Abstract
Purpose
Sorption studies of organic micropollutants to sediments are useful for predicting their fate and transport. They allow for a better understanding of the migration of contaminants through environmental media, the exposure of aquatic organisms to such chemical and their affect on human health.
Materials and methods
This study examined the sorption of carbamazepine, ketoprofen, diclofenac, bisphenol A and triclosan onto sediments sampled along a dam reservoir. The sediments differed in structure, chemical composition and particle size distribution. The potential effect on the quality of drinking water through the removal of micropollutants by sorption was also estimated.
Results and discussion
Sorption isotherms of micropollutants were constructed at pH 7.6 on three natural sediments. The sorption points were determined by measuring the concentrations of analytes in both the solution and sediment samples. The results of partitioning coefficients suggest that triclosan (187.5–1248.5 μg1-1/n l1/n kg−1) and bisphenolA (11.4–51.0 μg1-1/n l1/n kg−1) exhibited relatively higher sorption, whereas ketoprofen (1.3–2.0 l kg−1), diclofenac (4.8–5.5 l kg−1) and carbamazepine (2.9–5.5 l kg−1) did not sorb to the studied sediments. The affinity of all micropollutants was higher for the finest sediment with the highest content of organic carbon and clay minerals, collected in the deepest part of the reservoir near the water intake.
Conclusions
The low values of partitioning coefficients suggest that all of the tested micropollutants, except triclosan, are predominantly freely dissolved, which can have an adverse effect on the quality of drinking water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pharmaceuticals and personal care products (PPCPs) represent an important class of emerging organic micropollutants. These compounds are introduced to surface waters mainly by discharges of wastewater treatment plants (WWTP) and by runoff from agricultural fields (Ternes 1998; Daughton and Ternes 1999; Tixier et al. 2003; Kasprzyk-Hordern et al. 2008).
Five emerging organic micropollutants of relatively high potential ecological risk and high consumption have been selected for the subject of this study. Carbamazepine is a drug used for the treatment of epilepsy, trigeminal neuralgia, bipolar affective disorder and acute mania. It is considered an environmentally recalcitrant compound which exhibits only limited removal efficiency in municipal wastewater treatment plants (WWTPs) (Stamatelatou et al. 2003). Pharmaceuticals, such as diclofenac and ketoprofen, are non-steroidal anti-inflammatory drugs with pronounced antirheumatic, anti-inflammatory, analgesic and antipyretic properties. These pharmaceuticals have been frequently detected in municipal sewage, surface water samples and even ground water (Castiglioni et al. 2005; Tauxe-Wuersch et al. 2005). Triclosan is a very common antimicrobial agent, currently used in a large number of consumer products such as toothpastes, detergents, shampoos, body washes, deodorants, lotions and dishwashing liquids. The relatively high lipophility of triclosan can lead to sorption to particles. Besides, sorption to solid phase accounts for most of the removal of triclosan in a typical activated sludge treatment process (Bester 2003, 2005; Guang-Guo and Kookana 2007). Among anthropogenic chemicals, bisphenol A, which is listed as an endocrine disrupting chemical (EDCs), is widely used in the plastic industry as a monomer for the production of polycarbonate and epoxy resin (Michihiko et al. 2002; Min-Yu et al. 2002).
The ultimate fate of organic micropollutants in the aquatic environment is a current environmental issue (Loffler et al. 2005; Shareef et al. 2006; Styszko et al. 2010; Caldwell et al. 2014; Martínez-Hernández et al. 2014). Sorption of organic micropollutants is determined by different factors: the chemical nature of micropollutants, the ionization state, water solubility, the properties of the sorbent and temperature. The effect of thermal stratification on water quality reservoirs has been investigated by field observations and statistical analysis (Elçi 2008). It was observed that turbidity peaked mostly in the thermocline region, closely related to the location of the maximum density gradient and thus low turbulence stabilizing the sediments in the vertical water column (Elçi 2008).
The work described in this paper examines the sorption of emerging organic micropollutants to natural sediments collected along the Dobczyce dam reservoir (southern Poland). The sediments from the inflow and outflow of the reservoir vary in structure, grain size and chemical composition. Furthermore, because the reservoir is one of the main drinking water sources for Krakow and other small cities around it, experiments were conducted to answer the following question: to what degree does sorption of emerging organic micropollutants reduce the potential for exposure and, in consequence, what is the influence of their sorption on drinking water quality and accumulation in sediments. It is assumed that environment pollutant exchange between reservoir sediment and overlaying water could be limited for organic pollutants. Hydrophobic organic compounds enter waterways after sorbing to sediments and suspended solids, after which they settle out to the reservoir bottom. Water soluble compounds with low affinity for solids would most likely remain in the overlaying water during the summer period in the deepest part of reservoir, when stratification is evident. The research was novel for Polish waters, particularly for Krakow.
2 Materials and methods
2.1 Materials
Table 1 provides the chemical structures and the physical and chemical properties of the following: two non-steroidal anti-inflammatory drugs (NSAIDs), diclofenac (DCF), ketoprofen (KTP); an antiepileptic, carbamazepine (CBZ); an endocrine disruptor, bisphenol A (BPA) and a common antimicrobial agent, triclosan (TCS), investigated in the current study. Compounds of high purity grade (>90 %) were obtained from Sigma-Aldrich (Saint Louis, USA). A four ring polycyclic aromatic phenol, 1-pyrenol (98 %), was used as an internal standard and purchased from Sigma-Aldrich (Saint Louis, USA). Methanol was used to prepare solutions of the compounds both singly and as mixtures. A stock standard solution of 1000 μg ml−1 for each compound was used to prepare a mixture of standard solutions. The mixtures were used to spike water samples. Analytical grade reagents CaCl2, KH2PO4, HCl, ammonia solution (25 %), CH3COOH (99.5 %) and acetonitrile (HPLC super gradient grade), methanol (HPLC grade), ethyl acetate (HPLC grade) were obtained from POCH (Gliwice, Poland). Deionized water (<0.07 S/cm), used to prepare sample solvents for the HPLC system and solid-phase extraction (SPE), was obtained from the HLP5 pure water system (Hydrolab, Gdansk, Poland). Oasis HLB extraction cartridges, (3 cc/60 mg) for SPE, were purchased from Waters (Wexford, Ireland).
2.2 Characterization of the reservoir and its catchment
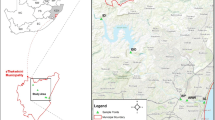
The sediments were collected from the Dobczyce drinking water reservoir (southern Poland) which supplies >60 % of the tap water to the city of Krakow and other, smaller towns around it. The reservoir is located 270 m above sea level south of the city of Krakow on the Raba river at 60 km from the source and has a volume of 125 × 106 m3 and a surface area of 970 ha. As it is shown in Fig. 1, the two main parts of the reservoir are the Myslenice basin and the Dobczyce basin. The main contaminants originate from agriculture and municipal activities with smaller industrial contributions.
2.3 Sediments
Sediment samples from the upper layer to a depth of 18 cm were collected in 2007 from areas with different types of hydrological conditions along the dam reservoir. Water depths at the sampling points were 3.5, 14.0 and 21.0 m. Exact sampling locations and the morphology of the sediments are presented in Fig. 1. The sediment samples were collected using a custom-designed sediment sampling device (LIMNOS, Komorow, Poland). Samples of sediments were taken three times at each sampling site. The layers (0–6 cm) taken from each individual sample have been combined to form one mean and representative sample for further processing and experimental measurements. Samples of sediments were stored in a frozen state until required, then dried by liophilization, sieved through a 2-mm sieve and stored at 4 °C. Sediments <2 mm were used in the experiments. The characteristics of the sediments are given in Table 2. Particle size distributions of sediment samples were analysed using a Beckman-Coulter LS 230 Laser Granulometer (Fullerton, USA), (0.04–2000 mm). Morphology was analysed by scanning electron microscopy (JEOL 5400 (EDS) with a Link ISIS 300 analyser, Oxford Instruments, UK). The microscopic images confirmed previously obtained particle size distributions for each sampling point (see Fig. 1). Total organic carbon (TOC) was quantified using the ELTRA CS500 elemental analyser (Neuss, Germany) after the removal of carbonate. The specific surface areas of the sediments were analysed using the BET method (Beckman Coulter SA3100 Analyser, USA). The ammonium acetate method, as described by Chapman (Chapman 1965), was used to determine the cation exchange capacity (CEC) of the samples. The pH of the sediments was measured by Multimeter (Crison Instruments, Barcelona, Spain).
2.4 Kinetic experiment
The batch sorption experiments of the selected compounds (Table 1) were conducted in dark glass bottles with 50 ml of 0.01 M CaCl2 and 10 g of sediments. To make sure the partitioning experiments were performed under equilibrium conditions, a kinetic study was performed. This kinetic experiment was performed with a concentration of 0.04 μg ml−1 for carbamazepine, diclofenac, bisphenol A, triclosan and 0.08 μg ml−1 for ketoprofen. The solutions were sampled at pre-defined time intervals: 3, 6, 18 and 24 h. After 24 h, the concentrations of the biocides in the liquid phase did not change any longer, i.e. equilibrium had been reached.
2.5 Batch sorption experiments
Batch experiments were conducted as specified by OECD Guideline 106 (OECD Guidelines for the Testing of Chemicals 2006). All experiments were done in 0.01 M CaCl2 solution. Ten grams of dried and sieved sediments, fraction under 2 mm, were mixed with 50 ml of 0.01 M CaCl2 solution, in dark glass bottles, spiked with appropriate working standard solutions and shaken for 24 h using a horizontal shaker at 50 rpm. Five initial concentrations in the aqueous phase for carbamazepine, diclofenac, bisphenol A and triclosan were 0.02, 0.04, 0.08, 0.16 and 0.32 μg ml−1. In the case of ketoprofen, the applied initial concentrations were doubled to obtain fractional uptakes at the sorption points above 20 %. Analytes were spiked in small volumes of methanol, less than 1 volume percent of total solution volume. At 24 h, pharmaceutical concentrations were quantified in the sediment and water phases.
After 24 h, the sediment suspensions were separated by centrifuging at 4000 rpm for 5 min. The supernatants were analysed using the method described below. After the removal of the supernatants, sediment samples were frozen and dried by lyophilisation. Dried sediment samples were extracted (extraction from 8 g of sediment) with 20 ml of methanol for 3 h using a horizontal shaker at 50 rpm and centrifuged. The final extracts (approximately 20 ml) were transferred to 500 volumetric flasks, followed by the addition of deionized water, the pH was adjusted to 2 and the extracts were analysed by the method described below.
2.6 Chemical analysis
The water samples obtained after centrifugation and acidification to pH 2 were extracted by using 60-mg Oasis HLB columns. Before use, the SPE cartridges were conditioned successively with 3 ml ethyl acetate, 3 ml methanol and 3 ml of water adjusted to pH 2. The water samples were loaded under gravitation. After loading, the cartridges were dried under vacuum for 30 min. After elution with 3 ml of ethyl acetate, the collected extracts were evaporated to dryness with a gentle stream of nitrogen and redissolved in a 100-μl solution of internal standard (20 μg ml−1) in methanol. Chromatographic analyses were performed with a Varian HPLC system consisting of the 9012 pump, 9050 UV-Vis detector and 9100 auto-sampler. The separation was carried out using a LiChrospher® 100 RP-18 (125 mm × 4 mm; particle diameter = 5 μm) cartridge column (Merck, Darmstadt, Germany) protected by a LiChrospher® 100 RP-18 (4 mm × 4 mm; particle diameter = 5 μm) guard column (Merck). The mobile phases were as follows: (A) 50 mM KH2PO4 and (B) acetonitrile (ACN). The HPLC method began with 10 % ACN content of the mobile phase and was increased to 70 % within 30 min. Afterwards, the column was equilibrated for 10 min at 10 % ACN. The injection volume was 20 μl, and flow rate 1 ml/min. The column eluent was analysed at wavelength λ = 220 nm.
2.7 Recoveries and limit of quantification
Control samples, without sediment, but spiked with tested substances were conducted in the same steps as the test system. Blank samples were included in each sample series. Absolute compound recoveries for water samples were determined by comparing the peak area of the/each control sample, to the area obtained from detecting a standard solution prepared in the same solvent mixture as the sample extract. Recoveries for each analyte over the entire method were determined by spiking wet sediment samples (8 μg of carbamazepine, diclofenac, bisphenol A, triclosan and 16 μg of ketoprofen). Subsequently, the samples were shaken for 2 h to mix sediment with the added standard solution, then frozen and dried by lyophilisation. Absolute recoveries were determined by comparing the peak area of a sediment extract (extraction from 8 g of sediment), which went through the whole method, to the area of the standard solution.
The quantification limits (LOQ) of the method for all analytes ranged from 0.04 to 20 μg ml−1 and 18 to 25 μg g−1 in aqueous and solid samples, respectively (see Table 3). Depending on the compound, the absolute recoveries ranged from 72 to 95 % in aqueous samples and from 61 to 85 % in sediment samples (see Table 3).
3 Results and discussion
3.1 Kinetics of sorption
Initial sorption experiments as a function of contact time were conducted on sediment S1. Sorption of triclosan (TCS) and bisphenol A (BPA) reached equilibrium in a few hours. The apparent sorption equilibria of carbamazepine (CBZ), diclofenac (DCF) and ketoprofen (KTP) were established within 24 h. The data shows that the mass balances of all compounds were incomplete. The total analyte mass after 24 h for CBZ, TCS and BPA was reduced to approximately 80 % (see Fig. 2). The total mass of DCF was 89 %, and for KTP, it exceeded 92 % (Fig. 2). Sorption evaluation based on the monitoring of analyte concentration in just the aqueous phase would have led to an overestimation of solid-water distribution coefficients. As a result, dissipative losses would have falsely been attributed to the uptake of the analyte to the sediment. The sorption points were determined by measuring the concentrations of analytes in both the solution and sediment samples.
3.2 Sorption isotherms
Sorption isotherms were obtained to describe the sorption behaviours of selected micropollutants on sediments along the dam reservoir. The sorption isotherms of the tested compounds are shown in Fig. 3. The data points obtained after an equilibration time of 24 h were regressively analysed using the linear and Freundlich models. The values of Kd, Freundlich constants (KF), the slope (n) and regression coefficients (R 2) obtained for both models are presented in Table 4. Correlations were found with R 2 values of 0.8239–0.9989.
Sorption isotherms of carbamazepine (CBZ), bisphenol A (BPA), triclosan (TCS), ketoprofen (KTP) and diclofenac (DCF) to the sediments S1, S2 and S3. Lines represent the linear (CBZ, KTP, DCF) and Freundlich models (TCS, BPA) adapted to the sorption data. Cw (μg l−1) and Cs (μg kg−1) are the aqueous and the sorbed concentrations of micropollutants, respectively
Through analysing the correlation, it was found that the linear model can be accepted for ketoprofen, diclofenac and carbamazepine in the case of all tested sediments (see Table 4). It also confirmed the Freundlich parameters (n) of ketoprofen, diclofenac and carbamazepine to all sediments which were linear or close to it. Correlation coefficients found for isotherms of bisphenol A and triclosan indicated that the Freundlich model better describes their sorption. Also, the Freundlich parameter, slope (n) values for bisphenol A and triclosan were lower than one (0.6985–0.9896) and proved decreasing affinities with increasing analyte concentrations. For details, see Table 4.
For all sediments, ketoprofen was found to have the lowest Kd values while triclosan had the highest KF values (Table 4). In comparison to triclosan, the values of the partitioning coefficients of bisphenol A were up to two orders of magnitude lower and those of ketoprofen, carbamazepine, diclofenac up to three orders of magnitude lower. The analytes may be ranked in order of increasing sorption coefficients in natural sediments as follows: ketoprofen < carbamazepine < diclofenac < bisphenol A < triclosan.
The Kd and KF values for individual compounds, except triclosan, stayed on the same order of magnitude for all sediments. KF values of triclosan exhibited significant differences, up to one order of magnitude, between the values for sediments S1, S2 and S3. All compounds had the lowest values of partitioning coefficient for sediment S1 and the highest for S3. From the mineralogical point of view, the analysed sediments consist mainly of quartz and alumino-silicates (Gołaś et al. 2005; Macherzynski et al. 2008b). Sorption generally increases with increasing organic carbon content of sediments. It should be noticed that sediment S3 has a much more porous structure (see Fig. 1) and exhibited higher organic carbon and clay contents in comparison to sediments S1 and S2 (see Table 2). The more porous structure of sediment S3 also confirms the highest content of pore water of all the tested sediments (see Table 2). This could be related to higher values of the specific surface area and CEC of sediment S3 (see Table 2). It seems that higher clay content can improve the sorption capacity of sediment S3.
The resulting partitioning coefficient values normalized on organic carbon content (log KOC) were plotted against the octanol water partitioning coefficient (log KOW) values in Fig. 4. Taking into account the high log KOW value (4.76) (see Table 1) of triclosan, one could expect strong sorption of this analyte. Given that the log KOC of triclosan (see Fig. 4) obtained in this study was similar to the octanol-water partitioning, coefficient for triclosan suggests that sorption capacity could be related to organic carbon content. The similar correlation between log KOW (2.45) and log KOC (2.43–3.00) can be observed for carbamazepine (see Fig. 4). The results of log KOC (3.30–3.60) of bisphenol A, similar to log KOW (3.4), also suggest sorption to organic matter. Diclofenac also has a high value of log KOW (4.51), but it showed a markedly lower log KOC value (2.44–3.22). Diclofenac mainly occurs in its anionic form in the natural pH of sediments; so, one could speculate that electrostatic interactions occurred between silt/clay and the ionic form of the analyte. It can be seen that the sorption behaviour of diclofenac in these sediments was less predictable. Ketoprofen is also an organic acid, and its values of Kd were the lowest. It could be suggested that this phenomenon is similar to that occurring in the case of diclofenac.
The sorption coefficients obtained in this study are comparable to those reported by other research. For example, Xu et al. (2009) found that ketoprofen was not strongly adsorbed to the soils with Kd values ranging from 1.26 to 8.24 l kg−1. The Kd values are similar to those determined in this study. Scheytt et al. (2005) studied the adsorption of carbamazepine and diclofenac to natural sandy sediments. Distribution coefficient (Kd) determined by the batch experiments varied from 0.21 to 5.32 for carbamazepine and 0.55 to 4.66 for diclofenac. Yamamoto et al. (2009) investigated eight pharmaceuticals (including carbamazepine) in batch sorption experiments using river sediments and model soils. Their reported Kd values of carbamazepine (0.08–1.8) were similar to this study as regards the sediment with the lowest organic carbon content. Dobor et al. (2012) examined the sorption of selected anti-inflammatory drugs on biofilm-covered river sediments. The calculated Kd values increased in the sequence of ibuprofen, naproxen, ketoprofen and diclofenac and varied between 0.2–1.2 (ketoprofen) and 0.2–1.4 (diclofenac), depending on the characteristics of the sediments. Stein et al. (2008) examined the sorption of eight psychoactive drugs including carbamazepine, on two river sediments with 0.74 and 4.36 % organic carbon content. The linear partitioning coefficient (Kd) of carbamazepine was 1.7 and 12.3 l kg−1. Sorption of bisphenol A on soils, sediments, minerals and zeolite was reported by Pan et al. (2009). The Kd data varied widely for bisphenol A from 2.75 to 212.8 depending on the properties of the solid matrices. The results of partitioning coefficient obtained for triclosan using soils and sediments showed a significant affiliation of this compound to solid matrices. The obtained Kd values ranged from 220 to 1092 for sediments (Huang et al. 2015) and 231 to 344 for soils (Karnjanapiboonwong et al. 2010). Both degradation rate and toxic effects of TCS decreased in sediment with higher sorption capacity, which can be attributed to a reduced bioavailablity (Karnjanapiboonwong et al. 2010). As partially presented above, the sorption of selected pharmaceuticals to sediments and soils was moderately dependent on the organic carbon content and also suggest the large contribution of hydrophobic interaction between solid matrix organic matter and the hydrophobic portion of the compounds molecules.
3.3 Environmental relevance
The partitioning coefficients of five micropollutants were obtained for the sediments. The results of partitioning coefficients (from 1.3 to 1248 l kg−1, see Table 4) and content of pore water in sediments (from 0.70 to 2.09 l) obtained by Macherzynski et al. (2008a) allowed for the estimation of the distribution of micropollutants between sediments and pore water. The fraction of compounds associated with the solid phase is estimated according to
where Cs (kg l−1) is the concentration of the solid material.
Results of the fraction of micropollutants bonded with sediments in equilibrium with pore water are presented in Fig. 5 and are in the range of 49–99 %. Pore water content has an influence on the sorption of carbamazepine, ketoprofen and diclofenac (see Fig. 5). The higher content of clay minerals in sediment S3 influences its structure, which can be seen on the SEM images in Fig. 1. The presence of clay minerals in the sediments increases the amount of pore water. The fraction of micropollutants sorbed on sediments decreases with the increasing content of pore water. This could suggest that the high pore water content of sediment S3 facilitates the exchange of ketoprofen, diclofenac and carbamazepine between pore water and bottom water. The content of pore water does not affect the fraction of bisphenol A and triclosan bonded with sediments.
A similar sorption behaviour of compounds can be expected in the case of suspension. The average concentration of suspension in reservoirs ranges from 3 to 25 mg l−1. Additionally, the fraction fs (see Eq. (1)) is in the range of 0.0032 to 2.78 %. These calculations indicate that all compounds except triclosan are predominantly freely dissolved. Similarly, the low values of fraction of carbamazepine and other psychoactive drugs associated with the suspension were reported by Stein et al. (2008). Consequently, we can expect a low level of occurrence for self-cleaning processes of water by sorption. Negligible sorption of these micropollutants could have a negative influence on the quality of drinking water. The water intake is located in the Dobczyce basin (see Fig. 1), close to the dam, where the smallest grains were decanted. On the other hand, low sorption limits the accumulation of micropollutants in sediments.
Another aspect which affects sorption is the thermal stratification in the deepest part of the reservoir—the Dobczyce basin (locations S3)—from May to September (Starmach and Mazurkiewicz-Boroń 2000). In this period, water inflow to the Dobczyce basin is in the epilimnion and does not have contact with the sediments. Consequently, from May to September, sorption processes mainly occur in the suspension. In the Dobczyce basin, the water is fully mixed twice a year—during spring and autumn circulation, when an intensive exchange of micropollutants between water and sediments can be expected. The shallower part of the reservoir—the Myslenice basin (locations S1 and S2, see Fig. 1)—is polymictic, the mixing of water occurs throughout the year, with the exception of the period of ice cover. It should be noticed that the assessment of the increase in sediment volume per year is very difficult.
Of course, various other processes (e.g. oxidation–reduction, biological activities) require consideration in a refined assessment. For example, the study showed that diclofenac is rapidly degraded, most likely via direct photolysis (Buser et al. 1998). The seasons, the concentration of suspended material and the depth in different parts of the reservoir have effect on the light intensity in the water. We can observe that in the Dobczyce reservoir, light fades at the depth of 5–10 m. It could be expected that almost all input of diclofenac will be degraded at the inflow of the reservoir—the Myslenice basin, where the depth is 5–15 m.
4 Conclusions
Laboratory batch experiments were conducted for five emerging organic micropollutants, of relatively high potential ecological risk and high consumption, to examine the partitioning to sediments along an artificial reservoir which is the source of drinking water for Krakow and other, smaller towns around it.
The results of partitioning coefficient suggest that triclosan and bisphenol A exhibit relatively higher sorption, whereas ketoprofen, diclofenac and carbamazepine were persistent against bonding with solid phase. The highest partitioning coefficients of all compounds were observed in the case of the finest sediment. This can be attributed to the higher content of organic carbon and clay minerals. However, the structure of the sediment with a higher clay mineral content is associated with higher pore water content. The calculations showed that a higher content of pore water caused a decrease in the sediment-associated fraction of ketoprofen, diclofenac and carbamazepine. This facilitates the exchange process of micropollutants between pore and bottom water. Furthermore, the results of the suspension-associated fraction of micropollutants showed a negligible removal of all compounds except triclosan. The presented aspects of low sorption of selected micropollutants and the thermal stratification of the water in the reservoir hardly influence the concentration of micropollutants flowing into the reservoir, which has a negative impact on drinking water quality.
The load of selected micropollutants flowing into the reservoir and their fate will be the next step of research in the near future. The presence of micropollutants in drinking water will also be examined.
References
Bester K (2003) Triclosan in a sewage treatment process—balances and monitoring data. Water Res 37:3891–3896
Bester K (2005) Fate of triclosan and triclosan-methyl in sewage treatment plants and surface waters. Arch Environ Contam Toxicol 49:9–17
Buser H-R, Poiger T, Müller MD (1998) Occurrence and fate of the pharmaceutical drug diclofenac in surface waters: rapid photodegradation in a lake. Environ Sci Technol 32:3449–3456
Caldwell DJ, Mastrocco F, Margiotta-Casaluci L, Brooks BW (2014) An integrated approach for prioritizing pharmaceuticals found in the environment for risk assessment, monitoring and advanced research. Chemosphere 115:4–12
Castiglioni S, Bagnati R, Fanelli R, Pomati F, Calamari D, Zuccato E (2005) Removal of pharmaceuticals in sewage treatment plants in Italy. Environ Sci Technol 40:357–363
Chapman HD (1965) Methods of soil analysis. Agronomy: Am. Inst. Agronomy. Madison, Wisconsin, USA
Daughton CG, Ternes TA (1999) Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect 107(Suppl 6):907–938
Dobor J, Varga M, Záray G (2012) Biofilm controlled sorption of selected acidic drugs on river sediments characterized by different organic carbon content. Chemosphere 87:105–110
Elçi Ş (2008) Effects of thermal stratification and mixing on reservoir water quality. Limnology 9:135–142
Gołaś J, Kubica B, Reczyński W, Kwiatek WM, Jakubowska M, Skiba M, Stobiński M, Dutkiewicz EM, Posmyk G, Jones KW, Olko M, Górecki J (2005) Preliminary studies of sediments from the Dobczyce drinking water reservoir. Pol J Environ Stud 14:577–584
Guang-Guo Y, Kookana RS (2007) Triclosan in wastewaters and biosolids from Australian wastewater treatment plants. Environ Int 33:199–205
Huang X, Wu C, Hu H, Yu Y, Liu J (2015) Sorption and degradation of triclosan in sediments and its effect on microbes. Ecotoxicol Environ Saf 116:76–83
Karnjanapiboonwong A, Morse A, Maul J, Anderson T (2010) Sorption of estrogens, triclosan, and caffeine in a sandy loam and a silt loam soil. J Soils Sediments 10:1300–1307
Kasprzyk-Hordern B, Dabrowska A, Vieno N, Kronberg L, Nawrocki J (2008) Occurrence of acidic pharmaceuticals in the Warta River in Poland. Chem Anal 53:289–303
Loffler D, Rombke J, Meller M, Ternes TA (2005) Environmental fate of pharmaceuticals in water/sediment systems. Environ Sci Technol 39:5209–5218
Macherzynski M, Reczyński W, Parker A, Górecki J, Gołaś J (2008a) Distribution of Cr, Pb, Cu, Cd and Zn in sediment samples from the Dobczyce dam reservoir. Proc ECOpole 2:281–289
Macherzynski M, Reczyński W, Sanecki J, Górecki J, Gołaś J (2008b) Sediment samples from the Dobczyce dam reservoir (Southern Poland). Arch Environ Prot 34:211–221
Martínez-Hernández V, Meffe R, Herrera S, Arranz E, de Bustamante I (2014) Sorption/desorption of non-hydrophobic and ionisable pharmaceutical and personal care products from reclaimed water onto/from a natural sediment. Sci Total Environ 472:273–281
Michihiko I, Min-Yu C, Chang-Suk J, Masanori F (2002) Acute toxicity, mutagenicity, and estrogenicity of biodegradation products of bisphenol-A. Environ Toxicol 17:457–461
Min-Yu C, Michihiko I, Masanori F (2002) Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environ Toxicol 17:80–86
OECD Guidelines for the Testing of Chemicals (2006) Test No. 106: adsorption-desorption using a batch equilibrum method. doi:10.1787/9789264069602-en
Pan B, Ning P, Xing B (2009) Part V–sorption of pharmaceuticals and personal care products. Environ Sci Pollut Res Int 16:106–116
Scheytt T, Mersmann P, Lindstadt R, Heberer T (2005) Determination of sorption coefficients of pharmaceutically active substances carbamazepine, diclofenac, and ibuprofen, in sandy sediments. Chemosphere 60:245–253
Shareef A, Angove MJ, Wells JD, Johnson BB (2006) Sorption of bisphenol A, 17α-ethynylestradiol and estrone to mineral surfaces. J Colloid Interface Sci 297:62–69
Stamatelatou K, Frouda C, Fountoulakis M, Drillia P, Kornaros M, Lyberatos G (2003) Pharmaceuticals and health care products in wastewater effluents: the example of carbamazepine. Water Sci Technol Water Supply 3:131–137
Starmach J, Mazurkiewicz-Boroń G (2000) Dobczyce reservoir (Zbiornik Dobczycki, in polish) by Karol Starmach Institute of Freshwater Biology. Polish Academy of Sciences, Cracow
Stein K, Ramil M, Fink G, Sander M, Ternes TA (2008) Analysis and sorption of psychoactive drugs onto sediment. Environ Sci Technol 42:6415–6423
Styszko K, Sosnowska K, Wojtanowicz P, Golas J, Gorecki J, Macherzynski M (2010) Sorption of ibuprofen on sediments from the Dobczyce (Southern Poland) drinking water reservoir. Arch Environ Prot 36:81–91
Tauxe-Wuersch A, De Alencastro LF, Grandjean D, Tarradellas J (2005) Occurrence of several acidic drugs in sewage treatment plants in Switzerland and risk assessment. Water Res 39:1761–1772
Ternes TA (1998) Occurrence of drugs in German sewage treatment plants and rivers. Water Res 32:3245–3260
Tixier C, Singer HP, Oellers S, Muller SR (2003) Occurrence and fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofen, and naproxen in surface waters. Environ Sci Technol 37:1061–1068
Xu J, Wu L, Chen W, Chang AC (2009) Adsorption and degradation of ketoprofen in soils. J Environ Qual 38:1177–1182
Yamamoto H, Nakamura Y, Moriguchi S, Nakamura Y, Honda Y, Tamura I, Hirata Y, Hayashi A, Sekizawa J (2009) Persistence and partitioning of eight selected pharmaceuticals in the aquatic environment: laboratory photolysis, biodegradation, and sorption experiments. Water Res 43:351–362
Acknowledgments
This work was supported by AGH University Grant no 11.11.210.244. The author thanks the staff of “Municipal Waterworks and Sewer Enterprise in Kraków” for their support in the collecting of sediment samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Jan Schwarzbauer
Rights and permissions
About this article
Cite this article
Styszko, K. Sorption of emerging organic micropollutants onto fine sediments in a water supply dam reservoir, Poland. J Soils Sediments 16, 677–686 (2016). https://doi.org/10.1007/s11368-015-1239-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1239-7