Abstract
Purpose
Diesel fuel represents a permanent source of soil pollution, and its removal is a key factor for human health. To address the limitations of conventional remediation techniques, microwave (MW) heating could be employed due to its great potentiality. This work presents the lab-scale experiments performed to study the potential of MW processing for diesel-polluted soils treatment and related modeling for the optimization of MW systems operating conditions.
Materials and methods
A sandy soil was artificially contaminated with diesel fuel, moisturized with different amounts of water content, and thermally treated by MW radiation using a lab-scale apparatus to investigate the effect of soil moisture on soil temperature profiles and contaminant removal kinetics. An operating power, ranging from 100 to 1,000 W, and treatment times of 5, 10, 18, 30, and 60 min were investigated. Contaminant residual concentration values were fitted using the first order kinetic model, and desorption parameters were calculated for each soil at different operating powers.
Results and discussion
Main results show that the operating power applied significantly influences the contaminant removal kinetics, and the moisture content in soil has a major effect on the final temperature reachable during MW heating. Minimal contaminant concentrations were achievable by applying powers higher than 600 W for a treatment time longer than 60 min. For remediation times shorter than 10 min, which result in a soil temperature of about 100 °C, the effect of the distillation process increases the contaminant removal, whereas for longer times, soil temperature is the main key factor in the remedial treatment.
Conclusions
MW thermal desorption of diesel-polluted soil was shown to be governed by pseudo-first-order kinetics, and it could be a better choice for remediation of diesel-polluted soils, compared to several biological, chemical–physical, or conventional thermal treatments, due to its excellent removal efficiency. The results obtained are of scientific and practical interest and represent a suitable tool to optimize the treatment operating conditions and to guide the design and the scale-up of MW treatments for full-scale remediation activities of diesel-polluted soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Petroleum hydrocarbon contaminated sites is a widespread and relevant environmental problem. Over the last few years, a large amount of petroleum products has been released into the environment via leakage from storage tanks and pipelines or accidental spills, making the management of contaminated sites a major environmental challenge in many countries (Pazos et al. 2012). For instance, the number of potentially contaminated sites in Europe is approximately three million, including about 250,000 sites that are expected to be highly contaminated and consequently in need of urgent remediation (Tatáno et al. 2013). Among hydrocarbons, diesel fuel, a complex mixture of saturated (60–80 % of n-alkanes and naphthenes) and aromatic hydrocarbons (20–40 %), is widely used in the world and represents a permanent source of soil and water pollution (Fernández et al. 2011; Silva-Castro et al. 2013). Diesel-contaminated soil is unsuitable for human uses (i.e., agricultural, commercial, residential, or recreational), representing a threat to human health.
In the last decade, different chemical–physical and/or biological remediation technologies have been employed to remove diesel fuel from different soils (Bento et al. 2005; Do et al. 2009; Fernández et al. 2011; Khalladi et al. 2008; Pazos et al. 2012; Silva-Castro et al. 2013; Tsai et al. 2010). However, these treatments may be too expensive or lengthy (Chien 2012).
To address these limitations and to achieve a better hydrocarbon removal efficiency, thermal treatment could be applied due to its versatility, removal efficiency, and required time (Careghini et al. 2010; Falciglia et al. 2011a; Merino and Bucalá 2007; US-EPA 2004). In fact, it is well-known that thermal decontamination of diesel-polluted soils presents excellent contaminant removal percentage in a very short remediation time (Falciglia et al. 2011b; Lee et al. 1998). However, conventional thermal treatments may be expensive due to their excavation and transport or fuel costs; and to reduce these costs, microwave (MW) heating technology (Chien 2012) could represent an optimal choice to remedy diesel-polluted soils due to its advantages.
In recent years, thermal remediation using MW heating has attracted great attention in the environmental field because it represents a novel and optimal approach (Lin et al. 2010). MW heating does not rely on heat transfer, and consequently heating times can be up three orders of magnitude lower than with conventional heating. Therefore, MW offers the potential to significantly reduce treatment times, risk of contamination, and costs due to the direct interaction of microwave with the soil and the ability to overcome heat and mass transfer limitations (Robinson et al. 2008).
MWs are a separate band of electromagnetic radiation with frequencies in the range of 300 MHz to 300 GHz. The key factor of the remediation process is represented by the mechanism of partial dissipation of the electromagnetic field energy and its conversion into heat necessary for the thermal desorption of the contaminants. In fact, the internal temperature distribution of a material, such as the soil using conventional heating, is limited by its thermal conductivity, whereas in the case of microwave radiation, the alternating electromagnetic field induces the rotation of the dipoles of water and other polar or semipolar substances present in the soil. The intermolecular friction results in the generation of heat (Kawala and Atamaczuk 1998). Moreover, MW are absorbed by materials with a high dielectric loss factor (absorbing), while passing through the low loss (transparent) material, resulting in a selective, uniform, and rapid heating. Therefore, heating times can be significantly reduced compared with those required when using conventional heating methods (Robinson et al. 2009).
The power absorbed per unit volume (P, W m−3) and consequently the rate of heat generated (ΔT Δt−1, °C min−1) depends directly on the frequency of the applied electromagnetic field and on the dielectric properties of the treated medium, and it is obtained from Poynting's theorem (Clark et al. 2000):
where ω is the angular frequency (ω = 2πf, f microwave frequency); ε0 is the permittivity of free space (8.85 × 10−12 F m−1), ε' and ε" are the real part (dielectric constant) and the imaginary parts (dielectric loss factor) of the complex permittivity, respectively; E is the magnitude of the internal electric field (V m−1). The real and the imaginary parts of its complex structures are often expressed through the loss tangent parameter, tan δ, which is the ratio between the loss factor and the dielectric constant. The dielectric constant ε' denotes the electric energy storage capacity of the medium, while the dielectric loss factor ε" can be considered as the ability of the medium to convert electromagnetic energy into heat due to the dielectric polarization of the particles in an alternating electric field. Substances which exhibit a large value of loss factor are good microwave absorbers, whereas substances whose loss factor is close to zero can be considered to be microwave transparent (Jones et al. 2002). It is important to note that the majority of the absorbed microwave power is converted to heat within the materials; therefore, the rate of heat generated during the microwave irradiation, for given electric field and permittivity of the material, is quantified by the following equation (Li et al. 2009):
where c p is the heat capacity of the medium (KJ kg−1 °C−1) and ρ is its density (kg m−3).
The dielectric properties are also important parameters in determining the penetration depth (D p ), that is the depth to which microwaves penetrate into the medium. In particular, the penetration depth is defined as the distance from the emission point at which the power drops to 0.37 from its value at the emission point. For low loss dielectric materials (i.e., soil) (ε"/ε' < <1), D p is given by the simplified relation (Acierno et al. 2004):
where λ0 is the wavelength of the radiation in the free space (in meters).
Based on the above equations, it is clear that, for an effective remediation treatment, the selection of the correct radiation frequency is fundamental. As a matter of fact, in order to achieve the most effective and rapid heating, the highest possible frequency should be applied, but with increased frequency, the radiation range decreases, and for values too high, the radiation effect is noticeable only within a few centimeters from the emission point.
For a full-scale application of MW remediation, a schematic design of a microwave heating system has recently been reported by Ha and Choi (2010), Barba et al. (2011) and Chien (2012).
In the last few years, several studies on contaminated soil remediation by MW have been performed in order to understand the fundamentals of the treatment and the dielectric properties of contaminated (Darayan et al. 1998) and uncontaminated soils (Dobson et al. 1985; Hallikainen et al. 1985) or to investigate the effects of process parameters on the contaminant removal efficiency. The first experimental results that proposed the MW remediation technique as a promising treatment of hazardous wastes were shown by Dauerman et al. (1992) and George et al. (1995). Removal of PCBs from contaminated soil was performed by several authors (Abramovitch et al. 1998a; Huang et al. 2011; Liu and Yu 2006; Liu et al. 2008). Their results revealed that an improvement of contaminant removal was obtained with the addition of energy absorbents such as Cu2O, MnO2, NaOH, iron powder, graphite, or granular activated carbons, and that rates of PCBs removed were highly dependent on microwave power, soil moisture, and the amount of adsorbent materials added. Yuan et al. (2006) investigated the remediation of soil contaminated with hexachlorobenzene (HCB), using a domestic microwave oven and powdered MnO2 as a microwave absorber. Their results showed that a complete removal of HCB was obtained with 10 min microwave treatment. Similar results in terms of removal efficiency were also obtained by Kawala and Atamaczuk (1998) in a pilot-scale study for the remediation of a TCE-polluted soil, where a microwave power of 600 W was supplied intermittently for 75 h. After the treatment, the contaminant concentration decreased from 5,000–22,300 to 8–29 mg kg−1, confirming the possibility of the use of microwave heating as an in situ remediation technique of volatile and semivolatile compound-polluted sandy soils, and that the use of low power generators for the supply of microwave energy may help to reduce the costs of the full-scale remediation interventions. Microwave treatment was also shown to be efficient in a short time for the remediation of soil polluted by PAHs (Abramovitch et al. 1998b; Robinson et al. 2009), PCPs (Di and Chang 2001), antibiotics (Lin et al. 2010), and crude oil (Li et al. 2009).
The above-mentioned works suggest that treatment power, time, soil dielectric characteristics, and microwave absorbents used are key factors in remedial processes, and that MW remediation is very effective for a large number of polar and nonpolar volatile and semivolatile hydrocarbons; but in the case of nonpolar compounds, their dielectric properties could limit the treatment removal efficiency. However, for nonpolar organic compounds such as diesel or kerosene, defined as transparent (low dielectric loss material), it was showed that they could also be efficiently removed using water as the MW absorbing phase (Jones et al. 2002). However, the literature review provides no data concerning the efficiency of MW heating of soil polluted with diesel fuel.
This work aims to study the potential of MW processing for the treatment of diesel-polluted soils using an experimental bench-scale apparatus. The main goals of the work were: (1) to assess the influence of power treatment, heating time, and soil moisture on the temperature profiles generated by the MW irradiation and on the diesel residual contamination in soil and thus removal efficiency; (2) to model the experimental data in order to calculate the desorption parameters needed to optimize the treatment operating conditions and to guide the design and the scale-up of microwave treatment systems.
2 Materials and methods
2.1 Materials
Commercially available diesel fuel (Esso, Italy) was used to artificially contaminate the soil. All chemicals used in experiments were of analytical reagent quality. N-hexane (C6H14, purity 99 %) and anhydrous sodium sulfate (Na2SO4, purity 99 %) were purchased from Merck KGaA (Darmstadt, Germany). Activated carbons (RB1) used for the volatile compound capture system of the experimental apparatus were supplied by Norit Italia S.p.A. (Ravenna, Italy). A model fine-sandy soil (75–200 μm) (Che.Mi.Fil. s.r.l., Verona, Italy), free of anthropogenic contamination, was selected for the experiments. Diesel and soil properties are given in Table 1.
2.2 Soil contamination
Selected soil samples were artificially contaminated by diesel fuel. The contamination procedure was performed by introducing a pollutant solution of diesel fuel (80 mL) in n-hexane (200 mL) into a 500 mL round-bottom flask containing the selected soil samples (120 g) to obtain a representative contaminant concentration for a sandy soil (Falciglia et al. 2011b). Soil and pollutant solution were shaken for 48 h using an orbital shaker, then the n-hexane solvent was removed in 1 h, using a rotary evaporator, under slight vacuum, in order to obtain a homogeneous powdered soil. The contaminated soil was kept in a closed vessel and stored in a dark room at 4 °C for 15 days, then analyzed by n-hexane extraction and subsequently gas chromatography (GC) for contaminant content before microwave treatment. Contamination procedure was carried out in triplicates, and mean and standard deviation values of adsorbed contaminant concentration were calculated. After the contamination procedure, a number of soil samples were moisturized with deionised water to 8 and 12 %. Adsorbed diesel on soil (C 0) as n-alkanes fractions (C10–C25) for the spiked soil was 1,916.4 ± 83 mg kg−1.
2.3 Experimental apparatus and procedures
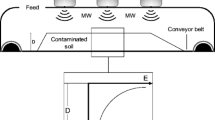
Contaminated soil samples were treated, simulating microwave thermal process conditions, using a bench scale apparatus. A 1,000 W modified domestic microwave oven (Panasonic NN-GD458W Inverter), at the frequency of 2,450 MHz with continuous adjustable power setting, was used. A schematic diagram of the experimental apparatus is shown in Fig. 1. A quartz reactor (h 100 mm, internal ∅ 80 mm) was installed into the microwave oven cavity, so that the contaminated soil sample occupied the center section of the cavity. The gas outlet section of the quartz reactor was connected to a condensing system, to a VOC capture system (activated carbon filters), and then to a vacuum pump to remove air in the reactor.
In the experiments, 20 g of polluted soil sample were placed inside the oven and treated by microwave heating for a time of either 5, 10, 18, 30, or 60 min, using an applied power ranging from 100 to 1,000 W. After a desired residence time, the microwave oven was turned off, and the temperature was immediately measured with a sheltered type-k thermocouple axially inserted up to the middle of the soil sample.
After treatment, soil samples were removed from the apparatus, cooled at room temperature (20 °C), and stored in a dark room at 4 °C prior to analyzing. At the same time, residual soil moisture content was measured, applying the D 2216—05 ASTM test method (ASTM 2008). The thermal treatment procedure was carried out in triplicates, and mean values of contaminant residual concentrations as a function of the treatment time were obtained for each selected power and soil moisture.
Removal efficiency (R) was also calculated by the following expression:
where C 0 (mg kg−1) is the initial contaminant concentration in soil, and C (mg kg−1) is the residual concentration of contaminant in soil after the thermal treatment.
2.4 Kinetic data modeling
Residual hydrocarbon concentration curves as a function of the desorber residence time follow a first order kinetic (Di and Chang 2001; Khalladi et al. 2008; Uzgiris et al. 1994), defining an exponential decay:
where C (mg kg−1) is the residual concentration in soil after a treatment time t (in minutes), C 0 (mg kg−1) represents the initial contaminant concentration, k (min−1) represents the rate of decay of the function, and d is the shape of the decay curve. k is temperature dependent and can be expressed as:
where E A (in Joules) is the activation energy of the system, A (in moles) is a frequency term, R (in Joule per kilogram per mole) is the gas constant, and T (in Kelvin) is the absolute temperature.
Residual concentration (C) results obtained during the experiments, expressed as C/C 0 ratio, were fitted using the first order kinetic model expressed by the logarithmic form of Eq. (5). Desorption parameter k was calculated for each soil at different power values, and the correlation was assessed as correlation coefficient R 2.
Obtaining the experimental parameters k, Eqs. (5) and (6) could represent a valuable tool in calculating residual concentration C or desorption rate at any given initial contaminant concentration in soil and in identifying the power and the time of treatment required to reach specific targeted levels of remediation.
2.5 Extraction and analysis
For each 20 g sample treated, a 2 g subsample was analyzed for hydrocarbon concentration. The subsample was mixed with n-hexane in a Soxhlet extractor for 6 h. Five milliliter of effluent were mixed with 2 mL of n-hexane in a separate funnel, stirred for 2 min, and then left at rest for separation. The supernatant phase was mixed with internal standard (ISM-560 Ultra Scientific, USA) and analyzed by gas chromatography.
Due to their high proportion (40 %) in diesel fuel, n-alkanes compounds (C10–C25) were chosen as representative components (Khalladi et al. 2008), and their total concentration in spiked and treated soil samples was taken as that of diesel fuel and expressed as mg/kgsoil.
The concentration of n-alkanes in soil samples was measured by GC (Agilent Technologies 6,890 N) equipped with a mass spectrometer (Agilent Technologies 5975), using the US-EPA 8270-C method. A capillary column (HP-5, 30 m length × 0.32 mm ID × 0.25 μm film thickness) was used. The GC was operated with a helium carrier gas flow rate of 1.5 mL min−1 and the oven temperature program starting at 40 °C (held for 4 min) and increasing at a rate of 10 °C min−1 to a maximum temperature of 310 °C. The temperature of the injector was 270 °C.
3 Results and discussion
3.1 Temperature profiles
The temperature (T) profiles of soil with time (t) during MW radiation at investigated power (P) series of 100, 250, 440, 600, and 1,000 W are shown in Fig. 2.
As expected, T increased with increasing time and P for all the samples of soil with different moisture content investigated. In all cases, T increased rapidly at the beginning of the treatment, and it stabilized after about 20 min; and it can be clearly seen that at higher P, T rose more rapidly. For the lowest P investigated (100 W), a slight T increase of 80 °C was observed. When P was as high as 440 W, T rose up to 184 °C, whereas a maximum increase of 240 °C (T = 260 °C) was reached for the 1,000 W treatment. Moreover, for all the P tested, a significant T increase was found for the soils with water content (8 and 12 %), respect to dry soil (contaminated soil without water content). For the treatment at 1,000 W, a difference of T up to 40 °C was observed between soils with 0 and 12 % water content.
This specific behavior strictly depends on the dielectric properties of the treated soils. As reported in the previous paragraph, the increase of T in time is linearly proportional to the dielectric constant defined loss factor (ε") that denotes the ability of the soil to convert electromagnetic energy into heat due to the dielectric polarization of the particles in an alternating electric field.
The trend for which the temperature profile rises more rapidly when the treatment starts and tends to stabilize after a fixed time is due to the reducing in the ability of the MW absorbent medium (soil) to convert energy into heat with a progressive increase in heat absorbed, and therefore, with an increase in temperature. As a matter of fact, it was clearly shown that ε", that at 20 °C is 0.8 (Robinson et al. 2012), decreases with T increasing, and that, for T values higher than 200 °C, it decreases towards a constant minimum value close to zero (Hallikainen et al. 1985; Robinson et al. 2009, 2012). A more rapid decrease of ε" could occur for temperature higher than 100 °C in the case of wet soil due the loss of moisture through evaporation (Li et al. 2009).
The highest T values found for the soil with water content depends on the improvement of dielectric properties of the medium containing water. Water is an excellent MW absorber, and its presence gives an increase of dielectric properties. Therefore, dry soil is a low energy absorbent, but its dielectric constant values allow it to reach minimal soil temperature of about 100 °C, whereas the moisture content in soil has a major effect on the further final temperature increase reachable during MW heating (Hallikainen et al. 1985; Li et al. 2009).
Results obtained are in agreement with other literature findings. Li et al. (2009) reported a maximum temperature of about 200 °C reached during the MW treatment of a crude oil-polluted soil (7.8 % oil content, 3.1 % water content) at 800 W for 10 min. Analog T profiles were also observed by Lin et al. (2010), who investigated the effect of MW heating on soil temperature and removal of chloramphenicol (CAP) from polluted soils, and Diprose (2001) who reported that the moisture content of a soil sample has an effect on the final temperature. Change in moisture content alters the conductivity and the permittivity of the sample, and hence the strength of the electric fields in the material and the power dissipated in it. Water has a high loss factor, so relatively small differences in moisture contents between samples result in temperature difference between them.
3.2 Kinetics of diesel removal
Figure 3 shows the dynamics of residual diesel concentration (C) represented by C10–C25 adsorbed on soil with time, at powers of 100, 250, 440, 600, and 1,000 W, respectively. As expected, contaminant concentration in the soil after MW heating decreased with time for all the soils, and the rate of diesel desorption increased with the power applied.
For all the water contents investigated, minimal C values (less than 200 mg kg−1) were achievable only when applying the power of 600 W for a treatment time longer than 60 min. The lowest C reached was observed for the wet soils (12 and 8 % water content).
It was clearly shown that soil temperature is the main key factor in the remedial process, and that residual diesel concentration strictly depends on the maximum temperature that the soil can reach during the MW treatment. As a matter of fact, for times longer than 10 min, the variation of the soil temperature caused by the several treatments investigated results in a similar variation of contaminant amount removed. In fact, a soil temperature higher than 180 °C, reachable by applying a power higher than 600 W, results in a residual concentration lower than 200 mg kg−1. Therefore, the presence of water in soil results in a significant increase in soil temperature and, therefore, determines the best contaminant removal.
This is consistent with other literature results about conventional thermal desorption treatment. In our previous study (Falciglia et al. 2011b), we found that a soil temperature of 175 °C was sufficient to remediate a diesel-polluted fine sandy soil at a final diesel ration of about 150 mg kg−1, and that temperatures higher than 250 °C are necessary in order to obtain residual concentration less than 10 mg kg−1. Merino and Bucalá (2007) and Lee et al. (1998) also showed that at about 300 °C, n-hexadecane and diesel can be removed completely from a fine sandy soil.
C data were fitted to the logarithmic form of Eq. (5), and rate of contaminant decay (k) values are shown in Fig. 3. R 2 values calculated were in the 0.70–0.99 range, and highest R 2 values were found for the highest powers applied. Results obtained show that the removal kinetics of diesel fits a pseudo first order kinetic model well. The apparent kinetic constant (k) was in the 0.0045–0.0368 min−1 range for the soil without water content, and it increased to 0.0049–0.0419 and 0.0057–0.0442 min−1 ranges for the soil, with moisture of 8 and 12 %, respectively.
As shown in Fig. 4, k values increased with increasing power for all the soils due to the nature of the thermal process. The slopes of the k trend were different for the three soils treated, but similar values were observed for both the wet soils. Moreover, a slope reduction was found for power higher than 600 W. This explains the minimal increase of the MW removal process for a power of 1,000 W, with respect to the 600 W treatment. The different slopes observed for the soils with diverse water contents imply that the activation energy is correlated with the nature of the soil. Specifically, the lowest activation energy is required for moist soils, while the highest for dry soils. This is due to the highest dielectric properties of the soils containing water that results in an improvement of the performance of the heating treatment (Acierno et al. 2003).
Kinetic data obtained are in agreement with that reported by previous authors. Huang et al. (2011) found k values between 0.003 and 0.068 min−1 for a MnO2-enhanced MW treatment of PCB-polluted soil, investigating power values between 200 and 800 W. Liu and Yu (2006) reported k values between 0.089 and 0.148 min−1 for a MW treatment of PCB polluted-soil, enhanced with GAC addition (5 %), investigating power values between 300 and 700 W.
Knowing the residual concentration values as a function of operating power and treatment time could be significant in assessing the change in energy efficiency and cost of a MW thermal remedial process.
3.3 N-alkanes fractions distribution
After the contamination procedure, sorbed diesel on soil (C 0), as n-alkanes fractions (C10–C25), was measured and related percentage calculated as the ratio between the concentration of the single n-alkanes fraction Cn and the total concentration C10–C25. Results are illustrated in Table 2. The percentage distribution of single n-alkanes fractions presented the highest percentage for the fractions ranging from C14 to C17, with a typical “bell curve type” shape. However, it did not reflect the percentage composition of diesel used for the contamination procedure (C10–C13 fraction was 37.1 % for diesel, while it was 18.6 for soil). This indicated that all fractions did not similarly adsorb onto the different soil matrices, and affinity was observed between specific n-alkanes fraction and soil.
Residual diesel concentration (C) values as n-alkanes fractions after the 440 W treatment for unmoisturized and moisturized soils are also reported in Table 2.
Results show that a preferential effect of the thermal treatment on the various n-alkanes present in diesel occurred. The distribution of the n-alkenes in soil after MW heating at different remediation time did not reflect the initial distribution (C 0) of sorbed diesel. Therefore, the MW treatment produced a major desorption of the lightest n-alkanes fractions, and the range of n-alkanes fractions removed depended on the treatment time and thus on soil temperature and on the presence of water in the soils. An increase of lightest n-alkanes fractions removal was recorded with increasing remediation time, as well as in the case of moisturized soil samples. Nonsignificant differences were recorded between 8 and 12 % soil moisture.
For instance, the treatment of the soil without moisture produced a total removal of C10–C11 fractions for a treatment time of 60 min, whereas for the soils with moisture content of 8 and 12 %, a shorter remediation time of 30 min was needed. This indicates that soil moisture influences a major desorption of the lightest n-alkanes fractions, probably due to both the soil temperature increase and evaporation-contaminant stripping phenomena, that present a major influence on the more volatile contaminant fraction.
Results are in agreement with our previous work (Falciglia et al. 2011a), where the effects of temperature on n-alkanes fractions selective desorption were investigated during a conventional bench-scale thermal desorption treatment. Main results showed that an increase of lightest n-alkanes fractions was recorded with increasing soil temperature.
3.4 Diesel removal efficiency
Based on residual diesel concentration adsorbed in soil, contaminant removal efficiency (R) vs. time was also calculated for all soils, and data obtained are shown Fig. 5. It can be seen that the higher MW powers lead to higher remediation efficiency. At all tested operating powers, maximum R values were observed for soils with 8 and 12 % water content. The lowest differences of R between the three soils were observed at the lowest power investigated and the highest for the 250 W treatment. When the power applied was higher than 600 W, a minimal difference of R was observed for the two moist soils. This specific behavior resulted in similar temperature profile for both the soils because the radiation energy applied at 600 W and over was sufficient to remove all the water (Table 3).
For moist soils, excellent efficiency was reached at power higher than 600 W for time longer than 30 min. A maximum R value of 95 % was reached for both the moist soils treated at 1,000 W for 60 min. A lower value of 90 % was reached for dry soil at the same operating conditions. For time shorter than 18 min, R was lower than 77 % (i.e., 77, 74, and 63 % for 12, 8, and 0 % water content, respectively).
Moreover, it is important to highlight that, at the highest powers, especially for the first 10 min, a significant contaminant removal increase was observed for the wet soils compared to dry soil due to the evaporation and contaminant stripping phenomena. This is clear by analyzing the residual soil moisture content after the MW treatment reported in Table 3. In fact, despite the soil temperature being in the same range for all soils, a significant difference in terms of contaminant removal was recorded. This was also observed for the treatments at the lowest powers of 100 and 250 W, for which, despite the soil temperature being about 100 °C, removals up to 60 % were achieved. For soils contaminated by polar compounds, this phenomenon could be the effect of a selective heating but, for nonpolar contaminants such as diesel, this could be ascribable just to a distillation process (Kawala and Atamaczuk 1998; Robinson et al. 2009).
Removal results are consistent with those found by Liu et al. (2008). They investigated the effect of a 10 min MW remediation at 750 W on a 20 g PCB-polluted soil sample, to which a microwave absorber had been added. They showed an increase in contaminant removal, with increasing soil moisture, to maximum values close to 100 % at a moisture content of 20 % (dry basis). A removal efficiency enhanced by water presence in soil was also observed by Yuan et al. (2006).
Overall, R values obtained in the performed experiment are in the same range as that reported by previous literature findings on MW treatment of hydrocarbon polluted soil, but the extremely different operating conditions adopted and the several dielectric materials used make a direct and effective comparison of the results very difficult (Robinson et al. 2012).
Huang et al. (2011) demonstrated that the maximum removal efficiency for a soil polluted by PCBs at 5 mg kg−1 and treated at 800 W for a period of 45 min, is about 95 %. Very high PCB removal was also found by Abramovitch et al. (1998a), investigating several operating conditions and the properties of the materials.
Removal efficiency of crude oil contaminant of 95 % (initial contaminant concentration in soil = 7.8 %) was observed for a 15 min at 800 W treatment enhanced by different microwave absorbers such as activated carbon powder or graphite fibers (Li et al. 2009). Contaminant removal higher than 90 % was also found by Lin et al. (2010), Calvert and Suib (2007), Robinson et al. (2009), and Kawala and Atamaczuk (1998), in studies aimed at investigating the effects of the MW heating on soils polluted by CAPs, HCB, PAHs, and TCE, respectively, in treatments enhanced by several MW absorbing materials or solutions.
High diesel removal efficiencies observed for MW heating remediation are hardly achievable using other economical treatments such as natural biodegradation or even using more expensive treatments such as oxidation with chemical agents or ozone. However, high removal efficiencies comparable to MW treatment can be reached by using soil washing with surfactants (Khalladi et al. 2008) or by using remediation treatments such as electrokinetic-Fenton oxidation (Tasi et al. 2010) or conventional thermal desorption remediation (Falciglia et al. 2011b), which require a higher energy consumption and consequently a higher cost.
In recent studies, Silva-Castro et al. (2013) reported a maximum removal of contaminant as n-alkanes of about 70 % after a combined treatment of oxidation and bioremediation, whereas Pazos et al. (2012) reported a maximum TPH removal of 78 % for an electrokinetic treatment. Łebkowska et al. (2011) found a 50 % removal of diesel as n-alkanes for a sandy soil polluted by diesel at 4,200 mg kg−1 of C10–C22 alkanes treated by ex situ biopile remediation for a period of about 1 month. Sprocati et al. (2012) carried out a study on bioaugmentation aimed at the remediation of a soil co-contaminated (spiked) with both diesel oil (1 %, v/w), and heavy metals (Pb and Zn), using microcosms in different experimental conditions. Authors reported an effective bioremediation of diesel ranging between 30 and 85 %, assessing the contamination dynamic by n-alkanes C15–C28 and C24. Fernández et al. (2011) observed a diesel removal of about 50 % for a soil contaminated at a low diesel rate of 5,800 μL kg−1 treated using a microcosm with plants and earthworms for a period of 6 months. Minimal percentages, not higher than 37 %, were observed for the highest contaminated samples. Li et al. (2009) reported that for a sandy soil spiked with diesel fuel at different rates (ranging from 500 to 50,000 mg kg−1), the TPHs natural biodegradation, for an incubation period of 110 days, reached a maximum value of about 73 % for the lowest contamination levels, whereas it was less than 70 % for the 5,000 mg kg−1 samples. Do et al. (2009) showed that an in situ chemical oxidation treatment of a diesel-polluted soil at 5,000 mg kg−1, using peroxymonosulphate/cobalt (PMS/CoII), was characterized by a maximum contaminant degradation of approximately 47 %, and that a sequential injection treatment using a large quantity of chemicals was needed to reach a contaminant degradation of 88 %. Moreover, Lee and Kim (2002) found that a diesel removal efficiency of 40 % (30 % as n-alkanes) was obtainable by oxidation with ozone of a heavily-contaminated sandy soil.
Therefore, MW heating could be a better choice for remediation of diesel-polluted soils.
4 Conclusions
The following conclusions have been drawn based on experimental results and discussion:
-
In MW remediation, the operating power applied significantly influences the contaminant removal kinetics, and the moisture content in soil has a major effect on the final temperature reachable during MW heating. Therefore, soil moisture is essential in order to reach high contaminant removal efficiency, but a water content of 8 % is enough to maximize the performance of the remedial process.
-
For all water contents investigated, minimal residual concentrations (less than 200 mg kg−1) were achievable only by applying powers higher than 600 W for a treatment time longer than 60 min.
-
For remediation times shorter than 10 min, which result in a soil temperature of about 100 °C, the effect of the distillation process increases the contaminant removals that reached values of about 60 % for wet soils due to the evaporation and contaminant stripping phenomena. For times longer than 10 min, soil temperature is the main key factor in the remedial process, and residual diesel concentration strictly depends on the maximum temperature that the soil can reach during the MW heating.
-
The pseudo first order kinetic model fits well the experimental data for residual concentration at all powers of treatment and for all tested soils. Kinetic parameters for the different experimental conditions could represent a valuable tool in calculating residual concentration or desorption rate at any given initial soil concentration and in identifying the operating power and the time of treatment required to reach specific targeted levels of remediation. This is fundamental in designing and scaling-up desorption systems and then in assessing the change in energy efficiency and cost of a MW remedial process.
-
MW remedial treatment could be a better choice for remediation of diesel-polluted soils, compared to several other biological, chemical–physical, or conventional thermal treatments due to its excellent removal efficiency.
References
Abramovitch RA, Bangzhou H, Davis M, Peters L (1998a) Decomposition of PCB's and other polychlorinated aromatics in soil using microwave energy. Chemosphere 37:1427–1436
Abramovitch RA, Bangzhou H, Abramovitch DA, Jiangao S (1998b) In situ decomposition of PAHs in soil and desorption of organic solvents using microwaves energy. Chemosphere 39:81–87
Acierno D, Barba AA, d’Amore M (2003) Microwaves in soil remediation from VOCs. 1: heat and mass transfer aspects. Environ Energy Eng 49:1909–1921
Acierno D, Barba AA, d’Amore M, Pinto IM, Fiumara V (2004) Microwaves in soil remediation from VOCs. 2: buildup of a dedicated device. Environ Energy Eng 50:722–732
ASTM (2008) Standard Test Method D 2216–05 for Laboratory Determination of Water (Moisture) Content of Soil and Rock by Mass. ASTM International, Philadelphia
Barba AA, Acierno D, d’Amore M (2011) Use of microwaves for in situ removal of pollutant compounds from solid matrices. J Hazard Mater 207–208:128–135
Bento FM, Camargo AO, Okeke BC, Frankenberger WT (2005) Comparative bioremediation of soil contaminated with diesel oil by natural attenuation, bio stimulation, and bioaugmentation. Bioresour Technol 96:1049–1055
Calvert CA, Suib SL (2007) An initial study into the use of microwave remediation of hexachlorobenzene-treated soil using selected oxidants and coated graphite rods. J Soils Sediments 7:147–152
Careghini A, Dastoli S, Ferrari G, Saponaro S, Bonomo L, De Propris L, Gabellini M (2010) Sequential solidification/stabilization and thermal process under vacuum for the treatment of mercury in sediments. J Soils Sediments 10:1646–1656
Chien Y (2012) Field study of in situ remediation of petroleum hydrocarbon contaminated soil on site using microwave energy. J Hazard Mater 199–200:457–461
Clark DE, Folz DC, West JK (2000) Processing materials with microwave energy. Mater Sci Eng A287:153–158
Darayan S, Liu C, Shen LC, Shattuck D (1998) Measurement of electrical properties of contaminated soil. Geophys Prospect 46:477–488
Dauerman L, Windgasse G, He Y, Lu Y (1992) Microwave treatment of hazardous wastes: feasibility studies. The Hazadous Management Research Center, New Jersey Institute of Technology Newark, New Jersey
Di P, Chang DPY (2001) Investigation of polychlorinated biphenyl removal from contaminated soil using microwave-generated steam. J Air Waste Manage Assoc 51:482–488
Diprose MF (2001) Some considerations when using a microwave oven as a laboratory research tool. Plant Soil 229:271–280
Do SH, Jo JH, Jo YH, Lee HK, Kong SH (2009) Application of peroxymonosulfate/cobalt (PMS(Co(II)) system to treat diesel-contaminated soil. Chemosphere 77:1127–1131
Dobson MC, Hallikainen MT, Ulaby FT, El-Rayes MA (1985) Microwave dielectric behavior of wet soil—part 2: dielectric mixing models. Ieee Transact. Geosci Remote Sens GE-23:25–34
Falciglia PP, Giustra MG, Vagliasindi FGA (2011a) Influence of soil texture on contaminant adsorption capacity and removal efficiency in ex situ remediation of diesel-polluted soil by thermal desorption. Chem Ecol 27–1:119–130
Falciglia PP, Giustra MG, Vagliasindi FGA (2011b) Low temperature thermal desorption of diesel-polluted soil: influence of temperature and soil texture on contaminant removal kinetics. J Hazard Mater 185:392–400
Fernández MD, Pro J, Alonso C, Aragonese P, Tarazona JV (2011) Terrestrial microcosms in a feasibility study on the remediation of diesel-contaminated soils. Ecotoxicol Environ Saf 74:2133–2140
George CE, Azwell DE, Adams PA, Rao GVN (1995) Evaluation of steam as a sweep gas in low temperature thermal desorption processes used for contaminated soil clean up. Waste Manag 15:407–416
Ha S, Choi K (2010) A study of a combined microwave and thermal desorption process for contaminated soil. Environ Eng Res 15:225–230
Hallikainen MT, Ulaby FT, Dobson MC, El-Rayes MA, Wu LK (1985) Microwave dielectric behavior of wet soil—part 1: empirical models and experimental observations. IEEE Trans Geosci Remote Sens GE-23:25–34
Huang G, Zhao L, Dong Y, Zhang Q (2011) Remediation of soils contaminated with polychlorinated biphenyls by microwave-irradiated manganese dioxide. J Hazard Mater 186:128–132
Jones DA, Lelyveld TP, Mavrofidis SD, Kingman SW, Miles NJ (2002) Microwave heating applications in environmental engineering: a review. Resour Conserv Recycl 34:75–90
Kawala Z, Atamaczuk T (1998) Microwave-enhanced thermal decontamination of soil. Environ Sci Technol 32:2602–2607
Khalladi R, Benhabiles O, Bentahar F, Moulai-Mostefa N (2008) Surfactant remediation of diesel-polluted soil. J Hazard Mater 164:1179–1184
Łebkowska M, Zborowska E, Karwowska E, Miaskiewicz-Peska E, Muszynski A, Tabernacka A, Naumczyk J, Jeczalik M (2011) Bioremediation of soil polluted with fuels by sequential multiple injection of native microorganisms: field-scale processes in Poland. Ecol Eng 37:1895–1900
Lee BT, Kim KW (2002) Ozonation of diesel fuel in unsaturated porous media. Appl Geochem 17:1165–1170
Lee JK, Parka D, Kimb B, Dongb J, Lee S (1998) Remediation of petroleum-contaminated soils by fluidized thermal. Waste Manag 18:503–507
Li D, Zhang Y, Quan X, Zhao Y (2009) Microwave thermal remediation of crude oil-contaminated soil enhanced by carbon fiber. J Environ Sci 21:1290–1295
Lin L, Yuan S, Chen J, Wang L, Wan J, Lu X (2010) Treatment of chloramphenicol-contaminated soil by microwave radiation. Chemosphere 78:66–71
Liu X, Yu G (2006) Combined effect of microwave and activated carbon on the remediation of polychlorinated biphenyl-contaminated soil. Chemosphere 63:228–235
Liu X, Zhang Q, Zhang G, Wang R (2008) Application of microwave irradiation in the removal of polychlorinated biphenyls from soil contaminated by capacitor oil. Chemosphere 72:1655–1658
Merino J, Bucalá V (2007) Effect of temperature on the release of hexadecane from soil by thermal treatment. J Hazard Mater 143:455–461
Pazos M, Plaza A, Martín M, Lobo MC (2012) The impact of electrokinetic treatment on a loamy-sand soil properties. Chem Eng J 183:231–237
Robinson JP, Snape CE, Kingman SW, Shang H (2008) Thermal desorption and pyrolysis of oil-contaminated drill cuttings by microwave heating. J Anal Appl Pyrol 81:27–32
Robinson JP, Kingman SW, Snape CE, Shang H, Barranco R, Saeid A (2009) Separation of polyaromatic hydrocarbons from contaminated soils using microwaves heating. Sep Purif Technol 69:249–254
Robinson JP, Kingman SW, Lester EH, Yi C (2012) Microwave remediation of hydrocarbon-contaminated soils—scale up using batch reactors. Sep Purif Technol 96:12–19
Silva-Castro GA, Rodelas B, Perucha C, Laguna J, González-López J, Calvo C (2013) Bioremediation of diesel-polluted soil using biostimulation as posttreatment after oxidation with Fenton-like reagents: assays in a pilot plant. Sci Total Environ 445–446:347–355
Sprocati AR, Alisi C, Tasso F, Marconi P, Sciullo A, Pinto V, Chiavarini S, Ubaldi C, Cremisin C (2012) Effectiveness of a microbial formula, as a bioaugmentation agent, tailored for bioremediation of diesel oil and heavy metal co-contaminated soil. Process Biochem 47(11):1649–1655
Tatáno F, Felici F, Mangani F (2013) Lab-scale treatability tests for the thermal desorption of hydrocarbon-contaminated soils. Soil Sediment Contam 22:433–456
Tsai TT, Sah J, Kao CM (2010) Application of iron electrode corrosion-enhanced electrokinetic-Fenton oxidation to remediate diesel-contaminated soils: a laboratory feasibility study. J Hydrol 380:4–13
US-EPA (2004) Treatment technologies for site cleanup: Annual status report (eleventh edition), Solid Waste and Emergency Response. EPA-542-R-03-009
Uzgiris EE, Edelstein WA, Philipp HR, Iben IET (1994) Complex thermal desorption of PCBs from soil. Chemosphere 30:377–387
Yuan S, Tian M, Lu X (2006) Microwave remediation of soil contaminated with hexachlorobenzene. J Hazard Mater B137:878–885
Acknowledgments
This research was funded by the Italian Ministry of Education, University and Research (MIUR), and “Research Program of Relevant National Interest” (conventional and novel remediation thermal desorption treatments of hydrocarbons polluted soil).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Willie Peijnenburg
Rights and permissions
About this article
Cite this article
Falciglia, P.P., Urso, G. & Vagliasindi, F.G.A. Microwave heating remediation of soils contaminated with diesel fuel. J Soils Sediments 13, 1396–1407 (2013). https://doi.org/10.1007/s11368-013-0727-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-013-0727-x