Abstract
Purpose
The Biomec process, a two-stage treatment based on a short mechanochemical (MC) pretreatment and then followed by an aerobic biological degradation, was developed and tested for detoxifying marine sediments that were largely contaminated by polychlorobiphenyls (PCBs).
Materials and methods
Clean marine sediment spiked with PCBs (Aroclor 1260) and, alternatively, with decachlorobiphenyl in slurry conditions was ultramilled for 1 min in a nutational high energy ball mill, then was treated aerobically in a bioreactor with a purposely selected commercial bacterium (Burkholderia xenovorans).
Results and discussion
With ∼66 % overall PCB biodegradation achieved in less than 3 months, laboratory experiments confirmed the remarkable effectiveness of Biomec process when compared to direct bioremediation. The investigation showed in particular that the MC pretreatment decreased the chlorination degree of high-chlorinated PCB congeners, and consequently their biorecalcitrance, through the substitution of some chlorine atoms with hydroxyl groups. This reaction eases the aerobic degradation of the hydroxyl-substituted PCBs by B. xenovorans, allowing bacteria to skip the cell stressing step of aromatic ring bi-hydroxylation along the biodegradation pathway.
Conclusions
After short MC treatment of the sediments, a common biological aerobic treatment can degrade PCB congeners, the highly chlorinated ones were included, in a fast, effective and cheap manner.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy sediment contamination by polychlorinated biphenyls (PCBs) and similar persistent pollutants is a major concern in industrial harbors due to their well-known toxicity and biomagnification in the food cycle (Frignani et al. 2001; Carpenter 2006; Sprovieri et al. 2007; Casado-Martinez et al. 2009).
This is the case of Taranto (South Italy), whose military and industrial harbors deserve national priority for reclamation (Italian Law 426/1998) as huge amounts of marine sediments largely contaminated by PCBs (and, to a lower extent, by PAHs and heavy metals) need to be dredged and disposed off as hazardous waste (European Waste Code 170505*). This prompted the search for technical and cost-effective methods capable of declassifying wastes to non-hazardous (EWC 170506) that could reduce the economic and environmental burden of their safe disposal.
The biological treatment of PCBs is cheap but very slow. Moreover, aerobic bacteria utilizing the biphenyl degradation pathway can degrade only the low chlorinated PCB congeners (i.e., with ≤5 Cl atoms) due to steric hindrance of catabolic enzymes (Focht and Brunner 1985; Borja et al. 2005; Field and Sierra-Alvarez 2008), while some anaerobic bacteria are able to use high-chlorinated PCBs as electron acceptors and may cause their slow dechlorination to lower chlorinated congeners (Wiegel and Wu 2000; Borja et al. 2005; Field and Sierra-Alvarez 2008). Two stage processes (i.e., anaerobic-aerobic) have been studied, but the very lengthy anaerobic stage slowed down the whole treatment (Maltseva et al. 1999; Tartakovsky et al. 2001; Patureau and Trably 2006).
Physicochemical PCB degradation (by ultrasonication, hydride reduction, hydrodechlorination, dissolving metal reduction, photochemical degradation, oxidation, etc.) requiring high heat, high pressure, radiation, special equipment, strongly basic, or similar uneasy conditions did not find large scale application so far (Ido et al. 2013). Therefore, a safer and more practical method, eventually based on integrated physicochemical-biological processes, for PCB detoxification is still desired.
Mechanochemistry (MC) is a well known technique capable to generate solid structure changes at nano-scale level promoting solid-solid and solid-liquid reactions at room conditions under the action of mechanical forces developed by collisions in suitably designed and properly operated high-energy ultramilling devices (Boldyrev and Meyer 1973; Butyagin 1994; Boldyrev and Tkacova 2000; Urakaev and Boldyrev 2000; Balaz 2010; Tumanov et al. 2011; Balaz et al. 2013). These devices consist essentially of a rapidly vibrating/rotating container into which the reactants and steel balls are put. The balls collide hundreds or thousands of times per second, compressing the reactants between each ball in an instant of extreme pressure and temperature and these myriad collisions make ball mill functional, inexpensive, and environmentally friendly. Used successfully in several fields (e.g., preparation of catalysts, functional ceramics, etc.), MC has also been proposed in the last decade for environmental protection (dehalogenation of hazardous waste) with promising results, although with high energy consumption (Korolev et al. 2003; Birke et al. 2004; Tanaka et al. 2004; Napola et al. 2006; Cangialosi et al. 2007a, b; Di Leo et al. 2013; Nikolic et al. 2014). In particular, MC allowed hydrophobic xenobiotics in soils and sediments to be oxidized at room temperature and solid state, i.e., without organic solvents (Nasser and Mingelgrin 2012). Products may be fast and effectively biodegraded by suitable bacteria.
A new, two-stage, process, named Biomec, has been proposed and tested in laboratory with marine sediments artificially contaminated by PCBs and PAHs (Cagnetta et al. 2009; Cagnetta 2013) wherein the waste (added with a cheap dechlorinating reagent as CaO in the case of PCBs) undergoes a very short MC pre-treatment in slurry conditions and the ultramilled material is submitted to aerobic biodegradation with selected microorganisms. To our knowledge, MC has never been combined with biological oxidation of toxic waste before.
In preliminary tests with marine sediments contaminated by PAHs, almost complete (>97 % MC + biological) degradation of PAHs was achieved (Cagnetta et al. 2013) while the results with PCBs, although encouraging (∼50 % overall degradation in 1 week) called for a deeper study, particularly in the relationship between MC and chlorination degree (i.e., PCB biorecalcitrancy) and in related reaction mechanism as addressed by this paper.
2 Materials and methods
2.1 Sample preparation and PCB analysis
Due to bureaucratic difficulties with military authorities, the collection of a small amount of poorly contaminated sediment (≈0.06 mmolPCB kg−1) was allowed inside Taranto harbor, used in some preliminary tests; the required amount of uncontaminated samples with similar physical characteristics (see Table 1) was further dredged by continuous drilling from a nearby unrestricted area. Core samples taken at ∼1-m depth of these latter were artificially contaminated to a much larger and more representative extent (6.5 mmolPCB kg−1) using Aroclor 1260 (Supelco, USA), a mixture of the 209 stable PCB congeners, with prevailing 6 and 7 chlorinated isomers (see Table 2). Its average formula (C12H3.6Cl6.4, MW 372 g mol−1) indicates that an average number (N ave) of 6.4 chloride ions should build up into solution by the complete dechlorination of one Aroclor 1260 molecule.
According to USEPA (2001), the sediment was artificially contaminated by dissolving Aroclor 1260 in hexane and pouring the solution on it to yield ∼2500 mg kg−1 (6.5 mmol kg−1) PCBs concentration. The sample was added with hexane until it was completely, wet and after homogenization by gentle stirring for 24 h in a glass beaker, the hexane was allowed to fully evaporate. According to USEPA (1995), the analysis of PCBs in contaminated samples was carried out by ultrasonic extraction for 30 min with hexane (20 mL g−1 of sediment), cleaning-up the extract from organic interference by means of Florisil (Sigma-Aldrich Co., USA), then with sulfuric acid (ca. 1 mL per 4 mL of extract) with final analysis by gas-chromatography (GC Mod.CP-3800 with ECD, Varian Inc., Palo Alto, USA) under the following operating conditions: capillary column 30-m long and 0.25-mm thick; initial temperature 75 C for 2 min, first ramp to 170 C (20 °C min-1), second ramp to 290 °C (2.5 °C min−1), maintained for 10.25 min; gas carrier Helium.
In order to assess the influence of chlorination degree on the efficacy of MC activation, decachlorobiphenyl (DCB, by Ehrenstorfer GmbH, Augsburg, D), the all-chlorinated (hence the most bio-recalcitrant) PCB, having chemical formula C12Cl10 and MW 499 g mol−1, was used as a unique sediment contaminant in some tests. During ultramilling of the sediment spiked with DCB, this latter undergoes almost complete physicochemical breakdown, forming 14 lower-chlorinated PCB congeners. In order to discriminate quali-quantitatively these congeners (especially in cases of geometric isomerism), GC analysis requires additional expensive tools and 209 congener standards, that cannot be allowed during this investigation. With the method and equipment in use, DCB exhibited the longest retention time (RT = 12.82 min) while the lower congeners formed by its ultramilling breakdown showed shorter RT, almost proportional to their chlorination degree (Table 3). Accordingly, RT was used by first approximation to identify each congener (or mixtures of PCBs with equal or contiguous chlorination degree), while the area below the peak provided a relative quantification of that congener.
The PCB degrading bacterium B. xenovorans st. LB400 (DSMZ, Braunschweig, D), suggested by Denef et al. (2005) being able to use biphenyl as unique source of carbon and energy, was selected. It was stored on Luria Bertani (LB) culture broth, prepared from commercial lyophilized mixture, while all tests were carried out with phosphate ammonium salts (PAS) minimum medium, composed of 75.5 mL of PA stock solution (56.77 g L−1 K2HPO4, 21.94 g L−1 KH2PO4, 27.61 g L−1 NH4Cl), 10 mL of PAS 100× stock solution (19.5 g L−1 MgSO4, 5 g L−1 MnSO4·H2O, 1 g L−1 FeSO4·7H2O, and 0.3 g L−1 CaCl2·2H2O), 50 mg of yeast extract (microbiological grade), and distilled water until the final volume of 1 L (all chemicals from Fluka, Milan, I) (Bedard et al. 1986). Sterilized PAS 100× solution was added once all other components had been sterilized.
Buildup of chlorides in the aqueous phase accompanying PCB dehalogenation was analyzed through a Mod. 761 Compact IC ion chromatography (Metrohm AG, Herisau, CH) with 1 mM NaHCO3 and 3.2 mM Na2CO3 as mobile phase.
2.2 Mechanochemical equipment and process
Contaminated sediment samples were ultramilled for ≤1 min in slurry conditions (90% v/w of water) into the Mod. 15 High Energy Nutational Mill (Hicom Technol., Pinkeba, AU) in the operating conditions summarized in Table 4, using CaO as dechlorinating reagent (30 % w/w of sediment).
2.3 Biological degradation setup
The ultramilled slurry, containing 200 g of sediment (collected through four ultramilling operations) and 2.3 L of aqueous solution, was rapidly transferred into the Esedra Plus 3.5 Bioreactor (Solaris Biotechnologies, Mantova, I) with 3.5 L capacity.
After decreasing the pH into the bioreactor to 6.8 by drop addition of 96 % sulfuric acid, the required volume of PA (194 mL), PAS 100× (25 mL) solution and yeast extract (125 mg) were supplemented to yield 2.52 L PAS medium with the milling aqueous solution. Humic acids (HA) by Fluka (Milan, I) were then added (1.5 % w/w of sediment) to improve PCBs bioavailability (Fava and Piccolo 2002; Alawi et al. 2008). Operating conditions in the bioreactor were kept constant at 30 °C, 500 rpm impeller rotation, and 100 % O2 saturation. The slurry was inoculated with 2.5 % v/v of a B. xenovorans culture grown with 1 g L−1 of biphenyl (added as crystals) in PAS medium. Initial concentration of chlorides in the slurry was accounted for. During each test, 10-mL slurry aliquots were withdrawn at given intervals, added with 0.5 mL of 2 M trichloroacetic acid to stop bacterial activity, and after centrifugation, the sediment and the supernatant solution were analyzed for PCBs and chloride concentration, respectively.
3 Results and discussion
3.1 Effect of MC on physical characteristics of the sediment
Macroscopic modifications of major physical properties of the material, varying with operating conditions, are known to be induced by ultramilling. The scientists do not fully agree with this yet, as it is based on the explanation of the underlying physicochemical activation and related reaction mechanism (Balaz et al. 2013). As shown in Fig. 1, the specific surface of sediment increased almost to the same extent (up to 40 %), at a different rate with the milling energy (i.e., the centrifugal acceleration of the steel balls, expressed as a multiple of gravity acceleration, g), in the very first minutes, then it kept decreasing consistently (up to 80 %) until a much lower value was achieved steadily. This may be explained with a sample of initial comminution (yielding higher specific surface) under the powerful pressure of colliding balls, followed by particle aggregation that promotes the eventual entrapment of heavy metals and other inorganic contaminants (Montinaro et al. 2007).
In the present experimental conditions (1 min milling at 40 g), a good compromise was reached between energy cost and specific surface increase for a satisfying diffusion of the reacting molecules in/outside the solid phase.
3.2 Effect of MC on PCB biodegradation
Figure 2 shows the decrease of PCB concentration during bioremediation of unmilled sediment, complemented by its apparent Cl stoichiometry (N) inferred by Cl¯ buildup in solution. As expected, PCB biodegradation occurred very slowly and poorly, with the initial rate (5.0 × 10−2 mmol kg−1day−1 for ≈2 weeks) decreasing to 5.8 × 10-3 mmol kg-1day-1 steady value in 3 months and overall PCB degradation amounting to ≈18 % (thus requiring ≈3.5 years theoretically to achieve full sediment bioremediation). Figure 2 also shows that Cl¯ buildup in solution complements PCB decrease in the sediment, confirming that this latter decrease is mainly due to bio-redox reaction, not just to physical loss (stripping). Quite interesting, however, no Cl¯ buildup occurred in the first few days of the biotreatment. This can be explained by assuming a short lag-phase of bacterial activity, during which PCB desorption from the solid phase is mainly due to physical actions, e.g., to their solubilization in the culture broth favored by the addition of humic acids.
Mass balance shows that, after 3 months, the average stoichiometry of Cl atoms removed per molecule of PCB biodegraded (N) is ≈3.7, indicating that biodegradation concerned preferentially the lower-chlorinated PCB congeners (i.e., with ≤5 Cl atoms), as expected.
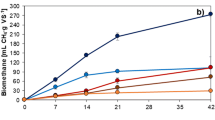
Bioremediation of ultramilled sediment in the same conditions was much more satisfying (Fig. 3). First of all, the very short (1 min) MC pretreatment per sè provided an appreciable decrease of PCB concentration in the solid phase (≈18 %, equivalent to the 3-month overall bioremediation of the unmilled sediment as in Fig. 2). Then, PCB biodegradation in the sediment took off and continued at the 5.0 × 10−2 mmol kg−1day-1 initial rate for 2 months when it slowly reached the 9.3 × 10−3 mmol kg−1day−1 steady value thereafter. In agreement with previous results (Cagnetta et al. 2009), the overall PCB biodegradation reached 66 % (17 % MC +49 % bio) in less than 3 months.
As shown in Fig. 4, a test carried out with real sediment in these operating conditions offered even better results. The overall PCB biodegradation reached 78 % (17 % MC +61 % bio) in only 3 weeks. Accounting for the lower initial PCB concentration, the biological degradation rate was obviously slower (1.4 × 10-3 mmol kg−1day−1), while the better overall performance was probably due to a lower inhibition by PCBs.
3.3 Effect of MC on bio-recalcitrance of various PCB congeners
Mass balance in Fig. 3 also shows that, after the initial bacterial lag-phase, Cl average release ranged steadily around the N = 6.4 stoichiometric value in Aroclor 1260; a similar result appears in Fig. 4 with real sediments (where the low PCB concentration made initial bacterial lag-phase negligible). This means that the biodegradation activity of B. xenovorans in ultramilled sediment addressed almost uniformly all PCB congeners, indicating that MC pre-treatment seemingly helped in making biodegradable also the higher-chlorinated congeners (i.e., with ≥5 Cl atoms), reportedly not biodegradable by aerobic bacteria, as already stated.
In order to investigate this important aspect, further tests with sediments contaminated with deca-chlorobiphenyl DCB, the full-chlorinated (N = 10) PCB congener, were carried out. Contrary to expectation, as shown in Fig. 5, the experimental results were even better than with Aroclor 1260 (N ave = 6.4). Indeed, not only the overall DCB biodegradation increased (∼71 % after 2 weeks vs. ∼66 % in 3 months achieved in similar conditions with Aroclor 1260, see Fig. 3), but the contribution of MC activation per sè was much larger (∼47 % vs. ∼17 %). If one accounts for the expected DCB most bio-recalcitrancy, this result indicates that the Biomec treatment was most effective for the high-chlorinated (most bio-recalcitrant to aerobic bacteria) PCB congeners, made biodegradable by the short MC pre-treatment.
Biodegradation of individual congeners formed during ultramilling is shown in Fig. 6 (where the same abscissa of the following Figs. 7 and 8 is used for sake of comparison). It can be seen that both the rate and the extent of biodegradation increase with the chlorination degree (i.e., with RT), with DCB being biodegraded almost totally (∼90 %) in 1 week and with all the DCB-derivatives achieving poorer performance (75 to 25 % biodegradation in 4 weeks) as chlorination degree decreases. This highlighted the effectiveness of the MC pre-treatment: the most bio-recalcitrant congeners become the most biodegradable.
This conclusion was confirmed by the results obtained in similar conditions with unmilled sediment spiked with Aroclor 1260. As shown in Fig. 7, the low-chlorinated congeners underwent appreciable biodegradation (30 to 45 % overall), while the high-chlorinated ones exhibited almost no biodegradation (+10/-15 %), as expected: this explains the poor average yield with unmilled specimen (see Fig. 2).
With the ultramilled sample (Fig. 8), on the contrary, even the high-chlorinated congeners underwent fast and effective biodegradation, to a much greater extent (40 ÷ 80 % overall), throughout the test duration: again, a proportionally better performance was achieved by the more chlorinated congeners.
3.4 Biodegradation mechanism of MC-activated PCBs
The conclusion that MC activation is most effective with the most bio-recalcitrant PCB congeners calls for a deeper insight into the possible mechanism of the Biomec process. As anticipated, in spite of the consolidated industrial exploitation of MC technology, full understanding of reaction mechanism(s) only recently started to mark significant steps forward (Tolochko et al. 2013; Friscic et al. 2013; Balaz et al. 2013). It is now suggested that the following physicochemical phenomena occur during the MC + biodegradation treatment of the Biomec process:
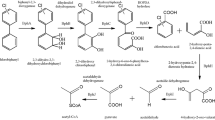
1) MC ultramilling promotes the nucleophilic substitution of Cl by OH in PCB molecules, creating polychlorinated polyhydroxylated biphenyls (PCPHBs) according to the following:
with consequent decrease of the overall PCB bio-recalcitrancy (especially for the high-chlorinated congeners, whose reaction is statistically more probable);
2) Higher bacteria selectivity for the more OH-substituted congeners permits fast biodegradation of PCPHBs.
As shown by Korolev et al. (2003), the OH¯ ions ensured at pH ≥12 by the CaO employed in ultramilling permit Cl nucleophilic substitution in PCBs thanks to the strong mechanical energy provided by the impacts between the milling bodies and the sediment particles. From a statistic point of view, such hydroxylation is favored in high-chlorinated congeners, where the probability of effective collisions with OH¯ ions is higher. Considering their MW, water solubility of hydroxylated compounds should not be enhanced substantially by the presence of OH groups in the molecule, especially for the high-chlorinated PCPHBs. This means that PCPHBs are still bound to the organic matter in the sediment after the ultramilling, and their solubility in water is enhanced by the addition of humic acids during the biological treatment. Moreover, because the GC method used permits to quantify total PCBs and not every single congener, high-chlorinated PCPHBs were very likely eluted with high RT, appearing at first glance as high chlorinated PCBs in Figs. 6, 7, and 8.
The easier biodegradation of high-chlorinated PCPHB congeners in ultramilled sediment can be explained by their different entry point during biodegradation by B. xenovorans (Fig. 9), whose pathway is the same for biphenyl and low-chlorinated PCBs (Denef et al. 2005). According to this pathway, PCB biodegradation requires an initiator enzyme (biphenyl dioxygenase, BDO) that promotes the hydroxylation of two adjacent aromatic positions, consuming NADH (Nicotinamide Adenine Dinucleotide, the well-known ubiquitarious cellular electron-acceptor co-enzyme) as well as O2 (Furukawa et al. 2004). The bi-hydroxylated moiety becomes the preferred (weakest) position for the cleavage of the aromatic ring, from where the decomposition of the remaining moiety may start to develop. By substituting the cell-stressing rate-determining BDO enzymatic hydroxylation with a much faster physicochemical reaction, the mechanical ultramilling permits to skip the first two steps of the catabolic pathway in Fig. 9 and allows for the biodegradation starting downstream of biphenyldihydrodiol dehydrogenase, where less selective enzymes can degrade PCPHBs with a bi-hydroxylated moiety (in position 2,3).
4 Conclusions
Biomec, a two-stage bioremediation process based on a very short (1 min) mechanochemical activation with high-energy ultramilling device, followed by conventional aerobic biodegradation by the purposely selected B. xenovorans aerobic bacterium, showed that PCB degradation in artificially contaminated marine sediments can be reached in a simple, fast and very effective way.
Furthermore, experimental evidence interestingly showed that the MC pretreatment makes the most chlorinated (expectedly the most bio-recalcitrant) PCB congeners most biodegradable by aerobic bacteria, according with the two-step reaction mechanism proposed. These results received preliminary confirmation with real sediments by experiments carried out at laboratory level.
Further tests are planned both at laboratory and at pilot (0.5 t/h) level using real contaminated sediments. If the reaction mechanism(s) proposed will receive due confirmation and if the pilot tests will be successful from the technical and economic points of view, new perspectives may open to full-scale application of MC technology also to the environment protection.
Accounting for today’s super high-energy industrial ultramills, capable to mechanically activate continuously up to 400 t/h of fines and to develop very high centrifugal energy, other hazardous waste (e.g., contaminated soils, industrial slag and sludge, fly ash, etc.) could be detoxified in technical, eco-friendly, and cost-effective manner.
References
Alawi AM, Khalili F, Elgani JA (2008) Interaction of PCBs with dissolved humic acid from Azraq (Jordan). Asian J Water Environ Pollut 5:45–48
Balaz P (2010) Mechanochemistry. In: Nanoscience and minerals enginering, Springer-Verlag Ed
Balaz P, Achimovicova L, Balaz M, Billik P, Cherkezova-Zheleva Z, Criado JM, Delogu F, Dutkova E, Gaffet E, Fj G, Kumar R, mitov I, Rojac T, Senna M, Streletskii A, Wieczorek-Ciurowa K (2013) Hallmarks of mechanochemistry: from nanoparticles to technology. Chem Soc Rev 42:7571–7637
Bedard DL, Unterman R, Bopp LH, Brennan MJ, Haberl ML, Johnson C (1986) Rapid assay for screening and characterizing microorganisms for the ability to degrade polychlorinated biphenyls. Appl Environ Microbiol 51:761–768
Birke V, Mattik J, Runne D (2004) Mechanochemical reductive dehalogenation of hazardous polyhalogenated contaminants. J Mat Sci 39:5111–5516
Boldyrev VV, Meyer K (1973) Festkörperchemie. beiträge aus forschung und praxis. Grundstoffindustrie Verlag, Leipzig
Boldyrev VV, Tkacova K (2000) Mechanochemistry of solids: past, present and prospects. J Mat Synth Proc 8:121–132
Borja J, Taleon DM, Auresenia J, Gallardo S (2005) PCBs and their biodegradation. Process Biochem 40:1999–2013
Butyagin PY (1994) Problems in mechanochemistry and prospects for its development. Russ Chem Rev 63:965
Cagnetta G (2013) A new process for rapid biodegradation of heavily contaminated marine sediments, PhD thesis, Technical University of Bari, Italy
Cagnetta G, Intini G, Liberti L, Notarnicola M, Spinosa L, Stellacci P (2009) Mechanochemical and biological degradation of PCBs in contaminated marine sediments. J Resid Sci Technol 6:139–144
Cagnetta G, Intini G, Liberti L, Lomovskiy OI, Boldyrev VV (2013) BIOMEC process for mechanochemical biodegradation of polycyclic aromatic hydrocarbons in marine sediments. Chem Sustain Dev 21:589–597
Cangialosi F, Intini G, Liberti L, Notarnicola M, Pastore T, Sasso S (2007a) Mechanochemical treatment of contaminated marine sediments for PAH degradation. Chem Sustain Dev 15:139–145
Cangialosi F, Intini G, Liberti L, Lupo L, Notarnicola M, Pastore T (2007b) Mechanochemical treatment of contaminated marine sediments for PCB degradation. Chem Sustain Dev 15:147–156
Carpenter DO (2006) PCBs: routes of exposure and effects on human health. Rev Environ Health 21:1–23
Casado-Martínez MC, Forja JM, DelValls TA (2009) A multivariate assessment of sediment contamination in dredged materials from Spanish ports. J Hazard Mater 163:1353–1359
Denef VJ, Patrauchan MA, Florizone C, Park J, Tsoi TV, Verstraete W, Tiedje JM, Eltis LD (2005) Growth substrate and phase-specific expression of biphenyl, benzoate and C1 metabolic pathways in Burkholderia xenovorans LB400. J Bacteriol 187:7996–8005
Di Leo P, Pizzigallo MDR, Ancona V, Di Benedetto F, Mesto E, Schingaro E, Ventruti G (2013) Mechanochemical degradation of pentachlorophenol onto birnessite. J Hazard Mater 244–245:303–310
Fava F, Piccolo A (2002) Effects of humic substances on the bioavailability and aerobic biodegradation of PCBs in a model soil. Biotech Bioeng 77:204–211
Field JA, Sierra-Alvarez R (2008) Microbial transformation and degradation of PCBs. Environ Pollut 155:1–12
Focht D, Brunner W (1985) Kinetics of biphenyl and PCB metabolism in soil. Appl Environ Microbiol 50:1058–1063
Frignani M, Bellucci LG, Carraro C, Raccanelli S (2001) PCBs in sediments of the Venice Lagoon. Chemosphere 43:567–575
Friscic T, Halasz I, Beldon PJ, Belenguer AM, Adams F, Kimber SAJ, Honkimaki V, Dinnieber RE (2013) Real-time and in situ monitoring of mechanochemical milling reactions. Nat Chem 5:66–73
Furukawa K, Suenaga H, Goto M (2004) Biphenyl dioxygenases: functional versatilities and directed evolution. J Bacteriol 186:5189–5196
Ido A, Ishihara S, Kume A, Nakanishi T, Monguchi Y, Sajiki H, Nagase H (2013) Practical method for PCB degradation using Pd/C-H2-Mg system. Chemosphere 90:57–64
ISPRA (2009) Valutazione dei risultati della caratterizzazione della Darsena Capitaneria di Porto ai fini della individuazione delle più appropriate modalità di gestione dei sedimenti. Port Authority of Taranto (in italian)
Italian Law 09/12/1998 n. 426, Gazzetta Ufficiale n. 291 (14/12/1998)
Korolev KG, Golovanova AI, Maltseva NN, Lomovskiy OI, Salenko VL, Boldyrev VV (2003) Application of mechanical activation to decomposition of toxic chlorinated organic compounds. Chem Sustain Dev 11:489–496
Maltseva OV, Tsoi TV, Quensen JF III, Fukuda M, Tiedje JM (1999) Degradation of anaerobic reductive dechlorination products of Aroclor 1242 by four aerobic bacteria. Biodegradation 10:363–371
Montinaro S, Concas A, Pisu M, Cao G (2007) Remediation of heavy metals contaminated soils by ball milling. Chemosphere 64:631–639
Napola A, Pizzigallo MDR, Di Leo P, Spagnuolo M, Ruggiero P (2006) Mechanochemical approach to remove phenanthrene from a contaminated soil. Chemosphere 65:1583–1590
Nasser A, Mingelgrin U (2012) Mechanochemistry: a review of surface reactions and environmental applications. Appl Clay Sci 67–68:141–150
Nikolic V, Komljenovic M, Bascarevic Z, Petrovic R (2014) Lead immobilization by geopolymers based on mechanically activated fly ash. Ceram Int 40:8479–8488
Patureau D, Trably E (2006) Impact of anaerobic and aerobic processes on PolyChloroBiphenyl removal in contaminated sewage sludge. Biodegradation 17:9–17
Sprovieri M, Feo ML, Prevedello L, Salvagio Manta D, Sammartino S, Tamburino S, Marsella E (2007) Heavy metals, PAH and PCB in surface sediments of Naples harbor (S Italy). Chemosphere 67:998–1009
Tanaka Y, Zhang Q, Saito F (2004) Mechanochemical dechlorination of chlorinated compounds. J Mater Sci 39:5497–5501
Tartakovsky B, Michotte A, Cadieux JCA, Lau PCK, Hawari J, Guiot SR (2001) Degradation of aroclor 1242 in a single-stage coupled anaerobic/aerobic bioreactor. Water Res 35:4323–4330
Tolochko BP, Sharafutdinov MR, Lyakhov NZ, Ten KA, Pruuel ER (2013) Mechanochemical process investigation using synchrotron radiation: model and real, IV Intern Conf on Fundamental Bases of Mechanochemical Technologies, Novosibirsk, Russia, 25-28 June
Tumanov IA, Achkasov AF, Boldyreva EV, Boldyrev VV (2011) Following the products of mechanochemical synthesis step by step. Cryst Eng Comm 13:2213–2220
Urakaev FK, Boldyrev VV (2000) Mechanism and kinetics of mechanochemical processes in comminuting devices 1. Theory Powder Technol 107:93–107
USEPA (1995) SW-846, tests methods for evaluating solid waste, physical/chemical methods, vol Update IV, 3rd edn. US GPO, Washington DC
USEPA (2001) Methods for collection, storage, manipulation of sediments for chemical and toxicological analyses: technical manual. National Service Center for Environmental Publications, Cincinnati
Wiegel J, Wu Q (2000) Microbial reductive dehalogenation of polychlorinated biphenyls. FEMS Microbiol Ecol 32:1–15
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Gijs D. Breedveld
Rights and permissions
About this article
Cite this article
Cagnetta, G., Intini, G., Liberti, L. et al. The Biomec process for mechanochemically assisted biodegradation of PCBs in marine sediments. J Soils Sediments 15, 240–248 (2015). https://doi.org/10.1007/s11368-014-1009-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-014-1009-y





 PCBs,
PCBs,  chlorides)
chlorides)
 PCBs,
PCBs,  chlorides)
chlorides)
 PCBs,
PCBs,  chlorides)
chlorides)
 PCBs,
PCBs, chlorides)
chlorides)


