon, organic carbon, and organic matter: laboratory methods. In: Sparks DL et al (eds) Methods of soil analysis. Part 3. Chemical methods. SSSA, Madison, Wisconsin, pp 961–1010
Novak JM, Busscher WJ, Watts DW, Laird DA, Ahmedna MA, Niandou MAS (2010) Short-term CO2 mineralization after additions of biochar and switchgrass to a Typic Kandiudult. Geoderma 154:281–288
Obia A, Mulder J, Martinsen V, Cornelissen G, Børresen T (2016) In situ effects of biochar on aggregation, water retention and porosity in light-textured tropical soils. Soil Till Res 155:35–44
Ouyang L, Wang F, Tang J, Yu L, Zhang R (2013) Effects of biochar amendment on soil aggregates and hydraulic properties. J Soil Sci Plant Nutr 13:991–1002
Paz-Ferreiro J, Fu S, Méndez A, Gascó G (2014) Interactive effects of biochar and the earthworm Pontoscolex corethrurus on plant productivity and soil enzyme activities. J Soils Sediments 14:483–494
Peng X, Ye LL, Wang CH, Zhou H, Sun B (2011) Temperature- and duration-dependent rice straw-derived biochar: characteristics and its effects on soil properties of an Ultisol in southern China. Soil Till Res 112:159–166
Rillig MC, Aguilar-Trigueros CA, Bergmann J, Verbruggen E, Veresoglou SD, Lehmann A (2015) Plant root and mycorrhizal fungal traits for understanding soil aggregation. New Phytol 205:1385–1388
Rogovska N, Laird D, Cruse R, Fleming P, Parkin T, Meek D (2011) Impact of biochar on manure carbon stabilization and greenhouse gas emissions. Soil Sci Soc Am J 75:871–879
Singh R, Babu JN, Kumar R, Srivastava P, Singh P, Raghubanshi AS (2015) Multifaceted application of crop residue biochar as a tool for sustainable agriculture: an ecological perspective. Ecol Eng 77:324–347
Six J, Elliott ET, Paustian K, Doran JW (1998) Aggregation and soil organic matter accumulation in cultivated and native grassland soils. Soil Sci Soc Am J 62:1367–1377
Skjemstad J, Clarke P, Taylor J, Oades J, Mcclure S (1996) The chemistry and nature of protected carbon in soil. Soil Res 34:251–271
Sohi SP, Krull E, Lopez-Capel E, Bol R (2010) A review of biochar and its use and function in soil. Adv Agron 105:47–82
Tisdall JM, Oades JM (1982) Organic matter and water-stable aggregates in soils. J Soil Sci 33:141–163
Verheijen FGA, Graber ER, Ameloot N, Bastos AC, Sohi S, Knicker H (2014) Biochars in soils: new insights and emerging research needs. Eur J Soil Sci 65:22–27
Warnock D, Lehmann J, Kuyper T, Rillig M (2007) Mycorrhizal responses to biochar in soil—concepts and mechanisms. Plant Soil 300:9–20
Weyers SL, Spokas KA (2011) Impact of biochar on earthworm populations: a review. Appl Environ Soil Sci ID:541592
Zhang Q, Wang Y, Wu Y, Wang X, Du Z, Liu X, Song J (2013) Effects of biochar amendment on soil thermal conductivity, reflectance, and temperature. Soil Sci Soc Am J 77:1478–1487
Zhang Q, Du Z, Lou Y, He X (2015) A one-year short-term biochar application improved carbon accumulation in large macroaggregate fractions. Catena 127:26–31
Zong Y, Xiao Q, Lu S (2015) Acidity, water retention, and mechanical physical quality of a strongly acidic Ultisol amended with biochars derived from different feedstocks. J Soils Sediments. dio:10.1007/s11368-015-1187-2
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31000250, 31570439) and the S&T Innovation Program of the Chinese Academy of Agricultural Sciences. We also thank the two anonymous referees for their helpful comments and suggestions that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Yong Sik Ok
Rights and permissions
About this article
Cite this article
Du, ZL., Zhao, JK., Wang, YD. et al. Biochar addition drives soil aggregation and carbon sequestration in aggregate fractions from an intensive agricultural system. J Soils Sediments 17, 581–589 (2017). https://doi.org/10.1007/s11368-015-1349-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1349-2