Abstract
Purpose
Sediments have been shown to contribute to the microbial quality of the water column during resuspension and serve as reservoirs for potentially pathogenic organisms. Currently, definitive guidelines regarding microbial indicators that need to be assessed in order to monitor faecal pollution in sediments do not exist. In this study, Escherichia coli (a well-established indicator) and Clostridium perfringens were monitored to determine their suitability as indicators for faecal pollution of sediments.
Materials and methods
Enumeration of E. coli in water was performed using the ColilertTM 18/Quantitray-2000 system from IDEXX. Identification and enumeration of C. perfringens in water was conducted using the boil method followed by the pour plate technique. Real-time polymerase chain reaction (RT-PCR) was used to confirm isolates. E. coli and C. perfringens were enumerated in sediment by firstly using the water displacement approach to dislodge organisms from sediment and then subsequently followed by the same methods as those used for detection and enumeration of the two potential indicators in water.
Results and discussion
The highest concentrations of E. coli and C. perfringens were obtained along the main stem of the Apies River which was characterised by the presence of wastewater treatment works, animal farmlands and informal settlements with inadequate sanitary facilities. The lowest concentration of both organisms was observed along the tributaries of the river, where there was minimal faecal pollution-related activity. Due to the difference in biological characteristics and survival patterns, concentrations of E. coli in sediments fluctuated (higher concentrations in the wet season) during the entire sampling period while concentrations of C. perfringens remained stable. There was a positive correlation between temperature and the presence of both organisms in the sediment, indicating the enabling environment of sediment to aid in bacterial survival.
Conclusions
E. coli and C. perfringens are both suitable indicators of faecal pollution in riverbed sediments. However, both organisms need to be monitored together for accurate assessment of the faecal pollution of sediments. E. coli remains a good indicator of recent faecal pollution and provides insight into the short-term impact of faecal pollution, while C. perfringens gives an indication of the long-term impact of faecal deposition in riverbed sediments due to the organisms’ persistence in the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A large and diverse nature of microorganisms can be present at any given time in the aquatic environment. An attempt to enumerate all the pathogens in water and sediments can be a time-consuming and financially demanding task that would appear unrealistic to execute for monitoring purposes (Izbicki et al. 2009). The World Health Organisation (WHO) recommends the analysis of faecal indicator microorganisms for assessing microbial water quality of any water intended for drinking; though in some cases, like in disease outbreaks, analysis may also include assessment of specific pathogen densities. Several studies have often referred to these indicator organisms as ‘microbial indicatorsʼ (Lisle et al. 2004; Shibata et al. 2004; Costán-Longares et al. 2008; Zhang et al. 2013). Considering the ambiguity of the term microbial indicator, a reclassification was undertaken which was more reflective of the function of each indicator group (Table 1).
As recommended by the WHO, certain criteria need to be met for an organism to be considered as an indicator of faecal pollution. Some of these criteria include the universal presence of this organism in high numbers in humans and other warm-blooded animals’ faeces, the simplicity of the method used for the detection of the organism and the inability of the organism to grow in natural water (WHO 2008).
There have been great advances in molecular methods allowing for the detection of the presence of pathogens in water and sediments. However, limitations such as the cost of instrumentation and the inability of most of these molecular methods to detect the viability of cells make a number of these methods unsuitable for routine monitoring purposes (Ashbolt et al. 2001; Klein 2002; Noble and Weisberg 2005). Thus, the enumeration of indicator organisms of faecal pollution using low-cost methods that can detect microbial viability remains a valuable microbial risk assessment tool (Yates 2007).
Several indicator organisms have been used in different studies around the world (Table 2). Total coliforms, faecal coliforms, Escherichia coli, faecal streptococci and enterococci have been the most commonly tested indicators of faecal pollution in water. However, in their review, Figueras and Borrego (2010) determined the strengths and shortcomings of indicators of faecal pollution and illustrated that none of these indicator organisms fulfilled all the required criteria necessary for assessing water quality. Thus, relying on a single indicator organism for predicting faecal pollution could be inadequate for setting protection measures of public health importance (Tyagi and Chopra 2006).
International bodies like the WHO, US Environmental Protection Agency (USEPA) and the European Union (EU) have recommended the use of E. coli as a suitable indicator (Figueras and Borrego 2010), despite the limitations discussed above. In addition to E. coli, the USEPA also recommends the use of Enterococci for marine and fresh waters. The organism (E. coli) has been historically and widely used, often as a singular indicator, to provide conclusive evidence of faecal pollution in water (Tallon et al. 2005; WHO 2008). The large number of E. coli in the human gut and the organism’s absence in other environments, as well as the user-friendly nature of the detection methods for the organism, are characteristics that favour E. coli use over other faecal indicator bacteria (Edberg et al. 2000). However, despite these positive attributes of E. coli as an indicator of faecal pollution, it has been found to be a poor index organism for some bacterial pathogens (Kong et al. 2002; Voytek et al. 2005), viruses (Moresco et al. 2012; Lee et al. 2014) and protozoa (Edge et al. 2013; Xiao et al. 2013).
Other microorganisms that have been suggested as indicators of faecal pollution in the aquatic environment include Enterococcus spp. (Miescier and Cabelli 1982), coliphages (Payment and Franco 1993; Gantzer et al. 1998) and Clostridium perfringens (Payment and Franco 1993; WHO 2008). C. perfringens forms spores that are very resistant to conventional wastewater treatment procedures and to environmental stress. The vegetative cells of C. perfringens do not reproduce in the environment and the spores are largely of faecal origin (Davies and Long 1995; Venczel and Arrowood 1997). The spore-forming ability of C. perfringens is a positive attribute that will be required of an indicator which will be used in determining microbial sediment quality. C. perfringens will persist longer in the environment than conventional indicators, thus making it suitable as an indicator of both recent and previous faecal contamination (Davies and Long 1995; Graziano et al. 2005). Davies and Long (1995) reported that C. perfringens could survive for up to 85 days in marine and freshwater sediments while other indicator organisms like the faecal coliforms decreased to 10 % of their initial concentration within the same time period. Due to the characteristics of C. perfringens discussed earlier, the organism has previously been suggested as a possible indicator of faecal pollution in river sediments (Marcheggiani et al. 2008). However, very few studies have determined the robustness of C. perfringens as a suitable indicator (Marcheggiani et al. 2008). C. perfringens has also been identified as useful indicator for Cryptosporidium oocysts and Giardia cysts and thus could also serve as a model organism for the presence or absence of human pathogenic protozoans and viruses (Payment and Franco 1993; Tyagi and Chopra 2006).
Sediments within the aquatic ecosystem represent a more complex environment than the overlaying water column. Large numbers of pathogenic microorganisms including bacteria, protozoan cysts and viruses which are the main cause of enteric diseases to humans (Taylor et al. 2001; Leclerc et al. 2002; Ashbolt 2004; Eisenberg et al. 2006; Kumar et al. 2006) are able to survive in the aquatic environment due to attachment to suspended sediment particles (Karim et al. 2004; Rehmann and Soupir 2009; Cho et al. 2010; Taylor et al. 2011; Kunkel et al. 2013). Several studies have reported that natural events like storms and floods, and/or human activities such as recreation, may lead to the resuspension of the attached bacteria in the sediment leading to an increase in bacterial concentration in the water column (An et al. 2002; Sinigalliano et al. 2007; Turkmen et al. 2012; Campos et al. 2013; Walters et al. 2014).
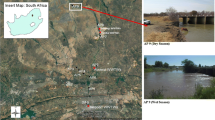
In South Africa and several other developing countries, surface waters remain an alternative water source for drinking and other household uses due to a lack of access to treated pipe water (Gemmell and Schmidt 2013). In some cases, water collected is used without prior treatment (DWAF 1996). Several studies have indicated that most of these surface water bodies, especially rivers (water column and sediment), are heavily polluted with faecal matter (Khan and Khan 2012; Britz et al. 2013; Sibanda et al. 2013; Gemmell and Schmidt 2013; Teklehaimanot et al. 2014). The resuspension of bacteria from sediments into the water column could possibly lead to an increased health risk for users of these contaminated rivers. Furthermore, guidelines do not currently exist to determine the microbial quality of sediments; hence, the health risk faced by users cannot be accurately determined. The development of guidelines to determine microbial sediment quality involves identifying suitable indicators of faecal pollution. Thus, the present study was carried out to investigate the suitability of E. coli and C. perfringens as indicators of faecal pollution in the riverbed sediments of the Apies River, Gauteng, South Africa.
2 Materials and methods
2.1 Location of study site
The Apies River located in Pretoria, Gauteng Province, South Africa, falls within the Crocodile (West) Marico Water Management Area (Fig. 1). The river falls within the Apies River basin with a total flow >500 m3 year−1, about 12 % of which comes from wastewater treatment works around the river (Venter 2007). The river begins in the Fountains Valley, Pretoria and follows through Gauteng, North-West and Limpopo provinces to eventually join the Limpopo River. There are a number of land use activities that occur along the river (Table 3), and these activities have an impact on the quality and quantity of the river. The flow in the Apies River is controlled by different processes such as the outflows from the four wastewater treatment works (WWTWs), the extraction of water for different usages and the total rainfall and runoff reaching the river. The contribution of WWTWs to the total flow in the river and the extraction of water for household and agricultural usages depend on how dry (low rainfall) or wet (high rainfall) the season is.
2.2 Sample collection and treatment
A total of 10 sampling sites (Table 3) were selected over an approximate 90 km stretch of the Apies River: sites DAS and AP1 were located upstream from the Bon Accord dam inlet; sites AP6, AP2 and AP7 were between the Bon Accord dam outlet and the Babelegi dam inlet; and sites AP8 and AP9 were downstream from the Babelegi dam outlet. Three tributaries to the Apies River (AP3, AP4 and AP5) were also included in the sampling. Dry season samples (May–August 2013) and wet season samples (January–February 2014) were collected from the 10 sampling sites along the Apies River. During the entire sampling period, a total of 1116 (558 water and 558 sediment) samples were collected and analysed for E. coli and C. perfringens.
Water samples were aseptically collected using sterile 1-l containers following standard procedures. Collection was done approximately 15 to 20 cm below the water surface to avoid collection of surface debris. All bottles were hermetically closed and transported to the laboratory on ice.
Using a sterile polypropylene scooper, grab samples of approximately 250 g of sediment were collected from the top 5 cm of the riverbed at the point directly below the site where the water sample was collected. The scooper was slightly tilted to allow the collected water to drain out and grab sediments samples were then transferred into sterile 100 ml polypropylene containers and firmly closed. The sediments were transported to the laboratory in cooler boxes with ice.
All samples were collected in triplicate and were analysed within 6 h upon arrival at the laboratory. Samples that could not be analysed on the same day were kept at 4 °C and were analysed within a 24 h period of collection.
2.3 Enumeration of indicator organisms in water samples
2.3.1 Enumeration of E. coli in water samples
E. coli were enumerated using the ColilertTM 18 Quanti-Tray/2000 system from IDEXX (IDEXX Laboratories (Pty) Ltd., Johannesburg, South Africa). Prior to analysis, the bottle was inverted several times, thus ensuring proper mixing of the river water. Analysis was performed as per the manufacturer’s instructions. Briefly, a 100-ml portion of the river water from the sampling point was mixed with the Colilert18 reagent in a sterile container, transferred and sealed in a Quanti-Tray using the Quanti-Tray sealer and incubated at 37 °C for 18–24 h. Plates were visually examined for acid production and under UV for fluorescence. The E. coli concentration was then inferred from the statistical table provided with the reagent based on the number of large and small positive wells.
2.3.2 Enumeration of C. perfringens in water samples
C. perfringens were enumerated using the pour plate technique. Aliquots of 100 ml of each sample were transferred into 120 ml glass bottles and heated at 80 °C for 5 min in a water bath to kill the vegetative forms of organisms present (Araujo et al. 2004). One milliliter of the heated sample was transferred to a Petri dish, and 15 to 20 ml TSC agar (prepared following manufacturer’s instructions and cooled to about 45 °C) supplemented with d-Cycloserine (Biomerieux®, France) was poured into the dish, allowed to solidify and incubated anaerobically at 37 °C. Plates were examined after 21 ± 3 h for the presence of black or grey colonies, characteristic of C. perfringens. The concentration of C. perfringens was expressed in colony-forming units (CFU) per volume of sample.
2.4 Enumeration of E. coli and C. perfringens from sediment samples
Sediment samples were prepared for analysis using a water displacement approach as described by Abia et al. (2015). Briefly, sediments were gradually transferred into a 1-l Durham bottle containing 400 ml of 1 × PBS to obtain a total of 500 ml, giving a 1:5 (vol/vol) dilution. Bottles were then vigorously shaken manually for approximately 2 min allowed to stand briefly, and appropriate volumes of the supernatant extracted and analysed for both indicator organisms as described for the water sample.
2.5 Real-time PCR for confirmation of C. perfringens isolates
2.5.1 DNA extraction
Confirmation of C. perfringens isolates was done using real-time polymerase chain reaction (PCR). DNA was extracted as previously described (Das et al. 2012). Briefly, a single colony was peeled off a TSC plate transferred into an Eppendof tube containing 100 μl of Milli-Q water, vortexed for 10 s and boiled at 100 °C for 10 min. The tube was then centrifuged at 10,000 rpm for 5 min to remove cell debris and the top clear supernatant used as source of template DNA.
2.5.2 PCR conditions
The reaction was run targeting the cpa gene, and the primer sequence was as follows: Forward-GCTAATGTTACTGCCGTTGA and Reverse-CCTCTGATACATCGTGTAAG (Das et al. 2012). Primers were obtained from Inqaba Biotec, South Africa. The real-time PCR reaction was carried out on a Corbett Life Science Rotor-Gene 6000 Cycler (Qiagen, Hilden, Germany). Amplification reaction was performed in a total volume of 20 μl consisting of 10 μl of a 2× SensiFASTTM HRM Mix (final concentration, 1×) (Bioline GmbH, Germany), 1 μl (final concentration, 1 μm) of each primer (Forward and Reverse), 5 μl of DNA template and 3 μl of nuclease-free water. The PCR conditions were optimised as follows: an initial incubation step at 95 °C for 10 min, followed by a 45-cycle amplification program consisting of 95 °C for 10 s, 55 °C for 15 s, 72 °C for 20 s and a final extension step at 72 °C for 5 min. The amplification step was followed by a melting step which was carried out by slow heating from 72 to 95 °C after a 90-s hold for pre-melt conditions on the first step. Fluorescence acquisition was done at 1 °C intervals with a hold for 5 s at each increment. The reaction included three positive controls (C. perfringens ATCC® 13124; American Type Culture Collection (ATCC), Manassas, VA, USA) and three negative controls (reaction mixture without DNA).
2.6 Physical and chemical water parameters
Physical parameters were measured in situ during sampling. Water temperature (°C), dissolved oxygen (DO, mg l−1), electrical conductivity (EC, μs cm−1) and pH were measured using an HQ40d Portable Multi-Parameter Meter (Hach, USA). Turbidity (Nephelometric Turbidity Units or NTUs) was measured using a T100 portable turbidly meter (EUTECH Instruments, Germany).
2.7 Statistical analysis
Statistical Package for the Social Sciences (SPSS) Version 20 (IBM Corporation, Armonk, New York, USA) and Microsoft Excel 2010 were used for data analysis. The Mann–Whitney rank sum test was used to compare seasonal occurrence of indicator organisms in water and sediment. The means of the different environmental parameters for both seasons were compared using a one-way analysis of variance (ANOVA). The correlation between the environmental parameters studied and the abundance of each indicator organism were determined using the nonparametric Spearman’s rank correlation. All statistical tests were considered significant at a 95 % confidence limit.
3 Results
3.1 Concentrations of E. coli and C. perfringens in water and sediments
Both organisms were detected at varying concentrations at all sampling sites in both the water column and sediments during the entire sampling period. All the C. perfringens isolates were confirmed positive for the cpa gene using real-time PCR (Appendix A – Electronic Supplementary Material). The mean E. coli concentration at AP2 was 1.47E + 03 MPN/100 ml in the dry season and 3.37E + 04 MPN/100 ml in the wet season, while for C. perfringens it was 1.72E + 04 CFU/100 ml and 6.02E + 04 CFU/100 ml for the dry and wet seasons, respectively (Appendix B – Electronic Supplementary Material). Relatively high concentrations of E. coli (2.30E + 02 CFU/100 ml) and C. perfringens (3.09E + 04 CFU/100 ml) (Appendix B – Electronic Supplementary Material) in the sediments were also recorded at AP8 which is situated downstream from the Babelegi and Temba WWTWs.
3.2 Comparison of E. coli and C. perfringens concentrations and detection rates in sediments
For all sampling rounds, the mean sediment concentrations of C. perfringens were higher than those of E. coli. The same trend was recorded in the overlaying water (Fig. 2). While C. perfringens showed a 100 % detection rate throughout the study, there were days where E. coli was not detected in water and sediment for various sampling sites (Appendix B – Electronic Supplementary Material). The highest number of days in which E. coli was not detected occurred at the DAS sampling point. A statistically significant difference (p < 0.05) was observed between the mean water count and the mean sediment count for both indicator organisms. Of the two indicator organisms, C. perfringens counts were higher in the sediments than in the water for the entire study period. On the other hand, higher E. coli concentrations in the sediments were only observed at some sites (AP1, AP2, AP5 and AP9) during the dry season (Table 4).
3.3 Seasonal variation in the concentration of E. coli and C. perfringens in sediments
The concentrations of E. coli and C. perfringens were also influenced by the change in season with the wet season recording higher concentrations than the dry season for both indicator organisms in the sediments. Although the difference between the dry season and the wet season mean concentrations for both indicator organisms was statistically significant (p < 0.05), E. coli showed a higher increase in concentration levels during the wet season as compared to C. perfringens (Table 5).
3.4 Correlation between indicator organisms and physico-chemical parameters
A statistically significant difference (p < 0.05) was observed between the dry and wet season measurements. A positive correlation was observed between temperature and concentrations of both indicator organisms in water and sediments (p < 0.05). A negative correlation was observed between the concentration of both indicator organisms in sediments and the water turbidity (Table 6). No correlation was observed between the concentration of C. perfringens in sediment and pH. A summary of the physic-chemical parameters is given in Appendix C (Electronic Supplementary Material)
4 Discussion
4.1 Concentrations of E. coli and C. perfringens in water and sediments
In order to assess the suitability of E. coli and C. perfringens as possible indicators of faecal pollution in riverbed sediment, it was essential to first determine if both organisms were present in the water column and the sediments, and secondly, to determine if the observed concentrations of the organisms obtained at the different sampling sites along the Apies River was reflective of the faecal pollution that might be occurring at each site. Throughout the entire study, the highest mean concentration of E. coli and C. perfringens in the sediments was recorded at site AP2 which is situated immediately downstream from Rooiwal WWTW.
The Apies River has four WWTWs situated along it (Fig. 1). Like several water bodies in South Africa, the river is highly likely to experience a deterioration in microbial quality due the presence of these WWTWs (Kinge et al. 2010; Britz et al. 2013; Gemmell and Schmidt 2013; Sibanda et al. 2013; Teklehaimanot et al. 2014). In 2008, South Africa initiated and finally adopted an incentive-based regulation to identify, reward, ensure and promote excellence regarding wastewater management. According to the 2012 report (The Green Drop Report) of this initiative, most of the country’s WWTWs were functioning above their design and/or operational capacity with 72.9 % of the 831 WWTWs assessed falling within the medium-to-critical risk categories (DWA 2012). Non-functional or sub-optimally functioning WWTWs result in the discharge of incompletely treated waste or, at times, untreated waste during plant failure, directly into surrounding water bodies. Both E. coli and C. perfringens serve as suitable indicators of the contribution that WWTWs might be adding to the faecal pollution load in sediment as both organisms were present in high concentrations in faecally polluted water. However, each organism provides a different timeline with regard to the pollution occurring. E. coli is generally very susceptible to most wastewater treatment processes and does not survive outside the gut for long periods of time. A well-functioning treatment plant will have the ability to completely reduce E. coli loads in raw waste resulting in effluent that can be safely discharged into the river with negligible impact on the river (van Der Drift et al. 1977; Olańczuk-Neyman 2001; George et al. 2002). The high E. coli concentrations recorded at the sites located downstream of WWTWs therefore suggest the discharge of untreated or poorly treated waste into the river, thus indicating recent faecal pollution. Unlike E. coli, C. perfringens is more resistant to most WWTWs processes and is usually discharged together with the final effluent (Bisson and Cabelli 1980; Fujioka et al. 1985; Skanavis and Yanko 2001; Teklehaimanot et al. 2014). Considering that the organism does not grow in natural water, the presence of C. perfringens in high concentrations at these sites is therefore indicative of faecal pollution that has occurred over a period of time and not necessarily in one pollution event.
High concentrations of E. coli and C. perfringens were also observed at sites (AP1, AP7, AP8 and AP9) along the river that were not directly downstream of WWTWs. At these sites, with the presence of informal settlements and the use of the land for agricultural purposes, the microbial quality of the river is expected to be compromised. Informal settlements often lack sanitary facilities and hence the river is sometimes used as a dumping site for human waste. Also, runoffs from agricultural farms have been identified as important sources of faecal indicator organism (Walters et al. 2010; Liang et al. 2013). The possible contribution of agricultural practice to the microbial load in sediment was observed by the presence of E. coli and C. perfringens in high concentrations especially at site AP9.
The lowest mean E. coli and C. perfringens concentrations were recorded at sites AP3, AP4 and AP5. These sites are all located on the tributaries of the Apies River and had very little land use activity and no WWTWs. Very little to no faecal pollution occurred at these sites. As expected, very low concentrations of both indicator organisms at the tributary sites were recorded, demonstrating their suitability as indicators of sediment faecal pollution even when pollution levels are very low. The suitability of both organisms as indicator organisms was further highlighted when during the wet (rainy) season samples obtained from these same tributary sites now had elevated levels of organisms. During the wet (rainy) season, these tributary sites receive large amounts of run-off from a neighbouring informal settlement characterised by high population density and inadequate sanitation facilities.
4.2 Comparison of E. coli and C. perfringens concentrations and detection rates in sediments
The location of site DAS downstream from the outlet of the Daspoort WWTW suggests that the absence of E. coli could be due to the inability of the cells to grow in the culture media because of injury from the treatment process. Thus, drawing conclusions based on the E. coli concentration alone could be misleading. C. perfringens forms stress-resistant spores that can survive for long periods of time in the environment. Also, C. perfringens has been used to check for the efficiency of water treatment processes as the organism’s spore-forming ability makes it resistant to conventional treatment methods (Bisson and Cabelli 1980; Hill et al. 1996; Skanavis and Yanko 2001; Wéry et al. 2008). As a result, it would be necessary to include C. perfringens as an indicator of faecal pollution in the sediments alongside E. coli especially at sites influenced by WWTWs. Furthermore, E. coli has been found to survive in sediments for shorter periods than many human enteric pathogens like Cryptosporidium spp. and Giardia spp. (Harwood et al. 2005). As such, the absence of E. coli is not conclusive of the absence of faecal pollution. However, this limitation could be overcome by including C. perfringens whose spore-forming ability allows it to survive longer than E. coli and also makes it a better indicator of long-term accumulation of faecal pollution (Desmarais 2002).
The observed high concentration of E. coli at sites AP1, AP2, AP5 and AP9 during the dry season could be mainly due to the various land uses around these areas. In AP9, for example, during the dry season, farmers water their cows directly in the river. During this process, the animals release waste directly into the water and because of the slow flowing conditions of the river during this period, the faeces rapidly settle into the sediments, thus polluting it. At site AP2, the WWTW could have contributed to the sediment E. coli during the dry season. Considering that the river receives little or no runoff during the dry season, the WWTW upstream contributes a greater percentage of the total river flow at this point and the presence of E. coli at these sites could indicate recent faecal pollution.
However, the overall higher concentrations of C. perfringens in the sediments compared to that of E. coli observed in this study (Fig. 3) could be due to the higher ability of C. perfringens to survive in the cold winter periods of the dry season. C. perfringens has been reported to survive in sediments long after pollution has occurred (Figueras and Borrego 2010; Devane et al. 2014). In such case, relying on C. perfringens alone in the absence of E. coli may lead to a false alarm of recent faecal pollution. Although clay has been demonstrated to aid survival of microorganisms (Santamarı and Gary 2003; Brennan et al. 2014), the higher counts of both indicator organisms in the sediments of the Apies River were not influenced by the sediment type, as other factors such as nutrient availability and available pollution source could affect the presence of the organisms.
4.3 Seasonal variation in the concentration of E. coli and C. perfringens in sediments
Rainfall increases the concentration of indicator organisms within water bodies through processes like surface runoff from surrounding areas (Guber et al. 2006; Walters et al. 2010; Liang et al. 2013; Martinez et al. 2014). The higher increase in the concentration of E. coli compared to that of C. perfringens could be due to the fact that E. coli is found in the faeces of many warm-blooded animals including ruminants and birds (Schierack et al. 2007; Figueras and Borrego 2010). Even though the high increase in the concentration of E. coli due to seasonal changes observed in this study could indicate faecal pollution, it does not necessarily mean pollution of human origin. Also, a good faecal indicator organism is not supposed to grow in unpolluted environments. However, E. coli has been reported to survive and grow with very low die-off rates even in unpolluted environments, meaning that its presence in the environment could also be of non-faecal origin (Martinez 2009; Figueras and Borrego 2010). These shortcomings could therefore limit the use of E. coli as the sole indicator of faecal pollution in the sediments of the Apies River during the wet season. On the other hand, C. perfringens has been found in the faeces of carnivorous animals and humans, but not herbivores (Vierheilig et al. 2013). However, due to the low number of carnivores, it is unlikely that these predators could be a source of marked faecal pollution in the aquatic environment due to runoff during the wet season (Mueller-spitz et al. 2010; Vierheilig et al. 2013). Therefore, the lower influence of the wet season on the concentration of C. perfringens compared to that of E. coli could imply that human sources might be the potential explanation for the increased C. perfringens counts observed during the wet season, especially at the tributaries (AP4 and AP5) and areas with agriculture (AP9) and informal settings (AP7 and AP8). As such, including C. perfringens together with E. coli in sediment monitoring during the wet season could give a better indication of the presence or absence of faecal pollution.
4.4 Correlation between indicator organisms and physico-chemical parameters
Several factors have been reported to affect the abundance of microorganisms in sediments. Temperature has been found to be one of the most important physical factors influencing growth and survival of microorganisms in the environment (Ross et al. 2003; Blaustein et al. 2013; Pachepsky et al. 2014). The negative correlation observed between the abundance of the indicator organisms in the sediments and turbidity of the water column could suggest that sediment disturbance would lead to the resuspension of organisms from the sediments to the water column.
5 Conclusions
The detection of both E. coli and C. perfringens at all the sampling sites (water and sediment) indicates possible faecal pollution in the Apies River. Sediments might enable E. coli to thrive for a longer period than in the water column, thus making it a suitable indicator for monitoring riverbed sediments. However, E. coli is still only an indicator of recent faecal pollution in sediment, as the organism is susceptible to environmental conditions and may not survive long enough to indicate a faecal pollution event has occurred. C. perfringens persists longer in the environment as compared to E. coli and was found to be present when faecal pollution had occurred even in the absence of E. coli. However, because C. perfringens can survive in sediments long after pollution has occurred, its presence alone cannot be used to predict a recent faecal pollution. Both organisms need to be monitored together in order to obtain an accurate assessment of the impact of faecal pollution in the sediments.
References
Abhirosh C, Sherin V, Thomas A (2010) Potential exposure risk associated with the high prevalence and survival of indicator and pathogenic bacteria in the sediment of Vembanadu Lake, India. Water Qual Expo Heal 2:105–113
Abia ALK, Ubomba-Jaswa E, Ssemakalu CC, Momba MNB (2015) Development of a rapid approach for the enumeration of Escherichia coli in riverbed sediment: case study, the Apies River, South Africa. J Soils Sediments. doi:10.1007/s11368-015-1081-y
An Y, Kampbell DH, Breidenbach GP (2002) Escherichia coli and total coliforms in water and sediments at lake marinas. Environ Pollut 120:771–778
Araujo M, Sueiro R, Gómez M, Garrido M (2004) Enumeration of Clostridium perfringens spores in groundwater samples: comparison of six culture media. J Microbiol Methods 57:175–180
Ashbolt N (2004) Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 198:229–238
Ashbolt N, Grabow W, Snozzi M (2001) Indicators of microbial water quality. In: Fewtrell L, Barthram J (eds) Water quality: guidelines, standards and health. IWA Publishing, London, pp 289–316
Bisson JW, Cabelli VJ (1980) Clostridium perfringens as a water pollution indicator. Water Pollut Control Fed 52:241–248
Blaustein RA, Pachepsky Y, Hill RL, Shelton DR, Whelan G (2013) Escherichia coli survival in waters: temperature dependence. Water Res 47:569–578
Brennan F, Moynihan E, Griffiths B (2014) Clay mineral type effect on bacterial enteropathogen survival in soil. Sci Total Environ 469:302–305
Britz TJ, Sigge GO, Huisamen N, Kikine T, Ackermann A, Lötter M, Lamprecht C, Kidd M (2013) Fluctuations of indicator and index microbes as indication of pollution over three years in the Plankenburg and Eerste Rivers, Western Cape, South Africa. Water SA 39:457–466
Campos C, Kershaw S, Lee R (2013) Environmental influences on faecal indicator organisms in coastal waters and their accumulation in bivalve shellfish. Estuar Coasts 36:834–853
Cho KH, Pachepsky YA, Kim JH, Guber AK, Shelton DR, Rowland R (2010) Release of escherichia coli from the bottom sediment in a first-order creek: experiment and reach-specific modeling. J Hydrol 391:322–332
Costán-Longares A, Montemayor M, Payán A, Méndez J, Jofre J, Mujeriego R, Lucena F (2008) Microbial indicators and pathogens: removal, relationships and predictive capabilities in water reclamation facilities. Water Res 42:4439–4448
Coulliette AD, Noble RT (2008) Impacts of rainfall on the water quality of the Newport River Estuary (Eastern North Carolina, USA). J Water Health 6:473–482
Das A, Mazumder Y, Dutta B (2012) Molecular typing of Clostridium perfringens isolated from diarrhoeic cattle. J Anim Sci Adv 2:226–229
Davies C, Long J (1995) Survival of fecal microorganisms in marine and freshwater sediments. Appl Environ Microbiol 61:1888–1896
Desmarais T (2002) Influence of soil on fecal indicator organisms in a tidally influenced subtropical environment. Appl Environ Microbiol 68:1165–1172
Devane ML, Moriarty EM, Wood D, Webster-Brown J, Gilpin BJ (2014) The impact of major earthquakes and subsequent sewage discharges on the microbial quality of water and sediments in an urban river. Sci Total Environ 485–486:666–680
DWA (Department of Water Affairs, Republic of South Africa) (2012) 2012 Green Drop Progress Report, South Africa
DWAF (Department of Water Affairs and Forestry) (1996) South African water quality guidelines, Volume 1. Domestic Use, South Africa
Edberg SC, Rice EW, Karlin RJ, Allen MJ (2000) Escherichia coli: the best biological drinking water indicator for public health protection. J Appl Microbiol 88:106S–116S
Edge TA, Khan IUH, Bouchard R, Guo J, Hill S, Locas A, Moore L, Neumann N, Nowak E, Payment P, Yang R, Yerubandi R, Watson S (2013) Occurrence of waterborne pathogens and Escherichia coli at offshore drinking water intakes in lake Ontario. Appl Environ Microbiol 79:5799–5813
Eisenberg JNS, Hubbard A, Wade TJ, Sylvester MD, LeChevallier MW, Levy DA, Colford JM (2006) Inferences drawn from a risk assessment compared directly with a randomized trial of a home drinking water intervention. Environ Health Perspect 114:1199–1204
Ferguson CM, Coote BG, Ashbolt NJ, Stevenson IM (1996) Relationships between indicators, pathogens and water quality in an estuarine system. Water Res 30:2045–2054
Figueras MJ, Borrego JJ (2010) New perspectives in monitoring drinking water microbial quality. Int J Environ Res Public Health 7:4179–4202
Fujioka RS, Shizumura LK, Fujioka S, Shizumura LK (1985) Clostridium perfringens, a reliable indicator of stream water quality. Water Pollut Control Fed 57:986–992
Gantzer C, Maul A, Audic JM, Schwartzbrod L (1998) Detection of infectious enteroviruses, enterovirus genomes, somatic coliphages, and Bacteroides fragilis phages in treated wastewater. Appl Environ Microbiol 64:4307–4312
Gemmell ME, Schmidt S (2013) Is the microbiological quality of the Msunduzi River (KwaZulu-Natal, South Africa) suitable for domestic, recreational, and agricultural purposes? Environ Sci Pollut Res Int 20:6551–6562
George I, Crop P, Servais P (2002) Fecal coliform removal in wastewater treatment plants studied by plate counts and enzymatic methods. Water Res 36:2607–2617
Graziano G, Twardock P, Myers R, Dial R, Scheel D (2005) The use of Clostridium perfringens as a fecal indicator to detect intertidal disposal at Backcountry Marine Campsites in Prince William Sound Alaska. For Serv Proc RMRS:565–572
Guber A, Shelton DR, Pachepsky YA, Sadeghi AM, Sikora LJ (2006) Rainfall-induced release of fecal coliforms and other manure constituents: comparison and modeling. Appl Environ Microbiol 72:7531–7539
Harwood VJ, Levine AD, Scott TM, Chivukula V, Lukasik J, Farrah SR, Rose JB (2005) Validity of the indicator organism paradigm for pathogen reduction in reclaimed water and public health protection. Appl Environ Microbiol. doi:10.1128/AEM.71.6.3163
Hill RT, Straube WL, Palmisano AC, Gibson SL, Colwell RR (1996) Distribution of sewage indicated by Clostridium perfringens at a deep-water disposal site after cessation of sewage disposal. Appl Environ Microbiol 62:1741–1746
Ibekwe A, Murinda S, Graves A (2011) Microbiological evaluation of water quality from urban watersheds for domestic water supply improvement. Int J Environ Res Public Health 8:4460–4476
Izbicki J, Swarzenski P, Reich C, Rollins C, Holden P (2009) Sources of fecal indicator bacteria in urban streams and ocean beaches, Santa Barbara, California. Ann Environ Sci 3:139–178
Karim M, Manshadi F, Karpiscak M, Gerba C (2004) The persistence and removal of enteric pathogens in constructed wetlands. Water Res 38:1831–1837
Khan S, Khan W (2012) Isolation and identification of bacterial pollutants from the Berg and Plankenburg Rivers in the Western Cape, South Africa. Water SA 38:819–824
Kinge C, Ateba C, Kawadza D (2010) Antibiotic resistance profiles of Escherichia coli isolated from different water sources in the Mmabatho locality, north-west province, South Africa. S Afr J Sci 106:44–49
Klein D (2002) Quantification using real-time PCR technology: applications and limitations. Trends Mol Med 8:257–260
Kong RYC, Lee SKY, Law TWF, Law SHW, Wu RSS (2002) Rapid detection of six types of bacterial pathogens in marine waters by multiplex PCR. Water Res 36:2802–2812
Kumar S, Ratho RK, Chawla YK, Chakraborti A (2006) Virological investigation of a hepatitis E epidemic in North India. Singapore Med J 47:769–773
Kunkel EA, Privette CV, Sawyer CB, Hayes JC (2013) Attachment of Escherichia coli to fine sediment particles within construction sediment basins. Adv Biosci Biotechnol 4:407–414
Leclerc H, Schwartzbrod L, Dei-Cas E (2002) Microbial agents associated with waterborne diseases. Crit Rev Microbiol 28:371–409
Lee CM, Lin TY, Lin C-C, Kohbodi GA, Bhatt A, Lee R, Jay JA (2006) Persistence of fecal indicator bacteria in Santa Monica Bay beach sediments. Water Res 40:2593–2602
Lee SC, Lee C, Marion J, Wang Q, Saif L, Lee J (2014) Occurrence of human enteric viruses at freshwater beaches during swimming season and its link to water in flow. Sci Total Environ 472:757–766
Liang Z, He Z, Zhou X, Powell CA, Yang Y, He LM, Stoffella PJ (2013) Impact of mixed land-use practices on the microbial water quality in a subtropical coastal watershed. Sci Total Environ 449:426–433
Lisle JT, Smith JJ, Edwards DD, Mcfeters GA (2004) Occurrence of microbial indicators and Clostridium perfringens in wastewater, water column samples, sediments, drinking water, and Weddell Seal feces collected at McMurdo. Appl Environ Microbiol 70:7269–7276
Luther K, Fujioka R (2004) Usefulness of monitoring tropical streams for male-specific RNA coliphages. J Water Health 2:171–181
Marcheggiani S, Iaconelli M, Annamaria D, Pierdominici E, La Rosa G, Muscillo M, Equestre M, Mancini L (2008) Microbiological and 16S rRNA analysis of sulphite-reducing clostridia from river sediments in central Italy. BMC Microbiol. doi:10.1186/1471-2180-8-171
Martinez JL (2009) Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ Pollut 157:2893–2902
Martinez G, Pachepsky YA, Whelan G, Yakirevich AM, Guber A (2014) Rainfall-induced fecal indicator organisms transport from manured fields: model sensitivity analysis. Environ Int 63:121–129
Miescier J, Cabelli J (1982) Enterococci and other microbial indicators in municipal effluents. J Wat Pollut Control Fed 54:1599–1606
Moresco V, Viancelli A, Nascimento MA, Souza DSM, Ramos APD, Garcia LAT, Simões CMO, Barardi CRM (2012) Microbiological and physicochemical analysis of the coastal waters of southern Brazil. Mar Pollut Bull 64:40–48
Mueller-spitz SR, Stewart LB, Klump JV, Mclellan SL (2010) Freshwater suspended sediments and sewage are reservoirs for enterotoxin-positive Clostridium perfringens. Appl Environ Microbiol 76:5556–5562
Noble RT, Weisberg SB (2005) A review of technologies for rapid detection of bacteria in recreational waters. J Water Health 3:381–392
Olańczuk-Neyman K (2001) Evaluation of indicator bacteria removal in wastewater treatment processes. Polish J Environ Stud 10:457–461
Pachepsky YA, Blaustein RA, Whelan G, Shelton DR (2014) Comparing temperature effects on Escherichia coli, Salmonella, and Enterococcus survival in surface waters. Lett Appl Microbiol 59:278–283
Payment P, Franco E (1993) Clostridium perfringens and somatic coliphages as indicators of the efficiency of drinking water treatment for viruses and protozoan cysts. Appl Environ Microbiol 59:2418–2424
Rehmann CR, Soupir ML (2009) Importance of interactions between the water column and the sediment for microbial concentrations in streams. Water Res 43:4579–4589
Ross T, Ratkowsky DA, Mellefont LA, McMeekin TA (2003) Modelling the effects of temperature, water activity, pH and lactic acid concentration on the growth rate of Escherichia coli. Int J Food Microbiol 82:33–43
Santamarı J, Gary Æ (2003) Enteric pathogens and soil: a short review. Int Microbiol 6:5–9
Schierack P, Walk N, Reiter K, Weyranch KD, Wieler LH (2007) Composition of intestinal Enterobacteriaceae populations of healthy domestic pigs. Microbiology 153:3830–3837
Shibata T, Solo-Gabriele HM, Fleming LE, Elmir S (2004) Monitoring marine recreational water quality using multiple microbial indicators in an urban tropical environment. Water Res 38:3119–3131
Sibanda T, Chigor VN, Okoh AI (2013) Seasonal and spatio-temporal distribution of faecal-indicator bacteria in Tyume River in the Eastern Cape Province, South Africa. Environ Monit Assess 185:6579–6590
Sinigalliano CD, Gidley ML, Shibata T, Whitman D, Dixon TH, Laws E, Hou A, Bachoon D, Brand L, Amaral-Zettler L, Gast RJ, Steward GF, Nigro OD, Fujioka R, Betancourt WQ, Vithanage G, Mathews J, Fleming LE, Solo-Gabriele HM (2007) Impacts of Hurricanes Katrina and Rita on the microbial landscape of the New Orleans area. Proc Natl Acad Sci U S A 104:9029–9034
Skanavis C, Yanko WA (2001) Clostridium perfringens as a potential indicator for the presence of sewage solids in marine sediments. Mar Pollut Bull 42:31–35
Tallon P, Magajna B, Lofranco C, Leung KT (2005) Microbial indicators of faecal contamination in water: a current perspective. Water Air Soil Pollut 116:139–166
Taylor L, Latham S, Mark E (2001) Risk factors for human disease emergence. Philos Trans R Soc B 356:983–989
Taylor P, Pachepsky YA, Shelton DR (2011) Escherichia coli and fecal coliforms in freshwater and estuarine sediments. Crit Rev Environ Sci Technol 41:1067–1110
Teklehaimanot GZ, Coetzee MA, Momba MNB (2014) Faecal pollution loads in the wastewater effluents and receiving water bodies: a potential threat to the health of Sedibeng and Soshanguve communities, South Africa. Environ Sci Pollut Res 21:9589–9603
Turkmen C, Ayyildiz O, Akbulut M, Kaya H (2012) Microbial quality in coastal waters of Dardanelles in relation to the pollution sources and transport pathways. Clean Soil Air Water 40:1320–1325
Tyagi V, Chopra A (2006) Alternative microbial indicators of faecal pollution: current perspective. Iran J Environ Health Sci Eng 3:205–216
Van Der Drift C, van Seggelen E, Stumm C, Hol W, Tuinte J (1977) Removal of Escherichia coli in wastewater by activated sludge. Appl Environ Microbiol 34:315–319
Venczel L, Arrowood M (1997) Inactivation of Cryptosporidium parvum oocysts and Clostridium perfringens spores by a mixed-oxidant disinfectant and by free chlorine. Appl Environ Microbiol 63:1598–1601
Venter A (2007) Prioritization of river basins in the Tshwane area with reference to faecal coliform bacteria for the purpose of the identification of candidate. Unpublished MSc Thesis. University of the Witwatersrand, South Africa
Vierheilig J, Frick C, Mayer R, Kirschner AKT, Reischer G, Derx J, Mach RL, Sommer R (2013) Clostridium perfringens is not suitable for the indication of fecal pollution from ruminant wildlife but is associated with excreta from nonherbivorous animals and human sewage. Appl Environ Microbiol 79:5089–5092
Voytek M, Ashen J, Kirshtein J, Landa E, Fogarty L (2005) Detection of Helicobacter pylori and fecal indicator bacteria in five North American rivers. J Water Health 3:405–422
Walters SP, Thebo AL, Boehm AB (2010) Impact of urbanization and agriculture on the occurrence of bacterial pathogens and stx genes in coastal waterbodies of central California. Water Res 45:1752–1762
Walters E, Schwarzwälder K, Rutschmann P, Landa E, Fogarty L (2014) Influence of resuspension on the fate of fecal indicator bacteria in large-scale flumes mimicking an oligotrophic river. Water Res 48:466–477
Wéry N, Lhoutellier C, Ducray F, Delgenès J-P, Godon J-J (2008) Behaviour of pathogenic and indicator bacteria during urban wastewater treatment and sludge composting, as revealed by quantitative PCR. Water Res 42:53–62
WHO (2008) WHO guidelines for drinking-water quality. 3rd edition. Volume 1 Recommendations. WHO Press, Geneva
Xiao G, Qiu Z, Qi J, Chen J, Liu F, Liu W, Luo J, Shu W (2013) Occurrence and potential health risk of Cryptosporidium and Giardia in the Three Gorges Reservoir, China. Water Res 47:2431–2445
Yates MV (2007) Classical indicators in the 21st century-far and beyond the coliform. Water Environ Res 79:279–286
Zhang W, Wang J, Fan J, Gao D, Ju H (2013) Effects of rainfall on microbial water quality on Qingdao No. 1 Bathing Beach, China. Mar Pollut Bull 66:185–190
Acknowledgments
This work received funding from the Water Research Commission (WRC), South Africa (Projects K5/2169 and K5/2147) and the National Research Foundation (NRF). Opinions expressed and conclusions arrived at are those of the author and are not necessarily to be attributed to the NRF.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Nives Ogrinc
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 84 kb)
Rights and permissions
About this article
Cite this article
Abia, A.L.K., Ubomba-Jaswa, E., du Preez, M. et al. Riverbed sediments in the Apies River, South Africa: recommending the use of both Clostridium perfringens and Escherichia coli as indicators of faecal pollution. J Soils Sediments 15, 2412–2424 (2015). https://doi.org/10.1007/s11368-015-1209-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-015-1209-0