Abstract
Mining industry generates large volumes of waste known as mine tailings, which contain heavy metals (HMs) that generate a risk to environmental health. Thus, remediation of HM pollution requires attention. In this study, HM bioaccumulation, genotoxic damage, and morphological and physiological changes in the tree species Prosopis laevigata were evaluated in order to assess its potential for remediation of mine tailings. P. laevigata plants were established in two treatments (reference substrate and tailing substrate) under greenhouse conditions. Every 2 months, six individuals were selected per treatment for 1 year. From each individual, macromorphological (height, stem diameter, and number of leaves), micromorphological (stomatal coverage and stomatal index), and physiological parameters (chlorophyll content) were evaluated, as well as the concentration of Pb, Cu, Cd, Cr, Fe, and Zn in root and foliar tissue. Genetic damage was assessed by the comet assay in foliar tissue. These parameters were evaluated in adult individuals established in mine tailings. Roots bioaccumulated significantly more HM compared to foliar tissue. However, the bioaccumulation pattern in both tissues was Fe > Pb > Zn > Cu. The plants in tailing substrate reduced significantly the morphological and physiological characters throughout the experiment. Only the bioaccumulation of Pb affected significantly the levels of genetic damage and the number of leaves, while Zn reduced plant height. The percentage of plants that have translocation factor values greater than 1 are Cu (92.9) > Fe (85.7) > Pb (75.0) > Zn (64.3). P. laevigata has potential to phytoremediate environments contaminated with metals, due to its dominance and establishment in abandoned mine tailings, and its ability to bioaccumulate HM unaffecting plant development, as well as their high levels of HM translocation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining is a very important traditional and economic activity in Mexico, contributing 5% to the nominal gross domestic product (INEGI 2016). Currently, Mexico stands out as the leading silver producer worldwide. However, when this activity is carried out, thousands of tons of waste are generated, known as mine tailings, that contain high concentrations of heavy metals (HMs) (Salas-Luévano et al. 2017).
Mine wastes containing HMs are an important source of environmental pollution (Ibarra-García et al. 2017). In addition, due to their characteristics and properties, they are considered potentially toxic elements for organisms exposed to them (Salas-Luévano et al. 2017), causing negative effects in all levels of biological organization (Mussali-Galante et al. 2013).
When HMs are bioavailable in the soil, they can be absorbed by plant roots and be bioaccumulated in different plant tissues (Talavera et al. 2005; Dinu et al. 2020; Mei et al. 2020). Subsequently, when these plant tissues are consumed by different herbivores, HM enters into the trophic networks and the quantities of HM within organisms increase. Later, these metals can be found in higher concentrations than those registered in the environment (Mussali-Galante et al. 2013), affecting human health negatively (DalCorso 2012; Tovar-Sánchez et al. 2016; Tovar-Sánchez et al. 2018).
It has been documented for plants that the bioaccumulation of HM promotes cytotoxic and genotoxic damage, which generates cellular, physiological, and morphological alterations (Nagajyoti et al. 2010). These effects have been detected through the use of biomarkers that are tools for measuring the effects of HM exposure in exposed organisms (Gold-Bouchot and Zapata-Pérez 2004; Ayeni et al. 2010).
Some of the effects that have already been reported using biomarkers in plants are the following: the generation of oxidative stress by the production of reactive oxygen species (Ercal et al. 2001), also for the double and single breaking strand of DNA, which can generate genetic damage (Sharma and Agrawal 2005; DalCorso 2012, Tovar-Sánchez et al. 2018). For example, it is common to employ genotoxic techniques such as alkaline single-cell electrophoresis or comet assay to evidence the genetic damage as a biomarker of early effects due to HM exposure. This is a widely accepted technique due to its sensitivity, simplicity, and speed to analyze the breaking of single strands of DNA in individual cells (Tovar-Sánchez et al. 2018). Similarly, the reduction of macromorphological (plant height, stem diameter, and the number of leaves (Kabata-Pendias 2011; Tovar-Sánchez et al. 2018)) and micromorphological characters (stomatal coverage and stomatal index (Tovar-Sánchez et al. 2018; Santoyo-Martínez et al. 2020)) and alterations in chloroplasts with implications in the photosynthetic process (Arena et al. 2017; Gonçalves Jr. et al. 2020) have been useful biomarkers for showing the adverse effects of HM exposition on the development, growth, and physiology of plants exposed (Gonçalves Jr. et al. 2020; Santoyo-Martínez et al. 2020). For example, it has been documented that Zea mays plants growing in mine tailings in Mexico bioaccumulate HM, which promotes changes in macro- and micromorphological characters and leaf structure form, causing loss of plant biomass and alterations in coloration pattern, also DNA damage (Tovar-Sánchez et al. 2018).
In nature, there are plant species known as hyperaccumulators, which are distinguished by presenting the following attributes: (a) high tolerance to metalliferous soils without presenting important effects on their viability, growth, development, and reproduction; (b) ability to accumulate large amounts of HM in aerial organs; (c) fast HM translocation from roots to stems; (d) high detoxification capacity (Rascio and Navari-Izzo 2011; Shiqi et al. 2018).
Some plant species that have been reported as hyperaccumulators belong to the Brassicaceae, Asteraceae, Fabaceae families, among others (De la Rosa et al. 2008; Buendía-González et al. 2010; Navarrete Gutierrez et al. 2018). This hyperaccumulation ability and HM tolerance by plants is an attribute that can be used for bioremediation techniques (Mousavi Kouhi and Moudi 2020). Within these techniques, phytoremediation uses plant species for the elimination, reduction, and retention of soil pollutants, favoring the reduction of their toxicity (Sharma and Pandey 2014; Mousavi Kouhi and Moudi 2020). The phytoremediation process has different stages: one is phytoextraction, which consists of the translocation of pollutants contained in the soil matrix, from roots to the plant’s aerial parts where they are accumulated. Subsequently, aerial parts can be harvested and in this way the metals can be removed from the soil (Suman et al. 2018; Mousavi Kouhi and Moudi 2020).
Some advantages of using phytoremediation as a remediation strategy for soils polluted with HM are that it can be carried out in situ, so it is not necessary to transport the polluted substrate to a specialized facility to be decontaminated. Phytoremediation also removes organic and inorganic pollutants from soil. It improves physicochemical soil properties when vegetation cover is generated, or carried out with conventional agronomic activities. It can also be carried out in soil, water, sediments, and air obtaining recycled water, metals, and biomass. It is also considered a low-cost technique and environmentally friendly (Delgadillo-López et al. 2011; Capozzi et al. 2020; Chaudhry et al. 2020).
In order to carry out phytoremediation processes, it is necessary to use plant species that have the appropriate characteristics to survive in metalliferous soils and with the metabolic mechanisms that are able to deal with the exposure to HM, that is, a species with attributes of hyperaccumulation (Suman et al. 2018).
To determine if a species has a phytoremediation potential, it is necessary to evaluate how plants respond to HM when exposed to them. This can be implemented with experiments under greenhouse conditions and in natural systems. The first studies have the advantage that the environmental variation can be minimized, and the HM effects on organisms exposed to different exposure times can be measured more accurately (De la Cruz-Landero et al. 2010). On the other hand, studies in natural systems are very relevant because they permit the evaluation of HM on individuals affected by mine tailings in a more real way (Murillo-Herrera 2015); also, it allows knowing the long-term effects of exposure to HM on the parameters evaluated. Therefore, research that combines both study approaches offer the opportunity to obtain results based on reality as well as the effects of exposure time to metals on plant species.
Prosopis laevigata (Humb. & Bonpl. ex Willd.) M.C.Johnst. (Fabaceae) is a tree species that develops naturally in mine tailings and has been reported to bioaccumulate metals such as Al, As, Cu, Cd, Ni, Pb, V, Mg, Zn, Cr, and Cd in different tissues (Alcalá-Jáuregui et al. 2018; Ibarra-García et al. 2017; Buendía-González et al. 2019; Ramírez et al. 2019). Also, genetic damage has been documented in P. laevigata populations exposed to MP in field (Murillo-Herrera 2015). Despite this, the HM effects on macro- and micromorphological and physiological characters (chlorophyll concentration), as well as bioaccumulation in different plant tissues (root and leaf) to different exposure times, are unknown.
In the present study, experimental designs were carried out in situ and ex situ, in natural conditions (mine tailings) and under greenhouse conditions, with the objective of obtaining results more related to reality. Determining the HM bioaccumulation capacity of P. laevigata at different exposure times (short and long term) in the presence of a mixture of metals and evaluating the effects of such exposure on physiological, morphological, and genotoxic markers are poorly explored, so information generated in this study can be useful to determine the phytoremediation potential of this species in soils contaminated with mine tailings.
The following questions were asked: (1) What is the effect of the exposure time to mining waste on the kind and concentration of HM bioaccumulated in plant tissues of P. laevigata? (2) What effect does HM bioaccumulation have on morphological, physiological, and genotoxic parameters in individuals of P. laevigata? (3) Is the exposure time to HM a variable that modifies the levels of genetic damage? (4) What is the relative contribution that each HM has on the morphological, physiological, and genotoxic parameters in individuals of P. laevigata?
Material and methods
Study area
Exposed site
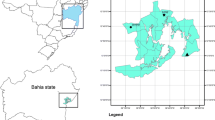
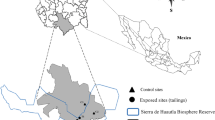
This study was carried out in Huautla, which is located in Tlalquitenango, in the state of Morelos, Mexico. It is part of the Sierra de Huautla Biosphere Reserve (REBIOSH) (Dorado et al. 2005). This town was an important mining district. However, after a long extraction process, the mines were abandoned leaving large amounts of waste or mine tailings, which are now in the open air (Velasco et al. 2004; SEMARNAT 2005; Mussali-Galante et al. 2013).
At present, there are three mine tailings that did not receive adequate treatment, with the risk of leaching or presenting runoff to other areas during the rainy season, which potentially pollutes the environment and the surrounding biota (SEMARNAT 2005).
There are two better conserved mine tailings; one is located in parallels 18° 26′ 36.37″ N–99° 01′ 26.71″ W and the other one is 18° 26′ 22.62″ N–99° 01′ 51.71″ W (Fig. 1); they are separated 500 and 100 m respectively from Huautla (Velasco et al. 2004). They have a similar chemical composition: pH from 7.85 to 8.37, electrical conductivity from 0.2 to 0.4 dS/m, percentage of organic matter from 0.52 to 0.84%, particle size < 45 μm. In addition, previous studies have shown the following pattern in terms of HM concentration: Fe > Zn > Pb > Mn > Cu > Cd (Solís-Miranda 2016); therefore, this area is contaminated with a mixture of metals.
Control site
The control site is in Quilamula, Morelos, a town located to the south-west of Tlalquitenango, Morelos, in parallel 18° 30′ 52″ N and 98° 59′ 59″ W (Fig. 1). This site was chosen because there are no records of contamination by mining activity. In addition, the geographical, climatic, and edaphic characteristics of the area are similar to the exposed sites.
Study species
Prosopis laevigata is a tree that belongs to the Fabaceae family and is commonly known as mezquite. It can reach up to 13 m high and has a diameter of 80 cm; it sheds its leaves in winter, and it blooms from February to May and fruits from June to July. It has a wide geographical distribution in Mexico (Rodríguez-Sauceda et al. 2014). It also grows naturally and abundantly in the mine tailings of Huautla, Morelos.
Seed sampling and germination of P. laevigata
Seeds were sampled in the two most preserved mine tailings of Huautla, Morelos. Fruits of established trees in tailings that presented complete pods were randomly selected, with no evidence of the presence of fungi or parasites. After that, seeds were removed from pods and confirmed that they were not parasitized. Finally, they underwent a mechanical scarification process to obtain more efficient germination. For germination, 100 seeds were placed in five Petri dishes (20 seeds per box) on cotton wool moistened with distilled water. Once seeds were germinated, they were placed in trays with peat moss substrate until plants reached a size between 4 and 5 cm. Later, 80 seedlings were transplanted into individual nursery polyethylene bags with treatment substrates (40 plants in tailing substrate and 40 in reference substrate). As control substrate, soil from Quilamula was used; it was filtered with a stainless steel sieve number 35 (Fiicsa), with a 0.5-mm mesh, in order to obtain a particle size similar to that of the mine tailing. As exposed substrate, the mixture of residues from tailings 1 and 2 was used. All the plants were kept under greenhouse conditions; they were watered twice a day, three times a week, and the temperature ranged from 32 to 35 °C. To evaluate HM bioaccumulation in plants (roots and leaves), the genetic damage, and the macro- and micromorphological characters of interest, the plants obtained were measured every 2 months until a year old. On each occasion, six individuals of P. laevigata growing in tailing substrate and six in the reference substrate were randomly taken for collecting data. Once all data were collected, statistical analysis was performed to evaluate each parameter.
Tailing substrate and reference substrate sampling
To carry out the experiment under greenhouse conditions, tailing substrate and reference substrate were collected through a superficial, simple, and random sampling in the two most preserved tailings located in Huautla, Morelos. After that, the material collected from both tailings was homogenized with a shovel, removing stones and root debris to obtain the substrate that was used to fill bags with a capacity of 4 L to transplant the P. laevigata seedlings exposed to HM. Reference substrate was sampled in Quilamula, where there are no reports of HM exposure from mining activity. This substrate was sieved to 3 mm to obtain a similar texture to mining substrate and it was used to transplant the reference seedlings of P. laevigata.
Selection of adult trees of P. laevigata established in mine tailings and control site
Six adult trees of P. laevigata established in the mine tailings were considered individuals exposed to HM. Also, six other trees growing in the control site (Quilamula) were considered the reference individuals. In both cases, trees with approximately the same height and diameter at chest height were selected randomly (individuals’ age is 25 years old, pers. obs.). Leaf samples were taken to measure chlorophyll content and bioaccumulation of heavy metals and to perform genotoxicity test (comet assay). It is worth mentioning that measurements made on naturally established trees in mine tailings were done to complement the data obtained in greenhouse conditions, that is, how P. laevigata seedlings exposed to HM develop over time from early age to adulthood.
Heavy metals’ concentration in plant tissue
Samples of root and leaf plant tissue were taken from six individual P. laevigata growing under greenhouse conditions in both treatments (tailing substrate and reference substrate). For individual plants that grow naturally in the mine tailings, only the foliar tissue of six plants was measured. The root tissue was washed with distilled water to remove the residue from the substrates. All the tissues were taken to a drying oven at 60 °C until they reached a constant weight. Each plant structure (0.25 g) was placed and pulverized in a container previously washed with HNO3. The samples were subjected to acid digestion using 10 ml of concentrated HNO3 (70%).
The samples were subjected to acid digestion using an accelerated reaction system microwave (CEM® MARS-5), using 10 mL of 70% HNO3 in closed Teflon pumps. Samples were dissolved and filtered in distilled water; this solution was dissolved at a final volume of 50 mL until analysis. A sample without tissue was processed simultaneously and was used as a control. The metals were then analyzed by atomic absorption spectrophotometry, a technique that determines the concentration of a metallic element in a sample, calibrating the spectrophotometer with standard solutions containing known concentrations of each analyzed element. The detection limits for all the elements analyzed in this work are shown in Table 1. For each measurement, the average value of three replicates was reported. All concentration values are reported in milligrams/kilogram in dry weight. For this study, the concentrations of three nonessential metals (Pb, Cd, and Cr) and three essentials (Fe, Zn, Cu) in foliar and root tissue of P. laevigata were analyzed.
Genetic damage in leaf tissue
The determination of genetic damage was performed in six individuals randomly chosen for each exposure time (2, 4, and 6 months) in both the tailing and reference substrates. We used the alkaline unicellular electrophoresis or comet assay technique, since this technique has a high degree of sensitivity, simplicity, and speed to analyze the breaking of single strands of DNA in individual cells (Rojas et al. 1999). Foliar tissue was used, which was rinsed with distilled water, dried and immersed in a glass petri dish with 20 mL phosphate-buffered saline (1 × PBS) for the isolation of the nuclei, which was performed by cutting the leaves immersed in PBS with a sharp razor, until the PBS turned greenish. Subsequently, 50 μL of cell suspension was taken and incorporated into an Eppendorf tube with 50 μL of low melting point agarose (1% LMPA). Then, 80 μL of the plant nucleus suspension was placed in a slide covered with a preformed monolayer of normal fusion agarose (NMA 1.0% Gibco). It was then covered with a coverslip and placed to cool on ice for 5 min. The coverslip was removed from the foil and a final layer of LMPA (0.5%) was placed at 4 °C for 5 min (Guo et al. 2020).
The gels were placed in a cold lysis solution (2.5 M NaCl, 100 mM EDTA, 10 mM Trisma-base pH 10) at 45 mL with 1% Triton-X and 10% dimethyl sulfoxide (DMSO) in Kopling vessels at 4 °C for 1 h. Subsequently, gels were placed in an electrophoresis chamber and covered with a cold alkaline buffer (NaOH (300 mM) + 1 mM EDTA) at pH 13.0 for 20 min for a DNA unwinding process. The electrophoresis was carried out at 300 mA and 25 V for 20 min under dark conditions.
Finally, the gels were washed three times with neutralizing buffer Tris (0.4 M pH = 7.5) for 5 min (Guo et al. 2020) and fixed with cold absolute ethanol for 10 min. For later reading of slides with the isolated nuclei, a staining with fluorochrome was made allowing the observation of individual cells (Mussali-Galante et al. 2005). The reading was done with the Comet IV software integrated in a fluorescence microscope with excitation filters from 515 to 560 nm, and a 590-nm filter barrier. Tree slides were made per individual, and 100 cells per sample were evaluated. For the naturally grown adult trees in the mine tailings, six individuals were randomly chosen for treatment (exposed to HM and control) and similarly, tree slides were made per individual, and 100 cells per sample were evaluated (Tice et al. 2000). In each nucleus, the length of the comet tail (DNA migration) was evaluated.
Micro- and macromorphometric procedure
Morphological characters were measured in six plants taken randomly from the greenhouse. These individuals were under two treatments (tailing substrate and reference substrate) and their data were registered every 2 months throughout 1 year (six data collection). The macromorphological characters evaluated were the height of the plant (cm), the diameter of the stem (cm), and the number of leaves. The micromorphological characters were stoma coverage (mm2) and the stomatal index. This was done through the replication technique with cyanoacrylate glue on three leaves belonging to three individuals per treatment (tailing substrate and reference substrate) of P. laevigata.
The stoma coverage
Three leaves were selected from each individual. We measured stoma length (μ), and stoma width (μ) to obtain stoma coverage (mm2). This was done by taking out the coverage of six stomata per individual. Stomatic coverage was calculated using this formula: [(Q1+ Q2)/4]2 × π, where Q1 is the smaller diameter and Q2 is the larger diameter.
The stomatic index
Stomas and epidermal cells were counted on the fields that were observed in order to calculate the stomatal index. The number of stoma fields was quantified taking into account only those stomata in which the two guard cells were observed complete and the number of epidermal cells was at least 60% of the field of vision (Paniagua-Ibáñez et al. 2015). With the data obtained, Salisbury’s formula (1968) was applied (Salisbury 1968).
where IE is the stomatic index, N.E. is the number of stomatic cells (guard cells) per unit of leaf, and N.C.E. is the number of ordinary epidermal cells per unit of leaf.
Determination of chlorophyll content
The chlorophyll content (mg/m2) was measured in six individuals (three leaves per individual) of P. laevigata for each treatment (tailing substrate and reference substrate) every 2 months for a year, with a chlorophyll-measuring device (Minota SPAD 502). Measurements were also made on six trees exposed to heavy metals, established in Huautla mine tailings, and six trees established in the town of Quilamula, as trees growing in control soil. The measurements are taken by simply inserting the foliar tissue for measuring into the head of the SPAD device and pressing to close it. These measurements are taken in living tissues, without causing damage, which allows the same leaf to be measured several times as it continues growing and developing.
Heavy metal enrichment and translocation factor
Two indices were used to evaluate the capacity of P. leavigata individuals to phytoextract HM: the bioconcentration factor (BCF) and the translocation factor (FT). The first determines the efficiency of the plant in the accumulation of substrate metals in its tissue (Yoon et al. 2006), and the second measures the efficiency of the plant in the transportation of metals from the root to the aerial parts (Yoon et al. 2006).
These indices are calculated as follows:
where Cfoliar is the metal concentration in the leaf tissue, Ctailing is the bioavailable metal concentration in the tailing, and Croot is the metal concentration in the root tissue. According to Yoon et al. (2006) and Covarrubias and Cabriales (2017), FT values > 1 indicate that the species is considered an accumulator of the analyzed metal.
Statistical analysis
All statistical analyses were performed with STATISTICA software version 8.0 (StatSoft 2000). We use the Shapiro-Wilk “W” test which is used to probe normality (Zar 2010). We performed a two-factor analysis of variance (Model I fixed effects, Zar 2010) to assess the effect of exposure time (T), treatment (t) (reference substrate and tailing substrate), and T × t interaction on bioaccumulation of root and leaf metals in P. laevigata individuals. Subsequently, a post hoc test (Tukey, P < 0.05) was performed to establish significant differences between pairs of averages (Zar 2010).
Also, two-factor analysis of variance was made to determine the effect of exposure time (T), treatment (t) (control and exposure), and interaction time × treatment on levels of genetic damage in leaves and roots of P. laevigata individuals. Subsequently, a Tukey test (P < 0.05) was carried out to determine significant differences between pairs of averages of the levels of genetic damage between exposure times (Zar 2010). In addition, a multiple regression analysis was used to assess the influence of lead, iron, zinc, and copper bioaccumulation on leaf tissue (Pb, Fe, Zn, Cu) on genetic damage levels (single-chain break) in P. laevigata.
To evaluate the effect of exposure time (T), treatment (t) (control and exposure), and interaction T × t on the variation in three macromorphological parameters (height, basal diameter, number of leaves), two micromorphological (stomatic index, stomatic coverage), and a physiological parameter (chlorophyll concentration) of P. laevigata individuals, a two-way analysis of variance was carried out. Subsequently, a Tukey test (P < 0.05) was performed to determine significant differences between pairs of the average values of the character evaluated over time, in both treatments (Zar 2010). For naturally grown adult trees in mine tailings, Student’s t tests were performed to evaluate the effect of the treatment (tailing substrate and reference substrate) on their chlorophyll content.
A multiple regression analysis was used to assess the influence of heavy metal bioaccumulation (Pb, Fe, Zn, Cu) on the variation of parameters in P. laevigata: three macromorphological characters (height, basal diameter, number of leaves), two micromorphological (stomatic index, stomatic coverage), and one physiological (chlorophyll concentration) (Zar 2010). Subsequently, a Tukey test (P < 0.05) was performed to determine significant differences between pairs of the average values of the character evaluated through the time of exposure to heavy metals (Zar 2010).
Results
Heavy metals’ concentration in the roots and leaves of Prosopis laevigata
Root
The presence of Fe, Pb, Zn, and Cu was detected in P. laevigata root plants growing under greenhouse conditions, while Cr and Cd were not detected (Table 1). The bioaccumulation pattern of metals was as follows: Fe > Pb > Zn > Cu. The two-way analysis of variance showed a significant effect of time (t), treatment (T), and interaction (t × T) on the bioaccumulation of four metals detected in P. laevigata root plants. The only exception was Fe; its concentration was not affected by the treatment or the interaction.
The bioaccumulation of the four metals detected in the plant roots growing in reference substrate remained constant over time. Likewise, Fe, Zn, and Cu had a higher concentration in plants growing in the reference substrate compared to the tailing substrate plants. In contrast, the concentration of metals in the roots of plants growing in tailing substrate showed an increase in Pb concentration, but a decrease in Fe, Zn, and Cu concentrations over time (Table 1). In particular, it is observed that Pb was the only metal that had the highest concentrations in plants growing in tailing substrate compared to plants growing in the reference substrate. Finally, it was observed that Fe is the metal that accumulates in greater concentration at the root in both treatments.
Leaf
The presence of Fe, Pb, Zn, and Cu was detected in P. laevigata leaves, growing under greenhouse conditions, as well as in adult individuals growing naturally in the mine tailings. In contrast, Cr and Cd were not detected (Table 1). The pattern of metal bioaccumulation in leaves was as follows: Fe > Pb > Zn > Cu. The two-way analysis of variance showed a significant effect of time (t), treatment (T), and interaction (t × T) on the levels of bioaccumulation of four metals (Pb, Fe, Zn, and Cu) in P. laevigata leaves. The exceptions were Pb and Fe that were not affected by time and the interaction.
The bioaccumulation of four metals detected in the foliar tissue of plants growing in reference substrate remained constant over time. However, Fe, Zn, and Cu had higher concentrations in the foliar tissue of plants growing in reference substrate than those in the tailing substrate. On the other hand, in plants growing in tailing substrate, Pb and Fe concentrations in leaf remain constant over time while the concentration of Zn and Cu increases over time (Table 1). In particular, it was observed that Pb was the only metal that had the highest concentration in plants growing in the tailing substrate while Fe, Zn, and Cu had higher concentrations in the foliar tissue of plants growing in reference substrate. Finally, the results show that Pb, Zn, and Cu bioaccumulate in greater concentration in foliar tissue than roots in both treatments (Table 1).
Genetic damage in Prosopis laevigata individuals growing in reference substrate and tailing substrate
Results show that there is a significant effect of time (t), treatment (T), and interaction (t × T) on genetic damage in individuals of P. laevigata (Table 2). In general, a statistically significant increase in genetic damage was observed over time in plants growing in both treatments (reference substrate and tailing substrate). However, the damage was significantly greater in plants growing in the tailing substrate.
Furthermore, results show that Pb is the only metal that presented a positive and significant relationship with the levels of genetic damage (single chain break) in leaf tissue of individuals of P. laevigata, explaining 19% of genetic damage found in individuals in both treatments (Table 3).
Morphological and physiological changes in Prosopis laevigata individuals growing on tailing substrate and reference substrate through time
In general, the results show that for all macro- and micromorphological characters, as well as the physiological (chlorophyll concentration) of P. laevigata plants growing for 12 months under greenhouse conditions, both treatments (tailing substrate and reference substrate) showed a significant effect on time (t), treatment (T), and interaction (t × T). The only exceptions were (1) the number of leaves where there was no effect of treatment and interaction, and (2) stomatic coverage where there was no effect of time, treatment, and interaction.
Macromorphological characters
In terms of plant size, it was found that in the reference substrate, there was an increase in plant height over time, while in the tailing-subtract differences in size were not statistically significant (Table 4). The same pattern was observed for stem basal diameter. Relating to number of leaves, it was observed that there was no significant effect over time in P. laevigata plants growing in the reference substrate. However, for individuals growing in tailing substrate, there was a significant reduction in this character (Table 4).
A multiple regression analysis was made to determine which metals are generating the greatest variation in the macromorphological parameters evaluated, and it was found that in general, HM affects only two structures of the plant: Zn affects the height (10.7%) and Pb affects the number of leaves (8.1%).
Micromorphological characters
In particular, an increase in the stomatic index was observed over time in plants growing in the reference substrate, while in those growing in tailing substrate there was a reduction. In the case of stomatic coverage, no significant differences were detected over time and between treatments (Table 4).
Physiological parameters
In general, an increase in chlorophyll concentration over time was observed in P. laevigata plants growing in both treatments (reference substrate and tailing substrate) (Table 4). However, over time periods of 6, 10, and 12 months, there was a significant reduction in chlorophyll content in plants growing in the tailing substrate compared to those that grew in the reference substrate.
Regarding the chlorophyll content of adult trees (average values (± SD) (reference substrate = 29.833 ± 7.019; tailing substrate = 28.725 ± 7.0198), Student’s t tests indicated that there is no effect of treatment on chlorophyll levels (t = − 0.5464, P > 0.05).
Heavy metal enrichment in roots and leaves and translocation factor in Prosopis laevigata individuals growing in substrate exposed to metals
Roots and leaves of P. laevigata plants growing in tailing substrate present enrichment or bioconcentration factor (BCF) of Zn was recorded. In contrast, no enrichment of Pb, Fe, and Cu (Table 5) was detected in any of the structures analyzed. On the other hand, the pattern of the average translocation factor (TF) values was as follows: Zn > Cu > Fe > Pb. Furthermore, the pattern obtained according to the percentage of plants that have TF values greater than 1 is the following (in parentheses, the maximum and minimum TF values are presented): Cu 92.9% (0.75–7.44) > Fe 85.7% (0.62–2.69) > Pb 75.0% (0.71–1.98) > 64.3% Zn (0.29–8.10).
Heavy metals’ effect over macro- and micromorphological and physiological parameters of Prosopis laevigata
In general, four metals were detected in P. laevigata tissues; only two of them, Zn and Pb, affect two plant structures. In particular, it was observed that Zn explained 10.7% of the variation detected in the plant height character. On the other hand, the Pb explained 8.1% of the variation in the number of leaves (Table 6).
Discussion
Studies are scarce on wild plant species that focus on the transport or accumulation of metals in different structures that use an approach combining an experimental design—both under greenhouse conditions and in natural conditions (mine tailings)—with the purpose of evaluating their potential to phytoremediate polluted environments with HM. According to our knowledge, this is the first study that simultaneously addresses the effect of exposure to metals over time on a combination of biomarkers (genetic, morphological, and physiological) and also to evaluate the potential of P. laevigata as a useful species for the purpose of phytoremediation, under this combined approach: in situ–ex situ.
Bioaccumulation of heavy metals in Prosopis laevigata
In this work, it was found that P. laevigata individuals that grew under greenhouse conditions in two treatments (reference substrate and tailing substrate) and adult individuals growing naturally in the mine tailings bioaccumulate Pb, Zn, Cu, and Fe in root and leaf tissue. Also, Fe, Zn, and Cu had a higher concentration in plants growing in the reference substrate compared to the tailing substrate plants. In general, the metal that most accumulates in P. laevigata plants in both structures and treatments was Fe, although its concentration remained constant over time. According to these results, a recent study shows that P. laevigata is a hyperaccumulator of Fe (Ramírez et al. 2019). In the literature, it has been reported that other plant species that bioaccumulate Fe are Sanvitalia procumbens (Lam.) (Asteraceae) (Rosas-Ramírez 2018), Lactuca sativa (L.) (Asteraceae), Brassica oleracea (L.) (Brassicaceae), Daucus carota (L.) (Apiaceae) and Raphanus sativus (L.) (Brassicaceae) (Casana and Beltrán 2017), and Cynara cardunculus (L.) var. altilis DC. (Asteraceae) (Capozzi et al. 2020). A possible explanation for the high concentrations of this metal in plants is that Fe is an essential metal and a structural component of porphyrin molecules, such as cytochromes (Rout and Sahoo 2015). In addition, this metal is involved in oxidation-reduction reactions in respiration and photosynthesis, playing an important role in enzyme systems related to chlorophyll synthesis (Kaya et al. 2020). Specifically, in this work, it was found that the highest concentration of this metal is in the root. A similar patron was detected in P. laevigata trees established near a river contaminated with HM (Ramírez et al. 2019) and in soybean plants (Glycine max (L.) Merr. (Fabaceae)) where Fe was detected in the root elongation areas during the maturation process, as well as in the young lateral roots (Ambler et al. 1971). This metal has also been detected in the root tissue of R. sativus (Brassicaceae) (Casana and Beltrán 2017) and in the macrophyte aquatic plant Phragmites australis (Cav.) Trin. (Poaceae) (Batty and Younger 2003). It has been suggested that the concentration of root metals is a mechanism of protection against the toxicity of these elements, preventing significant amounts from being translocated to the aerial parts (Batty and Younger 2003).
On the other hand, Pb remained constant in root plants of P. laevigata growing in both treatments. However, in leaf, it can be seen that the concentration increases over time in both treatments, which suggests that this metal is being translocated to aerial parts of plants. Previously, the presence of this metal in foliar tissue of P. leavigata individuals was recently reported (Alcalá-Jáuregui et al. 2018). In the literature, there are reports of other plant species that accumulate Pb in leaf tissue. For example, Brickellia veronicifolia (Kunth) Grey. (Asteraceae) accumulates Cd, Cu, Ni, and Pb (Hernández-Acosta et al. 2009) in its leaf tissue. Similarly, Buddleja scordioides Kunth (Scrophulariaceae) has high concentrations of Pb in leaf tissue also in Mimosa aculeaticarpa (Ortega) (Fabaceae) and Acacia schaffneri (S. Watson) F.J. Herm. (Fabaceae) (Salas-Luévano et al. 2009). More recently, the presence of Pb in Plantago lanceolate (L.) (Plantaginaceae) (Salas-Luévano et al. 2017) and Schinus molle (L.) (Anacardiaceae) (Alcalá-Jáuregui et al. 2018) in leaf tissue also has been reported. This could be because Pb inhibits the transport of essential metals such as Cu, Fe, and Zn (Patra et al. 2004), increasing its concentration over time and thus its translocation to the aerial part of the plant. Even a rapid translocation of the root to detoxify and sequester heavy metals in the leaves is a possible defense mechanism against herbivores (Rascio and Navari-Izzo 2011).
On the other hand, Zn bioaccumulates mostly in P. laevigata roots compared to leaves. These results are similar with other results found in Zea mays (L.) (Poaceae), where a greater bioaccumulation of Zn in root plants in comparison with leaves of corn is exposed to HM in mine tailings (Tovar-Sánchez et al. 2018). This could occur because the roots are the first organ with which soil metals have contact and can restrict the translocation of these metals to the leaves and fruits, sequestering and inactivating the metals and thus stabilizing their toxicity (Buendía-González et al. 2019; Dinu et al. 2020).
In the case of Cu, its concentration increases over time in both treatments. However, in general, it accumulates mostly in leaves rather than in roots. A higher concentration of Cu in the leaves suggests that this metal could also be translocated from roots to aerial parts over time. This result coincides with those reported in Z. mays (Benimeli et al. 2010) and C. cardunculus (Capozzi et al. 2020) where this metal was bioaccumulated in greater quantity in leaf tissue compared to root tissue. These results could be explained as Cu is an essential metal which can also be translocated to aerial parts of plants because it is an important component in regulatory proteins. It participates in the transport of electrons in chloroplasts and mitochondria of foliar cells. In addition, it acts as a cofactor of enzymes such as Cu-SOD and cytochrome oxidase and participates in different metabolic processes such as hormonal signaling, cell wall metabolism, and stress response (DalCorso 2012).
Interestingly, we found that Fe, Zn, and Cu had a higher concentration in plants growing in the reference substrate compared to the tailing substrate plants. In general, these results could be explained considering that Quilamula, Morelos, presents a natural richness of mineral soils (mainly sulfur minerals) of silver and lead. The most commonly found are arsenopyrite (FeAsS), galena (PbS), acanthite (Ag2S), and calclacita (Cu2S) (Volke et al. 2005). Therefore, the soils of the region may contain some metals.
Genetic damage in individuals of P. laevigata growing in greenhouse and mine tailings
The results obtained from individuals growing under greenhouse conditions in both treatments (reference substrate and tailing substrate) indicate a significant increase in genetic damage over time. However, this damage was significantly higher in plants growing in the tailing substrate. This same pattern was found in adult plants that grow naturally in reference substrate and tailing substrate. The increase in genetic damage over time may be possible because as plants grow, the amount of HM that they absorb and translocate to aerial parts also increases, as it was observed in this study for Pb. There is a study where an increase in genetic damage over time was detected in leaves of tobacco plants growing in a substrate from a soil contaminated with heavy metals (Gichner et al. 2006). In general, over time, metals can saturate the sequestration zones of these elements in cells (vacuoles and cell walls), a fact that can affect the cytoplasm and the nucleus by promoting genetic damage (García 2006; Sánchez-Pinzón 2010).
With reference to genetic damage present in individuals growing in reference substrate in greenhouse and in soil of the control site (Quilamula), it could be occurring because Huautla is an area naturally rich in minerals (Secretaria de Economia 2011) as previously mentioned. For example, the presence of metals has been reported in their bioavailable form in soil of this area, as in the case of Zn, Pb, Cu, and Fe (Solís-Miranda 2016).
Moreover, results indicated that genetic damage was significantly greater in P. laevigata plants exposed to HM from tailing substrate in greenhouses and in adult individuals that grow naturally in the study area. Previous studies with P. laevigata individuals that are established in the mine tailings of Huautla, Morelos, indicate that this species bioaccumulates Pb, Zn, and Cu and presents significantly greater genetic damage in individuals exposed to HM. Also, a positive and significant relation between the concentration of Cu and Pb accumulated in leaf tissue and genetic damage was detected (Murillo-Herrera 2015). Our results are similar, but in this case, it was found that of four metals detected in tissues of P. laevigata individuals, only Pb was related to the levels of genetic damage detected in both treatments. Comparable results were reported in tobacco plants exposed to Pb at different concentrations and exposure times, and greater genetic damage was found in exposed individuals compared to controls (Gichner et al. 2006). It has been documented that Pb can induce chromosomal aberrations because it binds to the components of cell walls or membranes, mineralizing the wall, changing its physicochemical properties, and disorganizing the microtubules (Eun et al. 2000). It has also been reported that this metal produces double- and single-strand breaks of DNA and can replace Zn in repair and replication enzymes with zinc fingers, as well as oxidative stress (Pourrout et al. 2013). Finally, it has been proposed that Pb is involved in the production of reactive oxygen species (Huihuia et al. 2020) causing genetic damage (Ercal et al. 2001). This information shows the possible mechanisms of action through which the Pb detected in the individuals of P. laevigata exposed to this metal may be causing the observed genotoxic damage.
Morphological and physiological changes in individuals of P. laevigata growing on tailing substrate and reference substrate over time
In general, the results showed a reduction in the macro- and micromorphological characters analyzed in P. laevigata plants growing in tailing substrate over time compared to individuals growing in the reference substrate. A similar response was detected with physiological parameters (chlorophyll content).
Macromorphological characters
The results obtained show that HM bioaccumulation has an effect on plant size, since individuals of P. laevigata growing under greenhouse conditions that were exposed to metals have lower values compared to those that grew in reference substrate. This is comparable with results of other studies where a reduction of height plants exposed to soils contaminated with HM was detected. For example, in Arundo donax (L.) (Poaeceae) growing in a soil contaminated with Pb, a higher concentration of this metal was found in its aerial parts, in addition to a significant reduction in size as well as a thickening of the base of stem (Guo and Miao 2010; Dinu et al. 2020). In the process of cell expansion, the lignification and stiffness of cell wall (Chaoui and El Ferjani 2005) increase, influencing strongly the elasticity and plasticity, increasing tissue stiffness and retarding plant growth (Kabata-Pendias 2011), which can affect the growth of different structures, such as leaves, generating a reduction in their size. This information is relevant because it allows us to explain that in this study, the number of P. laevigata leaves was significantly affected by the presence of Pb over time.
Interestingly, the results in terms of the leaves’ number revealed an enhanced growth of P. leavigata plants growing in the polluted substrate in comparison to individuals growing in the reference substrate. Similar results have been reported in basil Ocimum basilicum (L.) (Lamiaceae) (Dinu et al. 2020) under greenhouse conditions. The authors suggest that these results may occur because the presence of the metals at different concentration levels influenced the plant development, being involved in manifold metabolic and physiological processes. For example, the exposure to some metals like Cr, Cd, Pb, and Ni at low concentrations could positively influence the plant growth (Prasad et al. 2011). This process is called hormesis that is the stimulatory effect of sub-inhibitory concentrations of any toxic substances on any organism (Helmstädter 2008). The stimulation response can be seen as an adaptive compensatory process following an initial disruption in homeostasis. Metal ions can act as elicitors of defense responses that in turn can stimulate the growth of plants, particularly under stress conditions (Poschenrieder et al. 2013).
In addition, multiple regressions analysis showed that Zn has an influence on plant height. This result can be explained taking into consideration that one of the toxic effects of Zn is to inhibit the growth of roots and the emergence of lateral roots (DalCorso 2012). It has been documented that the growth and number of roots is essential for nutrient absorption, for water balance, and to support plants (Frankenberger and Arshad 2020). Also, zinc is an essential micronutrient that affects several metabolic processes of plants (Prasad et al. 2012). Therefore, if these processes are affected, the presence of Zn could compromise the size of the plants. In this context, our results indicate that Zn bioaccumulates in P. laevigata roots, a fact that could explain the reduction of the plants size exposed to metals. Other studies have evidenced a decreased growth and development and metabolic alterations in some plant species such as Lycopersicon esculentum L. (Solanaceae) (Ali et al. 2015), Zea mays and Oryza sativa L. (Poaceae) (Yang et al. 2015), and Brassica juncea (L.) Coss. (Brassicaceae) (Chaudhry et al. 2020). Finally, it has been documented that the translocation of HM to the aerial part of the plants promotes a break in the balance of nutrients in the root, which affects the process of cell expansion increasing the lignification and stiffness of the cell wall affecting the growth of the plants and, therefore, their size (Chaoui and El Ferjani 2005; Buendía-González et al. 2019).
Micromorphological characters
Stomata are specialized cells by which plants exchange gas, and which permit the entering of CO2 necessary for photosynthesis. Besides performing perspiration, stomata allow a loss of water vapor belonging to leaf cells and, at the same time, they rapidly diffuse drier air from the outside of each leaf (Kathpalia and Bhatla 2018). The results of this study show that there is a reduction in the stomatic index in P. laevigata individuals exposed to tailing substrate compared to individuals growing on the reference substrate in greenhouse. The same pattern was previously reported in individuals of this species growing in the mine tailings and control sites (Hernández-Lorenzo 2015) and in Beta vulgaris (L.) (Amaranthaceae) plants grown hydroponically (Sagardoy et al. 2010). This decrease in the number of stomata per unit area (mm2) prevents excessive perspiration of plants; it increases stomatic resistance (Cuypers et al. 2013). The literature indicates that this character can be reduced by exposure to HM, since occlusive cells are very sensitive to chemical stress and changes in position, and the number of stomata can be generated and leaves can fall earlier as a defense mechanism against the metal effects (Rajakaruna and Baker 2006; Rascio and Navari-Izzo 2011). For example, it has been documented that metals such as Pb affect cell walls and stomata and change tissue elasticity (Kabata-Pendias 2011), which could lead to a decrease in stomatic coverage. When plants are exposed to high concentrations of HM, there are direct effects on the guard cells generating hydroactive closure (Bhatla 2018). But, in this study, individuals exposed to metals did not show significant differences in stomatic coverage in comparison to plants growing in the reference substrate. In general, this may occur because plants exposed to metals may present anatomical and physiological changes as an adaptive response to these environmental conditions (Pedrosa-Gomes et al. 2011).
From this perspective, it is important to consider that P. laevigata is a species belonging to the Fabaceae family. Most of the representatives of this species family present adaptation mechanisms to deal with high concentrations of metals in soils (Mahar et al. 2016).
Physiological parameters
When chlorophyll content was evaluated in adult trees established naturally in mine tailings and also compared with trees in the control site, it was found that there are no significant differences. Despite this, in individuals growing under greenhouse conditions, there was an effect of the treatment (tailing substrate) on chlorophyll content, since there was a reduction of it in P. laevigata individuals exposed to HM. These results are similar with those observed in S. procumbens, where there was a reduction in chlorophyll content in plants exposed to Pb contained in tailing substrate (Rosas-Ramírez 2018). Similarly, a reduction in chlorophyll concentration in corn plants exposed to Pb was documented (Yllanes et al. 2014), besides a reduction in photosynthetic pigments in plants exposed to high concentrations of this metal (DalCorso 2012).
It has been documented that the accumulation of HM in toxic concentrations generates different adverse effects on plant physiology, such as inactivation of enzymes, chlorosis, weak growth, and blocking of metabolically important groups such as chlorophyll (Guala et al. 2010; Manara 2012); this can destabilize the cell wall and generate alterations in plant metabolism, affecting their photosynthetic activity (Rascio and Navari-Izzo 2011; Ruiz and Armienta 2012), due to thylakoid damage and modifying the cycle of Calvin, as well as pigmentation alterations that generate chlorosis and cell necrosis (Yadav 2010).
For example, it has been reported that Pb displaces Mg in chlorophyll affecting photosynthesis and electron transport (Singh et al. 2010). HMs also affect the photosynthetic functions of plants inhibiting chlorophyll biosynthesis (Aggarwal et al. 2012), decreasing the total proportion of chlorophyll a and b, which can cause a decrease in the photosynthetic rate (Cenkci et al. 2010; Pourrut et al. 2011). Even so, when chlorophyll in adult trees established in mine tailings and in soil of the control site was measured, no significant differences were observed between treatments, nor in stomatic coverage. This could indicate that over time in adult individuals, physiological adaptations are generated in response to different environmental conditions, which allows them to tolerate HM effects (Pedrosa-Gomes et al. 2011). In this case, these adaptations could be occurring in adult individuals of P. laevigata, generating tolerance to HM effects on chlorophyll concentration and stomatic coverage as a stress protection strategy caused by exposure to HM (Maestri et al. 2010; Rascio and Navari-Izzo 2011). This result is comparable with a study in which the chlorophyll content in leaves of 3-year-old trees of Olea europea (L.) (Oleaceae) species exposed to heavy metals was measured with a SPAD device and the results indicated the absence of chlorophyll content loss (Wilson and Pyatt 2007).
Potential of P. laevigata as a useful species for phytoremediate soils contaminated with heavy metals
P. laevigata is a tree species of wide geographic distribution in Mexico and is common in disturbed places such as metal-contaminated sites (Salas-Luévano et al. 2017). This species frequently inhabits arid and semi-arid areas and is established along with other species of the same family (Fabaceae) such as Vachellia farnesiana (L.) Wight & Arn. (Ibarra-García et al. 2017) and V. campechiana (Santoyo-Martínez et al. 2020). These species are closely related and there are reports that they also bioaccumulate HM such as Pb, Cu, and Zn (Murillo-Herrera 2015; Santoyo-Martínez et al. 2020). However, this is the first report that indicates that P. laevigata bioaccumulates Fe in root and foliar tissues in individuals growing in greenhouse; also, Fe was bioaccumulated in P. laevigata trees naturally established in the mine tailings. The last result is comparable with a recent study that indicates that P. laevigata trees established near a river contaminated with HM hyperaccumulated Fe (Ramírez et al. 2019). In general, it has been documented that plant species used to phytoremediate environments contaminated by metals belong to the following families: Asteraceae, Brassicaceae, Caryophyllaceae, Flacourtaceae, Lamiaceae, Poaceae, Violaceae, and Euphorbiaceae (Mahar et al. 2016; Salas-Luévano et al. 2017; Mei et al. 2020), these being mostly species of herbaceous life form. The results obtained in the present study propose P. laevigata as a tree species with potential use to phytoremediate contaminated sites for the following reasons: (1) P. laevigata is an accumulator species of the four metals analyzed (Zn, Pb, Fe, and Cu); therefore, according to literature, a plant can be considered an accumulator if its translocation factor (TF) is equal to or greater than 1 and hyperaccumulating if the values are > 5 (Olguín and Sánchez-Galván 2012; Ali et al. 2013). In this study P. laevigata showed the following average FT values: Pb (1119), Fe (1487), Cu (2410), Zn (2798). Considering these values, P. laevigata can be considered an accumulator of the four metals analyzed. However, when evaluating at the individual level, the following intervals are observed: Cu 92.9% (0.75–7.44) > Fe 85.7% (0.62–2.69) > Pb 75.0% (0.71 to 1.98) > 64.3% Zn (0.29–8.10). These data suggest that there may be differences in the ability of individuals to carry out this process and that even some plants may be hyperaccumulative as they had FT values > 5. In addition, the bioaccumulation factor for Zn, both in leaf and in root of P. laevigata individuals, was 8662 and 14,598, respectively. According to the TF values observed in this study, there are individuals of P. laevigata that can be accumulators of Pb and Fe, but also hyperaccumulators of Zn and Cu, which is of interest to phytoremediate polluted soils with these metals (Mousavi Kouhi and Moudi 2020). Plants that accumulate higher concentration of metals in aerial parts than their roots show great phytoextraction potential (Mojiri et al. 2013; Ibarra-García et al. 2017; Ramírez et al. 2019). (2) Although a reduction in morphological (macro and micro) and physiological (chlorophyll content) parameters was observed, as well as genetic damage in plants exposed to heavy metals, they did not present mortality. Even adult individuals, grown in mine tailings, have apparently adapted to the presence of metals without altering their chlorophyll levels. The above suggests that plants survive and fulfill a bioaccumulative function (Buendía-González et al. 2019) indicating that individuals of this species may be tolerant to the effects of HM (Ibarra-García et al. 2017; Buendía-González et al. 2019). (3) P. laevigata is a nitrogen-fixing plant with the potential to enrich the soil around it, promoting the growth of bushes associated with it and preventing soil erosion (Salas-Luévano et al. 2017). Moreover, it is a host plant for bird and rodent species (Golubov et al. 2001) and influences plant diversity and soil fertility (García-Sánchez et al. 2012). Therefore, it is of great ecological importance. These characteristics also make it a good candidate to be used in phytoremediation processes, as it offers other environmental services in addition to the bioaccumulation of HM. (4) As a plant widely distributed in arid and semi-arid areas of Mexico (Dorado et al. 2005; Salas-Luévano et al. 2017; Navarrete-Gutiérrez et al. 2018) and growing naturally in mine tailings, it is a candidate species to be used in phytoremediation processes in different areas contaminated by mine activity in Mexico (Ibarra-García et al. 2017; Buendía-González et al. 2019).
Due to greater amounts of metals found in roots compared to leaves, and when observing that nonessential metals such as Pb (which are of interest for phytoremediation) increase over time, the plants of P. laevigata do not bioaccumulate more of this metal even over 6 months of exposure. With this in mind, two proposals were generated for phytoremediation mine tailings: the first is to harvest all plants after 6 months of exposure during a phytoremediation process, because this species bioaccumulates a greater amount of Pb in roots, but also accumulates other metals in its structure (Fe, Zn, and Cu). The second proposal is to let plants grow until adults, and periodically harvest aerial parts of the trees, since all the metals analyzed are being translocated to aerial parts. Therefore, harvesting leaves periodically will allow the removal of metals. The information generated in this study suggests that P. laevigata is a species with potential for phytoremediation of soils contaminated by Fe, Pb, Zn, and Cu. Studies like this one are necessary for the implementation of integral phytoremediation processes that include the use of plant species like those that bioaccumulate different metals in contaminated soils with different metal mixtures.
Conclusion
In this study, P. laevigata chronically exposed to heavy metals in greenhouse/experimental conditions and in adult individuals established in mine tailings showed phytoremediation potential based on the calculated TF values and capacity to bioaccumulate HM in roots and leaves. Also, the biomarkers used in this study revealed that this tree species is sensitive to HM exposure because it showed a significant increase in genotoxic damage and changes in physiological and morphological characters as compared with plants growing without the presence of metals. However, P. leavigata develops and survives properly in polluted environments with HM. In conclusion, these results suggest that this tree species may be an appropriate candidate for its use in phytoremediation studies in polluted soils mainly for Fe, Zn, Cu, and Pb, due to their ability to establish in abandoned mine tailings and becoming one of the dominant plant species, without affecting plant development and survival, its ability to bioaccumulate HM in roots and leaves, and its high levels of HM translocation.
Finally, we consider that conducting phytoremediation studies with different plant species is an efficient approach to remove distinct metals from soils, so it is necessary to combine plant species to develop more effective strategies that can be used in polluted soil with complex HM mixtures.
References
Aggarwal A, Sharma I, Tripathi B, Munjal A, Baunthiyal M, Sharma V (2012) Metal toxicity and photosynthesis. Photosynthesis: overviews on recent progress and future perspectives, 229–236
Alcalá Jáuregui J, Rodríguez Ortíz JC, Hernández Montoya A, Filippini MF, Martínez Carretero E, Díaz Flores PE (2018) Capacity of two vegetative species of heavy metal accumulation. Rev FCA UNCUYO 50(1):123–139
Ali H, Khan E, Sjad MA (2013) Phytoremediation of heavy metals-concepts and applications. Chemosphere 91:869–881. https://doi.org/10.1016/j.chemosphere.2013.01.075
Ali MR, Mehraj H, Jamal Uddin AFM (2015) Effects of foliar application of zinc and boron on growth and yield of summer tomato. Biosci Agric Res 06:512–517. https://doi.org/10.18801/jbar.060115.61
Ambler JE, Brown JC, Gauch HG (1971) Sites of Iron reduction in soybean plants. Agron J 63:9597
Arena C, Figlioli F, Sorrentino MC, Izzo LG, Capozzi F, Giordano S, Spagnuolo V (2017) Ultrastructural, protein and photosynthetic alterations induced by Pb and Cd in Cynara cardunculus L., and its potential for phytoremediation. Ecotox Environ Safe 145:83–89. https://doi.org/10.1016/j.ecoenv.2017.07.015
Ayeni O, Ndakidemi PA, Snyman RG, Odendaal JP (2010) Chemical, biological and physiological indicators of metal pollution in wetlands. Sci Res Essays 5:1938–1949. https://doi.org/10.5897/SER
Batty LC, Younger PL (2003) Effects of external iron concentration upon seedling growth and uptake of Fe and phosphate by the common reed, Phragmites australis (Cav.) Trin ex. Steudel. Ann Bot-London 92(6):801–806. https://doi.org/10.1093/aob/mcg205
Benimeli CS, Medina A, Navarro CM, Medina RB, Amoroso MJ, Gómez MI (2010) Bioaccumulation of copper by Zea mays: impact on root, shoot and leaf growth. Water Air Soil Pollut 210(1–4):365–370. https://doi.org/10.1007/s11270-009-0259-6
Bhatla SC (2018) Light perception and transduction. In: Bhatla SC, Lal MA (eds) Plant physiology. Development and Metabolism Springer, Singapore, pp 519–558
Buendía-González L, Orozco-Villafuerte B, Cruz-Sosa F, Barrera-Díaz CE, Vernon-Carte EJ (2010) Prosopis laevigata, a potencial chromium (VI) and cadmium (II) hyperaccumulator desert plant. Bio/Technology 101:5862–5867. https://doi.org/10.1016/j.biortech.2010.03.027
Buendía González L, Cruz Sosa F, Rodríguez Huezo ME, Barrera Díaz CE, Hernández Jaimes C, Orozco Villafuerte J (2019) In vitro simultaneous acummulation of multiple heavy metals by Prosopis laevigata seedlings cultures. Rev Mex Ing Quím 18(3):1167–1177
Capozzi F, Sorrentino MC, Caporate AG, Fiorentino N, Giordano S, Spagnuolo V (2020) Exploring the phytoremediation potencial of Cynara cardunculus: a trial on an industrial soil highly cobtaminated by heavy metals. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-07575-9
Casana MM, Beltrán RA (2017) Bioacumulación de cobre, plomo, hierro y zinc en Lactuca sativa “lechuga”, Brassica oleracea “repollo”, Daucus carota “zanahoria” y Raphanus sativus “rabanito”. Conocimiento para el desarrollo 4(2) https://revista.usanpedro.edu.pe/index.php/CPD/article/view/167
Cenkci S, Cigerci IH, Yildiz M, Özay C, Bozdag A, Terzi H (2010) Lead contamination reduces chlorophyll biosynthesis and genomic template stability in Brassica rapa L. Environ Exp Bot 67:467–473. https://doi.org/10.1016/j.envexpbot.2009.10.001
Chaoui A, El Ferjani E (2005) Effects of cadmium and copper on antioxidant capacities, lignification and auxin degradation in leaves of pea (Pisum sativum L.) seedlings. C R Biol 328(1):23–31. https://doi.org/10.1016/j.crvi.2004.10.001
Chaudhry H, Nisar N, Mehmood S, Iqbal M, Nazir A, Yasir M (2020) Indian mustard Brassica juncea efficiency for the accumulation, tolerance and traslocation of zinc from metal contaminated soil. Biocatal Agric Biotechnol 23. https://doi.org/10.1016/j.bcab.2019.101489
Covarrubias SA, Peña Cabriales JJ (2017) Contaminación por metales pesados en México: Problemática y estrategias de fitorremediación. Rev Int Cont Amb 7-21. https://doi.org/10.20937/RICA.2017.33.esp01.01
Cuypers A, Remans T, Weyens N, Colpaert J, Vassilev A, Vangronsveld J (2013) Soil-plant relationships of heavy metals and metalloids. In: Alloway BJ (ed) Heavy metals in soils: trace metals and metalloids in soils and their bioavailability. Dordrecht, the Netherlands: Springer, 161–193
DalCorso G (2012) Heavy metal toxicity in plants. In: Furini A (ed) Plants and heavy metals SpringerBriefs in Molecular Science. Springer, Dordrecht pp 1–25. https://doi.org/10.1007/978-94-007-4441-7_1
De la Cruz-Landero N, Hernández VE, Guevara E, López-López MA, Santos AT, Ojeda-Trejo E, Alderete Chavez A (2010) Lipinus versicolor response in soils contaminated with heavy metals from a petroleum extraction field. J Appl Sci 10(8):694–609
De la Rosa G, Cruz G, Cano I, Fuentes R, Gardea JL (2008) Efecto de la edad de la planta y presencia de SS-EDDS en la tolerancia y absorción de Cr (III) por Helianthus annuus. Rev Mex Ing Quim 7:243–251
Delgadillo-López AE, González-Ramírez CA, Prieto-García F, Villagómez-Ibarra JR, Acevedo-Sandoval O (2011) Fitorremediación: Una alternativa para eliminar la contaminación. Tropic Subtropic Agroecosyst 14:597–612
Dinu C, Vasile GG, Buleandra M, Popa DE, Gheorghe S, Ungureanu EM (2020) Traslocation and accumulation of heavy metals in Ocimum basilicum L. plants grown in a mining-contaminated soil. J Soil Sedim 1–14. https://doi.org/10.1007/s11368-019-02550-w
Dorado O, Arias D, Ramírez R, Sousa M (2005) Leguminosas de la Sierra de Huautla Universidad Autónoma del Estado de Morelos Centro de Educación Ambiental e Investigación Sierra de Huautla 176
Ercal N, Gurer Orhan H, Aykin Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1(6):529–539. https://doi.org/10.2174/1568026013394831
Eun SO, Youn HS, Lee Y (2000) Lead disturbs microtubule organization in the root meristem of Zea mays. Physiol Plant 110:357–365. https://doi.org/10.1111/j.1399-3054.2000.1100310.x
Frankenberger WT Jr, Arshad M (2020) Phytohormones in soils microbial production & function. CRC Press
García-Sánchez R, Camargo-Ricalde SL, García-Moya E, Luna-Cavazos M, Romero-Manzanarez A, Montaño M (2012) Prosopis laevigata and Mimosa biuncifera (Leguminosae), jointly influence plant diversity and soil fertility of a Mexican semiarid ecosystem. Rev Biol Trop 60:87–103
García V (2006) Efectos fisiológicos y compartametalización radicular en plantas de Zea mays L. expuestas a la toxicidad por plomo. Dissertation, Universidad Autónoma de Barcelona
Gichner T, Patková Z, Száková J, Demnerová K (2006) Toxicity and DNA damage in tobacco and potato plants growing on soil polluted with heavy metals. Ecotox Environ Safe 65:420–426. https://doi.org/10.1016/j.ecoenv.2005.08.006
Gold-Bouchot G, Zapata-Pérez O (2004) Contaminación, ecotoxicología y manejo costero. In: El Manejo Costero en México. Rivera Arriaga E, Villalobos GJ, Azuz Adeath I, Rosado May F. (eds) Universidad Autónoma de Campeche, SEMARNAT, CETYS-Universidad, Universidad de Quintana Roo, Campeche, Mexico. 654: 277–286
Golubov J, Mandujano MC, Eguiarte LE (2001) The paradox of mesquites (Prosopis spp.): invading species or biodiversity enhancers? Bot Sci 69:23–30. https://doi.org/10.17129/botsci.1644
Gonçalves AC Jr, Schwantes D, Braga de Sousa RF, Benetoli da Silva TR, Guimar VF, Campagnolo MA, Soares de Vasconcelos E, Zimmermann J (2020) Phytoremediation capacity, growth and physiological responses of Crambe abyssinica Hochst on soil contaminated with Cd and Pb. J Environ Manag 262:110432. https://doi.org/10.1016/j.jenvman.2020.110342
Guala SD, Vega FA, Covelo EF (2010) The dynamics of heavy metals in plant–soil interactions. Ecol Model 221:1148–1152
Guo Z, Miao X (2010) Growth changes and tissues anatomical characteristics of giant reed (Arundo donax L.) in soil contaminated with arsenic, cadmium and lead. J Cent South Univ T 17:770–777. https://doi.org/10.1007/s11771−010−0555−8
Guo J, Shi R, Cao Y, Luan Y, Zhou Y, Gao Y, Tian Y (2020) Genotoxic effects of imidacloprid in human lymphoblastoid TK6 cells. Drug Chem Toxicol 43:208–212. https://doi.org/10.1080/01480545.2018.1497048
Helmstädter A (2008) Is there a tonic in the toxin? The Arndt–Schulze law as an explanation for non-linear dose–response relationships, In: Balz V, Schwerin A.v, Stoff H, Wahrig B (eds) Precarious Matters. The History of Dangerous and Endangered Substances in the 19th and 20th Centuries, Max Planck Institut für Wissenschaftsgeschichte, Berlin, pp. 29–37
Hernández-Acosta E, Mondragón-Romero E, Cristobal-Acevedo D, Rubiños-Panta JE, Robledo-Santoyo E (2009) Vegetación, residuos de mina y elementos potencialmente tóxicos de un jal de Pachuca, Hidalgo, México. Rev Chapingo Ser Cie 15(2):109–114
Hernández-Lorenzo B (2015) Análisis de la anatomía y morfología de Prosopis laevigata, por acumulación de metales pesados en la Sierra de Huautla, Morelos. Universidad Autónoma del Estado de Morelos, Dissertation https://documentcloud.adobe.com/link/track?uri=urn:aaid:scds:US:f1e6be7a-3573-45ac-8d0f-bd0dd2f859d1
Huihuia Z, Xina L, Zisonga X, Yueb W, Zhiyuanb T, Meijunc A, Yuehuic Z, Wenxud Z, Nanb X, Guangyub S (2020) Toxic effects of heavy metals Pb and Cd on mulberry (Morus alba L.) seedling leaves: photosynthetic function and reactive oxygen species (ROS) metabolism responses. Ecotox Environ Safe 195. https://doi.org/10.1016/j.ecoenv.2020.110469
Ibarra-García AR, Barceló-Quintal ID, García-Albortante J, López-Lafuente AL, González-Huecas C, Quintana-Nieto JR, Mugica-Alvarez V (2017) Phytoextraction of metals by native plants from mining wastes in Zacatecas, Mexico. Acta Hortic 1227:409–416. https://doi.org/10.17660/ActaHortic.2018.1227.51
INEGI (Instituto Nacional de Estadística y Geografía) (2016) Sistema de cuentas nacionales de México. http://www.inegi.org.mx/sistemas/bie
Kabata-Pendias A (2011) Trace elements in soil and plants, 4th edn. CRC Press, Boca Raton
Kathpalia R, Bhatla SC (2018) Plant water relations. In: Bhatla SC, Lal MA (eds) Plant physiology. Development and Metabolism Springer, Singapore, pp 37–81
Kaya C, Higgsb D, Ashrafc M, Alyemenid MN, Ahmadd P (2020) Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol Plant 168:256–277. https://doi.org/10.1111/ppl.12976
Maestri E, Marmiroli M, Visioli G, Marmiroli N (2010) Metal tolerance and hyperaccumulation: costs and trade-offs between traits and environment. Environ Exp Bot 68:1–13. https://doi.org/10.1016/j.envexpbot.2009.10.011
Mahar A, Wang P, Ali A, Awasthi MK, Lahori AH, Wang Q, Li R, Zhang Z (2016) Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: a review. Ecotoxicol Environ Saf 126:111–121. https://doi.org/10.1016/j.ecoenv.2015.12.023
Manara A (2012) Plant responses to heavy metal toxicity. In: Furini A (ed) Plant and heavy metals. Springer Science & Business Media, Pisa, Italy, pp 27–53
Mei Y, Zhou H, Gao L, Zuo YM, Wei KH, Cui NQ (2020) Accumulation of Cu, Cd, Pb, Zn and total P fromsynthetic stormwater in 30 bioretention plants. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-07731-6
Mojiri A, Aziz HA, Zahed MA, Aziz SQ, Selamat MRB (2013) Phytoremediation of heavy metals from urban waste leachate by southern cattail (Typha domingensis). Int J Sci Res Environ Sci 1:63–70
Mousavi Kouhi SM, Moudi M (2020) Assessment of phytoremediation potential of native plant species naturally growing in a heavy metal-polluted saline–sodic soil. Environ Sci Pollut Res 27:10027–10038. https://doi.org/10.1007/s11356-019-07578-6
Murillo-Herrera AI (2015) Detección de daño genotóxico en Prosopis laevigata de los jales de Sierra de Huautla, Morelos, México provocado por metales pesados. Universidad Nacional Autónoma de México, Dissertation https://documentcloud.adobe.com/link/track?uri=urn:aaid:scds:US:5d2461a0-1ec2-4d8a-96f3-1ea8ae5f5174
Mussali-Galante P, Ávila Costa MR, Piñón Zarate G, Martínez Levy G, Rodríguez Lara V, Rojas Lemus M, Fortoul TI (2005) DNA damage as an early biomarker of effect in human health. Toxicol Ind Health 21(5–6):155–166. https://doi.org/10.1191/0748233705th224oa
Mussali-Galante P, Tovar Sánchez E, Valverde M, Rojas del Castillo E (2013) Biomarkers of exposure for assessing environmental metal pollution: from molecules to ecosystems. Rev Int Contam Ambie 29:117–140
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8(3):199–216. https://doi.org/10.1007/s10311-010-0297-8
Navarrete Gutiérrez DM, Pons MN, Cuevas Sánchez JA, Echevarria G (2018) Is metal hyperaccumulation occurring in ultramafic vegetation of central and southern Mexico? Ecol Res 33:641–649. https://doi.org/10.1007/s11284-018-1574-4
Olguín E, Sánchez Galván G (2012) Heavy metal removal in phytofiltration and phycoremediation: the need to differentiate between bioadsorption and bioaccumulation. New Biotechnol 30:3–8. https://doi.org/10.1016/j.nbt.2012.05.020
Paniagua-Ibáñez M, López-Caamal A, Mussali-Galante P, Sánchez-Salinas E, Ortiz-Hernández LM, Ramírez-Rodríguez R, Tovar-Sánchez E (2015) Morphological variation of Cosmos bipinnatus (Asteraceae) and its relation to abiotic variables in Central Mexico. Rev Chil Hist Nat:2–13. https://doi.org/10.1186/s40693-015-0044-4
Patra M, Bhowmik N, Bandopadhyay B, Sharma A (2004) Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ Exp Bot 52(3):199–223. https://doi.org/10.1016/j.envexpbot.2004.02.009
Pedrosa-Gomes M, Lara Lanza T, Marques D, de Oliveira GM, de Castro E, Soares A (2011) Accumulation of heavy metal in Brachiaria decumbens. Sci Agrár 68:566–573. https://doi.org/10.1590/S0103-90162011000500009
Poschenrieder C, Cabot C, Martos S, Gallego B, Barceló J (2013) Do toxic ions induce hormesis in plants? Plant Sci 212:15–25. https://doi.org/10.1016/j.plantsci.2013.07.012
Pourrut B, Shahid M, Dumat C, Winterton P, Pinelli E (2011) Lead uptake, toxicity, and detoxification in plants. In: Whitacre D (ed) Reviews of Environmental Contamination and Toxicology Volume 213. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-9860-6_4
Pourrout B, Shahid M, Douay F, Dumat C, Pinelli E (2013) Molecular mechanisms involved in lead uptake, toxicity and detoxification in higher plants. In: Gupta DK (ed) Heavy Metal Stress in Plants. Springer, Heidelberg, Berlin pp 121–147. https://doi.org/10.1007/978-3-642-38469-1_7
Prasad A, Kumar S, Khaliq A, Pandey A (2011) Heavy metals and arbuscular mycorrhizal (AM) fungi can alter the yield and chemical composition of volatile oil of sweet basil (Ocimum basilicum L.). Biol Fertil Soils 47:853–861
Prasad TNVKV, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Reddy KR, Pradeep T (2012) Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J Plant Nutr 35(6):905–927. https://doi.org/10.1080/01904167.2012.663443
Rajakaruna N, Baker AJM (2006) Serpentine: a model hábitat for botanical research in Sri Lanka. Ceylon J Sci 32:1–19
Ramírez V, Baez A, López P, Bustillos B, Villalobos MA, Carreño R, Contreras JL, Muñoz Rojas J, Fuentes LE, Martínez J, Munive JA (2019) Chromium hyper-tolerant Bacillus sp. MH778713 assists phytoremediation of heavy metals by mesquite trees (Prosopis laevigata). Front Microbiol 10:1833. https://doi.org/10.3389/fmicb.2019.01833
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180(2):169–181. https://doi.org/10.1016/j.plantsci.2010.08.016
Rodríguez-Sauceda EN, Rojo Martínez GE, Valverde Ramírez B, Martínez Ruíz R, Cong Hermida MC, Medina Torres SM, Piña Ruíz HH (2014) Análisis técnico del árbol del Mezquite (Prosopis laevigata Humb. & Bonpl. ex Willd.) En México. Ra Ximhai 10(3):173–193
Rojas E, López MC, Valverde M (1999) Single cell gel electrophoresis assay: methodology and applications. J Chromatogr 722:225–254
Rosas-Ramírez ME (2018) Relación entre la bioacumulación de metales pesados y la concentración de clorofila en Sanvitalia procumbens. Universidad Autónoma del Estado de Morelos, Dissertation https://documentcloud.adobe.com/link/review?uri=urn:aaid:scds:US:b942e10e-6309-4556-bb54-1a8c395dd158
Rout GR, Sahoo S (2015) Role of iron in plant growth and metabolism. Rev Agric Sci 3:1–24. https://doi.org/10.7831/ras.3.1
Ruiz HEA, Armienta HMA (2012) Acumulación de arsénico y metales pesados en maíz en suelos cercanos a jales o residuos mineros. Rev Int Contam Ambie:103–117
Sagardoy R, Vázquez S, Florez-Sarasa ID, Albacete A, Ribas-Carbó M, Flexas J, Abadıa J, Morales F (2010) Stomatal and mesophyll conductances to CO2 are the main limitations to photosynthesis in sugar beet (Beta vulgaris) plants grown with excess zinc. New Phytol 187:145–158. https://doi.org/10.1111/j.1469-8137.2010.03241.x
Salas-Luévano MA, Manzanares-Acuña E, Letechipía-de León C, Vega-Carrillo HR (2009) Tolerant and hyperaccumulators autochthonous plant species from mine tailing disposal sites. Asian J Exp Sci 23(1):27–32
Salas Luévano MA, Mauricio-Castillo JA, González-Rivera ML, Vega-Carrillo HL, Salas-Muñoz S (2017) Accumulation and phytostabilization of As, Pb and Cd in plants growing inside mine tailings reforested in Zacatecas, Mexico. Environ Earth Sci 76: 806. https://doi.org/10.1007/s12665-017-7139-y
Salisbury FT (1968) Las plantas vasculares: forma y función. México: Herrero Hermanos Sucesores pp 598
Sánchez-Pinzón MS (2010) Contaminación por metales pesados en el Botadero de basuras de Moravia en Medellín: Transferencia a flora y fauna y evaluación del potencial fitorremediador de especies nativas producidas. Pontificia Universidad Javeriana, Colombia, Dissertation
Santoyo-Martínez M, Mussali-Galante P, Hernández-Plata I, Valencia-Cuevas L, Flores-Morales A, Ortiz-Hernández L, Flores-Trujillo K, Ramos-Quintana F, Tovar-Sánchez E (2020) Heavy metal bioaccumulation and morphological changes in Vachellia campechiana (Fabaceae) reveal its potential for phytoextraction of Cr, Cu, and Pb in mine tailings. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-07730-7
Secretaria de Economía (2011) Panorama Minero del estado de Morelos. Servicio Geológico Mexicano, serie panorama minero de los estados, Pachuca
SEMARNAT, Secretaria de Medio Ambiente y Recursos Naturales (2005) Dirección de Investigación en Residuos y Sitios Contaminados Subdirección de Investigación en Sitios Contaminados y Sustancias Tóxicas. Informe 42 anual de actividades. Evaluación de tecnologías de remediación para suelos contaminados con metales. Etapa II. [Fecha de consulta: 4 de noviembre de 2016]. http://www.inecc.gob.mx/descargas/dgcenica/metales_eii2005.pdf
Sharma P, Pandey S (2014) Status of phytoremediation in world scenario. Int J Environ Bioremediation & Biodegradation 2:178–191
Sharma RK, Agrawal M (2005) Biological effects of heavy metals: an overview. J Environ Biol 26(2):301–313. https://doi.org/10.12691/ijebb-2-4-5
Shiqi L, Yang B, Kou Y, Zeng J, Wang R, Xiao Y, Li F, Lu Y, Mu Y, Zhao C (2018) Assessing the difference of tolerance and phytoremediation potential in mercury contaminated soil of a nonfood energy crop, Helianthus tuberosus L. (Jerusalem artichoke). Peer J 6:1–18. https://doi.org/10.7717/peerj.4325
Singh R, Tripathi RD, Dwivedi S, Kumar A, Trivedi PK, Chakrabarty D (2010) Lead bioaccumulation potential of an aquatic macrophyte Najas indica are related to antioxidant system. Bioresour Technol 101:3025–3032. https://doi.org/10.1016/j.biortech.2009.12.031
Solís-Miranda BA (2016) Aislamiento de bacterias de jales mineros y análisis de su potencial para la remediación de sitios contaminados con metales pesados. Universidad Autónoma del Estado de Morelos, Dissertation https://documentcloud.adobe.com/link/track?uri=urn:aaid:scds:US:3a5f2873-da05-408d-b7d5-8b170013d30e
StatSoft (2000) Correspondence analysis. Tulsa, StatSoft Inc 2000 http://www.statsoftinc.com/textbook/stcoran.html
Suman J, Uhlik O, Viktorova J, Macek T (2018) Phytoextraction of heavy metals: a promising tool for clean-up of polluted environment? Front Plant Sci 9:1476. https://doi.org/10.3389/fpls.2018.0147
Talavera O, Yta M, Moreno R, Dótor A, Flores N, Durante C (2005) Mineralogy and geochemistry of sulfide-bearing tailings from silver mines in the Taxco, Mexico area to evaluate their potential environmental impact. Geofis Int 44:49–64
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H et al (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221. https://doi.org/10.1002/(SICI)1098-2280(2000)35:3<206::AID-EM8>3.0.CO;2-J
Tovar-Sánchez E, Mussali Galante P, Martínez-Pacheco M, Ortiz Hernández ML, Sánchez-salinas E, Olvera-Verona A (2016) Relationship between genotoxic damage and arsenic blood concentrations in individuals residing in an arsenic contaminated area in Morelos, Mexico. Rev Int Contam Ambie 32:101–117
Tovar-Sánchez E, Cervantes Ramírez T, Castañeda Bautista J, Gómez Arroyo S, Ortíz Hernández L, Sánchez Salinas E, Mussali Galante P (2018) Response of Zea mays to multimetal contaminated soils: a multibiomarker approach. Ecotoxicology 27:1161–1177. https://doi.org/10.1007/s10646-018-1974-9
Velasco J, De la Rosa D, Ramírez M, Volke T (2004) Evaluación de tecnologías de remediación para suelos contaminados con metales Etapa II. SEMARNAT-INE, México pp 46
Volke ST, Velasco TA, De la Rosa PA, Solórzano OG (2005) Evaluaciones de tecnologías de remediación para suelos contaminados con metales. Etapa II. Secretaria de Medio Ambiente y Recursos Naturales, México
Wilson B, Pyatt FB (2007) Heavy metal bioaccumulation by the important food plant, Olea europaea L., in an ancient metalliferous polluted area of Cyprus. B Environ Contam Tox 78(5):390–394. https://doi.org/10.1007/s00128-007-9162-2
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179. https://doi.org/10.1016/j.sajb.2009.10.007
Yang Z, Chen J, Dou R, Gao X, Mao C, Wang L (2015) Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants, maize (Zea mays L.) and rice (Oryza sativa L.). Int J Env Res Pub He 12:15100–15109
Yllanes P, Vélez A, Lozano S (2014) Efectos fitotóxicos del plomo en maíz híbrido Dekalb (Zea mays L.) en suelo arenoso y limoso. Biologist 12:337–248
Yoon J, Cao X, Zhou Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368:456–464. https://doi.org/10.1016/j.scitotenv.2006.01.016
Zar JH (2010) Biostatistical analysis. Prentice Hall, New Jersey
Acknowledgments
We thank the “Doctorado en Ciencias Naturales,” Autonomous University of Morelos State (UAEM), for the facilities granted to carry out this project. This research was supported by a CONACyT scholarship grant to M.S.M. (Grant: 429356). Also, we thank Rosalind Pearson Hedge for her comments and English edition that improved our manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muro-González, D.A., Mussali-Galante, P., Valencia-Cuevas, L. et al. Morphological, physiological, and genotoxic effects of heavy metal bioaccumulation in Prosopis laevigata reveal its potential for phytoremediation. Environ Sci Pollut Res 27, 40187–40204 (2020). https://doi.org/10.1007/s11356-020-10026-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-10026-5