Abstract
Purpose
Nitrate (NO −3 ) is often considered to be removed mainly through microbial respiratory denitrification coupled with carbon oxidation. Alternatively, NO −3 may be reduced by chemolithoautotrophic bacteria using sulfide as an electron donor. The aim of this study was to quantify the NO −3 reduction process with sulfide oxidation under different NO −3 input concentrations in river sediment.
Materials and methods
Under NO −3 input concentrations of 0.2 to 30 mM, flow-through reactors filled with river sediment from the Pearl River, China, were used to measure the processes of potential NO −3 reduction and sulfate (SO 2−4 ) production. Molecular biology analyses were conducted to study the microbial mechanisms involved.
Results and discussion
Simultaneous NO3 − removal and SO4 2− production were observed with the different NO −3 concentrations in the sediment samples collected at different depths. Potentially, NO −3 removal reached 72 to 91 % and SO 2−4 production rates ranged from 0.196 to 0.903 mM h−1. The potential NO −3 removal rates were linearly correlated to the NO −3 input concentrations. While the SO 2−4 production process became stable, the NO −3 reduction process was still a first-order reaction within the range of NO −3 input concentrations. With low NO −3 input concentrations, the NO −3 removal was mainly through the pathway of dissimilatory NO −3 reduction to NH +4 , while with higher NO −3 concentrations the NO −3 removal was through the denitrification pathway.
Conclusions
While most of NO −3 in the sediment was reduced by denitrifying heterotrophs, sulfide-driven NO −3 reduction accounted for up to 26 % of the total NO −3 removal under lower NO −3 concentrations. The vertical distributions of NO −3 reduction and SO 2−4 production processes were different because of the variable bacterial communities with depth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Anthropogenic activities have dramatically increased nitrogen (N) loading to aquatic ecosystems, particularly in the highly developing and densely populated regions (Galloway et al. 2004; Boyer et al. 2006). The N loading has resulted in considerable increase in nitrate (NO −3 ) concentrations in rivers, contributing to coastal eutrophication and hypoxia (Qiu et al. 2010). Nevertheless, most of the N loading to terrestrial soils and freshwater disappears before reaching coastal waters (Forshay and Stanley 2005; Seitzinger et al. 2006). These changes in N cycling lead to the question: what processes are involved in N removal from rivers?
Most attention has been devoted to study NO −3 removal by respiratory denitrification with organic carbon as an electron donor, and dissimilatory NO −3 reduction to ammonium (DNRA) by fermentative bacteria (Burgin and Hamilton 2007). Alternatively, autotrophic denitrifiers can use NO −3 to oxidize sulfide and elemental S to SO 2−4 and their biogeochemical importance has been recognized (Jørgensen and Gallardo 1999; Shao et al. 2010). Nevertheless, most research activities have been focused on sulfide-driven autotrophic denitrifiers in marine sediments (Fossing et al. 1995; Sayama et al. 2005; Zhang et al. 2009). Several taxa, including the members of the genera Thiobacillus, Thiocapsa, and Beggiatoa, have been established with diverse metabolic characteristics (Brettar and Rheinheimer 1991; Jørgensen and Gallardo 1999; Kojima and Fukui 2003). More recently, the importance of sulfide-driven autotrophic denitrifiers in freshwater ecosystems has been investigated. For example, Kamp et al. (2006) enriched the Beggiatoa from a NO −3 -rich stream and observed its ability to oxidize sulfide with NO −3 . Burgin and Hamilton (2008) studied the pathways of sulfide-driven NO −3 reduction in freshwater bodies. Payne et al. (2009) revealed the coupling respond of N and S in wetlands.
There are two pathways for NO −3 removal through sulfide oxidation: reducing NO −3 to N2 in the form of denitrification; and reducing NO −3 to NH +4 in the form of DNRA (Burgin and Hamilton 2008). Environmental factors in river sediment, such as sediment depth, NO −3 concentrations, and bacterial population and activities, should affect the NO −3 removal process with S oxidation and the associated pathways. However, the potential and mechanisms of sulfide-driven NO −3 reduction in river sediment are still poorly understood. Therefore, the objectives of this study were: (1) to investigate the potential rates of sulfide-driven NO −3 reduction with different NO −3 input concentrations in different depths of river sediment; and (2) to explore the dominate pathways of the sulfide-driven NO −3 reduction process.
2 Materials and methods
2.1 Sample collection
Sediment samples were collected from a drain outlet (113°17′1.66″E, 23°6′50.41″N) of the Pearl River in Guangzhou, China. The section Pearl River crossing Guangzhou City is close to the Pearl River Estuary, which links the highly developing urban area and the South China Sea, and represents an important ecosystem. In recent years, the Pearl River has been subjected to a high load of anthropogenic contaminants from wastewater runoff because of the increasing population and economic development in the Pearl River Delta (Jiang et al. 2009; Lu et al. 2009). The river is also affected by tides of the South China Sea.
On July 14th, 2010, two sediment cores were collected using a core sampler with a diameter of 7.0 cm and length of 50 cm. Water samples above the sediment–water interface, and the top 30 cm of the sediment cores were retained for the following experiments: (1) one of the sediment cores was used for flow-through reactor (FTR) experiments (described below); and (2) the other core was used to analyze chemical and microbial conditions of the sediment before the FTR experiments.
2.2 Flow-through reactor experiment
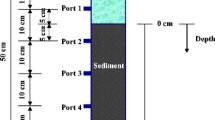
Six FTRs in this study were constructed following Pallud et al. (2007). The inner diameter of the reactor cell was the same as that of the sediment core (7.0 cm). A buffer room and a sintered disk were included to homogenize the influent. The 30 cm sediment core was cut into slices at depths of 0–2, 5–7, 10–12, 15–17, 20–22 and 25–27 cm. Each sediment slice was put into the polyvinyl chloride cell of the FTR with nitrocellulose filter (0.22 μm pore size) on each side.
The FTR experiments were conducted in an incubator, in which the temperature was set at 28 °C based on the temperatures of bottom water in the river (range from 12 to 28 °C). The influent of FTR was controlled at a constant flow rate (15 cm3 h−1) using a peristaltic pump. Input solutions included five NO −3 concentrations (0.2, 1, 5, 15 and 30 mM) and one Br− solution (1 mM). The NO −3 input concentrations were selected based on the literature (e.g., Laverman et al. 2007; Pallud et al. 2007). To ensure an anaerobic condition in the cell, each reactor was wrapped with airproof material and the input solution was vigorously purged with argon gas before entering the cell. For each input solution, the experiment was carried out until reaching a steady-state outflow concentration (about 20 h). The Br− breakthrough curves were used to obtain the transport parameters (Pallud et al. 2007).
The steady-state rate of NO −3 reduction or SO 2−4 production was calculated as follows:
where: C i is the input concentration; C o is the steady-state concentration in the outflow; Q is the volumetric flow rate; and V is the volume of the sediment slice in the reactor.
2.3 Analysis of environmental parameters
Water content of the sediment samples was measured using the gravimetric method. Concentrations of Br−, NO −2 , NO −3 and SO 2−4 of the water samples, interstitial water samples from the sediment sub-samples, and the outflow samples of the FTR experiments were measured using ion chromatography (Metrohm 882, Metrohm AG, Herisau, Switzerland), and NH +4 using spectrophotometric detection with Nessleri’s reagent. Total organic carbon content (TOC) was determined with the potassium dichromate dilution heat colorimetric method and the total nitrogen (TN) content was determined using a Foss Kjeltec 2300 Analyzer Unit (Foss Tecator AB, Höganäs, Sweden). Analyses of acid-volatile sulfur (AVS) concentrations in the sediment were based on the cold-acid purge-and-trap method (Chen et al. 2006).
2.4 Molecular biology analysis
2.4.1 DNA extraction and PCR amplification
Total DNA was extracted from each sediment slice with Fast DNA spin kit (Bio 101, Qbiogene Inc., CA, USA) following manufacturer’s instruction manual. The following primer sets were used for PCR amplification of the genes encoding 16S rRNA: the forward primer 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) labeled at the 5′ end with the dye carboxyfluorescein (FAM; synthesized within the primer by Genolab Co., Ltd., China.) and the reverse primer 1492R (5′-GGTTACCTTGTTACGACTT-3′). The PCR mixture contained 50 ng of extracted DNA, 2 μl of 5 mM concentrations of each primer, 3.2 μl of 2.5 mM concentrations of dNTP, 0.4 μl of 5 U/μl TaKaRa Taq DNA polymerase (TaKaRa Bio Inc., Shiga, Japan), and 5 μl of 10× PCR buffer for TaKaRa Taq, then replenish with ddH2O to 50 μl. The PCR amplifications were performed in a total volume of 50 μl in 0.2 ml reaction tubes using the PCR reactor (T-gradient, Biometra, USA) with the following procedure, 94 °C for 5 min and then 30 cycles each consisting of 30 s at 94 °C, 30 s at 55 °C; and 45 s at 72 °C; and finally 10 min at 72 °C to complete the primer extension. The presence of PCR products was shown using 1 % agarose gel electrophoresis.
2.4.2 Terminal-restriction fragment length polymorphism analysis
After the processes of purification and concentration of the PCR products with a Wizard SV gel and a PCR clean-up system (AxyPrep PCR clean kit, China), 5 μl of the amplicons were digested with 10 U of the restriction enzyme HaeIII, HhaI, and MspI (TaKaRa, Japan), respectively, in the manufacturer’s recommended reaction buffers for 4 h at 37 °C. Enzymes were subsequently inactivated by incubation at 65 °C for 20 min. Cleaved PCR products were purified by ethanol precipitation and dissolved in 20 μl of sterile ddH2O. Ten μl of the purified products together with 0.5 μl of internal standard (ROX-500) were denatured at 95 °C for 2 min, cooled on ice, and subject to electrophoresis on a ABI 3730XL DNA analyzer (Applied Biosystems, USA). After electrophoresis, the size of the fluorescently labeled terminal-restriction fragments (T-RFs) was determined by comparing with the size standard using the software GeneMarker 1.97. To avoid detecting primers and uncertainties in size determination, terminal-restriction fragment lengths smaller than 50 bp were excluded. The percentage of detected T-RF was calculated from the peak height to the total peak height (>1 %). T-RFs were identified by the T-RFLP analysis package built by the Center for Limnology, University of Wisconsin–Madison, USA (https://secure.limnology.wisc.edu/trflp), then the taxonomy information was searched from the U.S. National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov).
2.4.3 Real-time PCR analysis
The primers selected for amplification of the different genes encoding narG, nirS, nirK, nosZ and nrfA are narG328f–narG497r (Reyna et al. 2010), nirS3f–nirS5r (Braker et al. 1998), nirK2f–nirK3r (Thräback et al. 2004), nos1527f–nos1773r (Scala and Kerkhof 1998), and nrfA2F–nrfA2R (Smith et al. 2007). Each assay contained a standard using a serial dilution of known copies of PCR fragments of the respective functional genes, independent triplicate sediment DNA templates for each sediment slice, and triplicate no template controls. Experimental Q-PCR triplicates for each DNA sample were then averaged to obtain a single gene copy number. Real-time PCRs were carried out in LightCycler480 with Sequence Detection Software v1.4 (Applied Biosystems, USA). Each PCR mixture (10 μl) was composed of 5 μl of SYBR Premix Ex TaqTM II (2×), 0.4 μl 10 nM of each forward and reverse primers, 0.2 μl ROX Reference Dye II (50×) × 3, 3.2 μl ddH2O and 2.0 μl of template DNA (TaKaRa Biotechnology, Japan). PCR amplification and detection were performed in LightCycler480 Multiwell (384-well) reaction plates with optical cap (Applied Biosystems, USA). The PCR procedure was as follows, 30 s at 94 °C, 40 cycles of 5 s at 94 °C; 30 s at the specific annealing temperature (61, 57, 57, 57, and 60 °C for narG, nirS, nirK, nosZ, and nrfA, respectively); and 30 s at 70 °C. A melting curve analysis for SYBR Green assay was conducted after the amplification to distinguish the targeted from the non-targeted PCR product.
3 Results
3.1 Physical and chemical characteristics of the sediment core
Physical and chemical properties of the sediment core are listed in Table 1, including water content, porosities, concentrations of NO −3 , NH +4 , TN, TOC and AVS of the sediment, and concentrations of NO −3 and NH +4 in the interstitial water. The water contents and porosities decreased from 67 % to 29 % and from 82 % to 54 %, respectively, with increasing core depths. The NO −3 concentrations in the sediment and interstitial water reached the maximum values (0.056 mmol kg−1 and 0.0802 mM, respectively) at a depth of 10-12 cm, then decreased with the depth. The NH +4 concentrations were much larger than the NO −3 concentrations and the maximum NH +4 values in the sediment and interstitial water (39.0 mmol kg−1 and 5.48 mM, respectively) also occurred at a depth of 10-12 cm. The TOC and TN values were highly correlated with a coefficient of determination (R 2) of 0.934 (p < 0.05) and their peak values were also at 10–12 cm depth. The AVS concentration in the surface layer (12.0 mmol kg−1) was significantly lower than in deeper layers (see Table 1). The highest AVS (33.7 mmol kg−1) was in the 15–17 cm layer, while the AVS values in other layers ranged from 25.0 to 27.6 mmol kg−1.
3.2 Nitrate removal in the flow-through reactor
Nitrate removal was observed in the FTR experiments with the different NO −3 input concentrations. The NO −3 removal in the sediment samples at the different depths ranged from 72 to 91 %. Higher NO −3 removal percentages were observed with the lower input concentrations (0.2 and 1 mM). With the increase of input concentrations, the removal percentages of NO −3 decreased from 85 to 75 %. The variability of the NO −3 removal percentages among the different sediment depths was small, as indicated by the small range of the quartile intervals (0.68 to 1.58 %).
The potential NO −3 removal rates in the FTRs were linearly related to the NO −3 concentrations in the reactor with high R 2 values, ranging from 0.9974 to 0.9996. For all the sediment samples at the different depths, the linear relationships were almost identical with slopes ranging from 0.223 to 0.230 and intercepts ranging from 0.0365 to 0.0540. All the linear relationships can be represented by the following relationship:
where: R p is the potential NO −3 removal rates; and C r is the NO −3 concentration in the reactor, calculated by the average of the input and output NO −3 concentrations. The equation indicated that, in this range of input concentrations (0.2–30 mM), the potential NO −3 removal rates in all layers of the sediment were of a first-order reaction with the maximum removal rate of 3.877 mM h−1.
3.3 Sulfate production in the flow-through reactor
When the NO −3 input concentration increased from 0.2 to 30 mM, SO 2−4 production values increased correspondingly (Table 2). The SO 2−4 production rates in all sediment depths also increased significantly with the NO −3 removal (Fig. 1). The SO 2−4 production rates were more variable among the different depths compared to the NO −3 removal rates. In particular, when the NO −3 input concentrations ≥5 mM, the differences of SO 2−4 production rates among the depths became more obvious. At a depth of 20–22 cm, the SO 2−4 production rates increased significantly with the NO −3 input concentrations, while at a depth of 10–12 cm the SO 2−4 production rates did not respond to the NO −3 inputs as strongly as at other depths. The SO 2−4 production rates decreased from the surface to 10–12 cm then increased at deeper depths.
3.4 Ammonium production in the flow-through reactor
In contrast to the SO 2−4 production, NH +4 production did not increase with the increased NO −3 concentrations (Fig. 2). On average, the highest NH +4 production was about 0.342 mM and was reached at the low NO −3 input concentration (0.2 mM). Subsequently, NH +4 production decreased with increased NO −3 input concentrations and became stable (0.198 to 0.217 mM) with the higher NO −3 input concentrations.
3.5 The abundances of denitrification functional genes
The vertical distributions of denitrification functional genes (narG, nirS, nirK, and nosZ) before and after the FTR experiments were markedly different (Figs. 3a–d), while the nrfA gene could not be quantified due to its poor amplification efficiency. The narG gene encoded to NO −3 reductase significantly increased from 1.85 × 103 to 5.72 × 103 g−1 sediment on average after the FTR experiments, especially for the top three layers. The nirS gene encoded to nitrite (NO −2 ) reductase (a cytochrome cd1) increased after the FTR experiments within the top and bottom layers. In contrast, the nirK gene encoded to another type of NO −2 reductase (a Cu-containing enzyme) reduced by one to two orders of the magnitude, with very small variability with depth. Similarly, the nosZ gene encoded to nitrous oxide reductase declined dramatically from 1.21 × 103 to 0.39 × 103 g−1 sediment.
3.6 Analysis of terminal-restriction fragment length polymorphism
The microbial populations were characterized by the analysis of T-RFLP profiles using 16S rRNA. Its relative abundance was based on the T-RFLP profiles digested by restriction of the enzyme HaeIII. To identify the probable microbial species, the information on terminal-restriction fragment digested by HhaI and MspI was used conjointly with that for HaeIII. After the FTR experiment, at least one prominent peak (the length of the predominant T-RFs) became more prominent. Overall, two peaks, accounting for the most proportion of integral areas, appeared in five reactors except the one at 20–22 cm depth. Peak 329 bp in T-RFLP fingerprints was observed in the reactors with sediment samples at 0–2, 5–7, and 25–27 cm depth, accounting for 34, 12, and 1.4 % of their total integral areas, respectively. Peak 334 bp was observed in the reactors at 10-12 and 15-17 cm depth, accounting for 67 % and 72 % of total integral areas, respectively. Peaks of 329 and 334 bp matched well Lactobacillus. Highly similar gene fingerprints were observed after the FTR experiment, suggesting that the same population of bacteria had been colonized in the different sediment layers by the stimulation of NO −3 . In contrast to the ubiquitous peaks, the main T-RFs were observed only in some layers. For example, Thiobacillus and Thiocapsa matched with peaks of 78 and 243 bp in the layers at 10–12, 15–17, and 25–27 cm depth, while Beggiatoa (matched with peak 189 bp) appeared at 0–2 and 5–7 cm depth after the FTR experiments. A significant proportion of the different T-RFLP peaks were observed only at 20–22 cm depth. Spirochaeta halophila (M88722, matched with peak 206 bp), which was closely related to green sulfur bacteria (Woese et al. 1990), accounted for 8.9 % of total integral area. This was a high percentage compared with the sulfide oxidizing bacterium genus above.
4 Discussion
4.1 Vertical distributions of C, N, and S in the sediment core
The vertical distributions of nutrients should influence bacterial communities at different sediment depths (Fan et al. 2006; Huang et al. 2011). Long-term domestic wastewater pollution has resulted in organic matter accumulation in the sediment, which provides adequate nutrition for microbial growth. Thus, the high activities of microbes should influence the distributions and transformation of the inorganic N. Generally, NO −3 and TOC decrease with sediment depth, and the denitrification of deeper sediments can be neglected due to substrate limitation (Tiquia et al. 2006). However, the vertical distribution of inorganic N in our sediment had a peak value at 10–12 cm depth instead of at the surface. The NO −3 concentrations of the deep layers were similar to those of the top layers. Therefore, N cycling might also be active in the deeper sediment layers. Nitrite was below detection in most samples. Nevertheless, the NH +4 concentrations were higher than those in other sediments (Magalhães et al. 2005; Fan et al. 2006; Strous et al. 2006). The high NH +4 concentrations might result from active mineralization of organic N (Farías et al. 2004), which is typical for an urban river. In the sediment, the high concentrations of NH +4 , which is the substrate of nitrification and product of dissimilation, may influence the rate and pathway of microbial processes (Ma and Aelion 2005). The AVS concentrations in our sediment were comparable with those in other river sediments collected in the Pearl River Delta, China (Sheng et al. 2011) and were higher than those in the Pearl River Estuary (Chen et al. 2006).
4.2 Nitrate removal with sulfate production
Nitrate removal is generally considered to be a beneficial process in aquatic ecosystems (Brunet and Garcia-Gil 1996). Nitrate removal also enhances the process of sulfide oxidation. The NO −3 input in the FTR experiments showed high percentages of NO −3 removal and high NO −3 potential removal rates. The potential NO −3 removal rates reported here were higher than those in other fresh water sediments and in marine sediments (Hordijk et al. 1987; Laverman et al. 2006, 2007; Pallud et al. 2007). In the FTR experiments, the NO −3 removal rates remained as a first-order reaction when the NO −3 input concentrations increased 150 times (from 0.2 to 30 mM). The high initial concentration of TOC at the study site supported labile products from organic matter decomposition, which was beneficial to the NO −3 removal process. At our site in the subtropical zone, the high water temperature promoted the NO −3 removal process (Piña-Ochoa and Álvarez-Cobelas 2006; Silvennoinen et al. 2008; Li et al. 2010). Based on the literature, the wide range of the NO −3 input concentrations was selected to study the potential NO −3 removal rates in the sediment. In addition, NO −3 concentrations of overlying water in certain water bodies, such as in Pearl River (Dai et al. 2006) and Marne River, France (Laverman et al. 2010), are within the range of the NO −3 input concentrations in this study. Therefore, the results here should be useful to estimate the actual NO −3 removal rates.
Sulfate production coincident with NO −3 removal was observed at all depths and all NO −3 input concentrations in the FTR experiments (see Table 2), which was evidence of the sulfide-driven NO −3 reduction process. The SO 2−4 production was highly related to the NO −3 input, indicating that the biologically mediated process, instead of the O2-driven S oxidation process, promoted the sulfide-driven NO −3 reduction process. In addition, the experiments were conducted under an anaerobic condition. In our experiments, the NO −3 addition stimulated NO −3 reducers and acted as an electron acceptor in anaerobic metabolism for sulfide oxidizers, ultimately enhancing production of SO 2−4 . The sulfide-driven NO −3 reduction process was consistent with that reported in the literature (Burgin and Hamilton 2008; Payne et al. 2009). The common electron acceptors, in order from the highest to lowest efficiency of energy, are O2, NO −3 , Fe3+ and SO 2−4 . Microbes utilizing electron acceptors with a higher efficiency should be more competitive (Burgin and Hamilton 2007). In an anaerobic condition, NO −3 has a higher energy efficiency than SO 2−4 . Denitrifiers (i.e., sulfur oxidizers) can easily use NO −3 as an electron acceptor, oxidizing reduced S to SO 2−4 . At our study site, the load of reduced sulfur (such as AVS) from the protein mineralization in domestic wastewater might be the main reason for SO 2−4 production corresponding to NO −3 reduction.
We investigated the SO 2−4 production under different NO −3 input concentrations. As illustrated in Fig. 1, the SO 2−4 production rates did not increase with the NO −3 removal linearly. The results were explained as follows. Because of the high NO −3 removal rates, the electron acceptors (NO −3 ) were always rich in the reactions. Therefore, NO −3 was not a limiting factor for the processes of reducing NO −3 to N2 in the form of denitrification and for reducing NO −3 to NH +4 in the form of DNRA. Although some forms of reduced sulfur are not available for microbial NO −3 reduction (Haaijer et al. 2007), the amount of reactive reduced sulfur (AVS) in this study was sufficient for the sulfide-driven NO −3 reduction process. The process was mainly controlled by the electrons transferred in the reaction of S2− oxidizing to SO 2−4 by relative bacteria. The SO 2−4 production rates driven by NO −3 removal could be characterized by the Michaelis–Menten function as follows (Laverman et al. 2010):
where: R s is the SO 2−4 production rate; C is the concentration of NO −3 removal in the reactor; R max is the maximum reaction rate; and K m is the half-saturation concentration. As shown in Fig. 1, the data were fitted well by Eq. (3), with an R 2 of 0.900 to 0.988. From the fitting, we also obtained the maximum SO 2−4 production rates (R max) ranging from 0.196 to 0.903 mM h−1.
The molar ratio of SO 2−4 production to NO −3 removal (SP/NR) indicates the relative importance of SO 2−4 production in NO −3 removal (Burgin and Hamilton 2008). In this study, the ratio of SP/NR decreased from 26 to 1.9 % with the increase of NO −3 input concentrations. The results suggest that the sulfide-driven NO −3 reduction process could not be neglected because it accounted for up to 26 % of the total NO −3 removal under the low NO −3 condition (0.2 mM).
4.3 The pathways of nitrate reduction with sulfide oxidation
In the FTR experiments, the NO −3 removal reached 72 to 91 % and NO −2 was hardly detected from the FTR output. The increase of nirS genes also indicated active NO −2 reduction. There were three possible pathways of NO −3 and NO −2 reduction in this process (Burgin and Hamilton 2007), i.e.: denitrification (NO −3 ➔ NO −2 ➔ NO ➔ N2O ➔ N2); DNRA (NO −3 ➔ NO −2 ➔ NH +4 ); and anaerobic ammonia oxidation (ANAMMOX) (NO −2 + NH +4 ➔ N2H4 ➔ N2). ANAMMOX is restricted to low NO −3 concentrations and comparative low C/N ratios (Burgin and Hamilton 2007). Its contribution to N2 release only accounts for 8 % in estuary sediment and 2 % in eutrophic marine sediment (Thamdrup and Dalsgaard 2002; Trimmer et al. 2003). With the high NO −3 concentrations in the FTR experiments, ANAMMOX should not be the main process.
Compared to denitrification, DNRA is a relatively understudied pathway of microbial metabolism (Burgin and Hamilton 2007). The DNRA is considered to be promoted by fermentation (Tiedje 1988). The pathway is often coupled with sulfur cycling and iron transfer (Korom 1992; An and Gardner 2002; Weber et al. 2006), though it is still unknown whether the two processes are mutually exclusive (Burgin and Hamilton 2007). Organic-rich and NO −3 -limited conditions favor the DNRA pathway (Megonigal et al. 2004; Laverman et al. 2006), while NO −3 -sufficient conditions with a suitable C/N ratio favor denitrification (Silver et al. 2001). To study the DNRA results, we measured NH +4 concentrations from the water output. Interestingly, the NH +4 production decreased with increased NO −3 input concentrations (see Fig. 2), which was different from the general recognition that NO −3 addition stimulates the DNRA aggrandizement (An and Gardner 2002). The ratio of NH +4 production to NO −3 removal in our study (1.04 to 6.07 % over 1 mM NO −3 input) showed that NH +4 production only accounted for a small fraction of the NO −3 removal. The explanation of the result was related to environmental conditions (Burgin and Hamilton 2007). The low C/N resulted from the high labile carbon concentration (see Table 1) with the high NO −3 inputs leading to high efficiency of respiratory denitrification. As shown in Fig. 4, the ratio of NH +4 production rates to NO −3 reduction rates (APR/NRR) described the theoretical threshold lines for the different processes (Laverman et al. 2006). With the low concentration (0.2 mM) of NO −3 input, APR/NRR values at all depths (except for 10–12 cm) were larger than 1.15, indicating that the DNRA was the main pathway of NO −3 removal. However, with an increase of the NO −3 input concentrations, the processes tended to shift to denitrification and incomplete denitrification. Laverman et al. (2006) assumed that the end-product of incomplete denitrification was NO −2 . However, in our study, the absence of NO −2 in the FTR output and the increase of nirS gene after the FTR culture suggested that NO −2 was probably transformed to NO, then to N2O.
Sulfide reduction may interfere with both denitrification (Fossing et al. 1995) and DNRA (Sayama et al. 2005). A high H2S concentration can inhibit denitrification and lead NO −3 to transfer to NH +4 (An and Gardner 2002; Mazéas et al. 2008). A low H2S concentration should stimulate NO −3 removal and inhibit denitrification at the same time, which resulted in N2O accumulation (Senga et al. 2006; Aelion and Warttinger 2009; Beristain-Cardoso et al. 2009). On the other hand, elemental S and metal-bound sulfides often present in fresh sediments should spur on S oxidizers to participate in denitrification (Burgin and Hamilton 2007). In this study, the H2S might be quickly consumed in the early stage of the FTR experiment. The ratio values of SO 2−4 and NH +4 productions ranged from 13.7 to 197 % (calculated from the SP/AR) with the increase of NO −3 input concentrations. Therefore, sulfide might stimulate the DNRA process with the low NO −3 concentrations (0.2–1.0 mM) and be involved in the denitrification with the higher NO −3 inputs (over 5.0 mM).
4.4 Vertical distributions of the sulfide-driven nitrate reduction
The NO −3 removal capacity in each layer of the sediment was almost the same (Eq. 2), which was mainly attributable to the substrate concentrations and environment conditions, with sufficient NO −3 and organic matter at all sediment depths. Adequate substrates enhanced NO −3 reducers to gain a competitive advantage. Similar prominent peaks appeared in all the T-RFLP profiles after the FTR experiments. The peaks matched Lactobacillus, which is a NO −2 reducer with products of N2O and CO2 (Dodds and Collins-Thompson 1985). The predominating population in the FTR kept the high rates of denitrification and mineralization. The narG genes amassed at depths of 5–7 cm and 10–12 cm before the FTR experiments and became uniform after the experiments, which was another line of evidence for the similar NO −3 reduction rates in all sediment layers. However, with lower NO −3 input concentrations, the NO −3 reduction pathways were different in the different layers (see Fig. 4). The N and C aggregation layer (10–12 cm) resulted in denitrification process with 0.2 mM NO −3 input.
In this study, the amount of AVS in each sediment slice was sufficient for the sulfide oxidation process. Therefore, the difference of SO 2−4 production among the depths might be caused by the diversity of sulfide oxidizers in the different layers. Thiobacillus, Thiocapsa, and Beggiatoa identified in this study belong to the autotrophic denitrifiers (Zopfi et al. 2001; Kamp et al. 2006; Shao et al. 2010). However, Beggiatoa only appeared in the upper layers after the FTR experiments, while Thiobacillus and Thiocapsa only appeared in the deep layers. The relatively high abundance of S. halophila at 20–22 cm depth after the FTR experiments might be the reason for the highest SO 2−4 production among the layers. More accurate characterization of the microbial community among the layers is currently under investigation.
5 Conclusions
Flow-through reactor experiments with river sediment samples from depths of 0 to 30 cm and influent NO −3 concentrations of 0.2 to 30 mM were conducted to quantify the NO −3 removal process with SO 2−4 production. Molecular biology analyses were carried out to study the microbial processes involved. With the different NO −3 input concentrations and the different sediment samples, the NO −3 removal reached 72 to 91 % and increased with SO 2−4 production. The potential SO 2−4 production rates ranged from 0.196 to 0.903 mM h−1. With the NO −3 input concentration of 0.2 mM, the main pathway of NO −3 reduction in the FTR experiments was through DNRA, whereas with higher NO −3 input concentrations the main pathway of NO −3 reduction was through denitrification. The results of NO −3 removal at different depths were similar. Nevertheless, the results of SO 2−4 production along the depths were different because of the spatial variation of sulfide oxidation organisms. The results from this study should be useful to help understand the potential processes of N removal from rivers and their sediments.
References
Aelion CM, Warttinger U (2009) Low sulfide concentrations affect nitrate transformations in freshwater and saline coastal retention pond sediments. Soil Biol Biochem 41:735–741
An S, Gardner WS (2002) Dissimilatory nitrate reduction to ammonium (DNRA) as a nitrogen link versus denitrification as a sink in a shallow estuary (Laguna Madre/Baffin Bay, Texas). Mar Ecol Prog Ser 237:41–50
Beristain-Cardoso R, Texier A, Sierra-Álvarez R, Razo-Flores E, Field JA, Gomez J (2009) Effect of initial sulfide concentration on sulfide and phenol oxidation under denitrifying conditions. Chemosphere 74:200–205
Boyer EW, Howarth RW, Galloway JN, Dentener FJ, Green PA, Vörösmarty CJ (2006) Riverine nitrogen export from the continents to the coasts. Global Biogeochem Cycle 20:GB1S91
Braker G, Fesefeldt A, Witzel K (1998) Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl Environ Microb 64:3769–3775
Brettar I, Rheinheimer G (1991) Denitrification in the central Baltic: evidence for H2S-oxidation as motor of denitrification at the oxic–anoxic interface. Mar Ecol Prog Ser 77:157–169
Brunet RC, Garcia-Gil LJ (1996) Sulfide-induced dissimilatory nitrate reduction to ammonia in anaerobic freshwater sediments. FEMS Microbiol Ecol 21:131–138
Burgin AJ, Hamilton SK (2007) Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front Ecol Environ 5:89–96
Burgin AJ, Hamilton SK (2008) NO3-driven SO 2−4 production in freshwater ecosystems: Implications for N and S cycling. Ecosystems 11:908–922
Chen F, Yang Y, Zhang D, Zhang L (2006) Heavy metals associated with reduced sulfur in sediments from different deposition environments in the Pearl River estuary, China. Environ Geochem Health 28:265–272
Dai M, Wang L, Guo X, Zhai W, Li Q, He B, Kao S-J (2006) Nitrification and inorganic nitrogen distribution in a large perturbed river/estuarine system: the Pearl River Estuary, China. Biogeosciences 5:1227–1244
Dodds KL, Collins-Thompson DL (1985) Production of N2O and CO2 during the reduction of NO −2 by Lactobacillus lactis TS4. Appl Environ Microb 50:1550–1552
Fan LF, Shieh WY, Wu WF, Chen CP (2006) Distribution of nitrogenous nutrients and denitrifiers strains in estuarine sediment profiles of the Tanshui River, northern Taiwan. Estuar Coast Shelf Sci 69:543–553
Farías L, Graco M, Ulloa O (2004) Temporal variability of nitrogen cycling in continental-shelf sediments of the upwelling ecosystem off central Chile. Deep-Sea Res Pt Ii 51:2491–2505
Forshay KJ, Stanley EH (2005) Rapid nitrate loss and denitrification in a temperate river floodplain. Biogeochemistry 75:43–64
Fossing H, Gallardo VA, Jørgensen BB et al (1995) Concentration and transport of nitrate by the mat-forming sulfur bacterium Thioploca. Nature 374:713–715
Galloway JN, Dentener FJ, Capone DG et al (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Haaijer SCM, Lamers LPM, Smolders AJP, Jetten MSM, Op den Camp HJM (2007) Iron sulfide and pyrite as potential electron donors for microbial nitrate reduction in freshwater wetlands. Geomicrobiol J 24:391–401
Hordijk CA, Snieder M, Van Engelen JJM, Cappenberg TE (1987) Estimation of bacterial nitrate reduction rates at in situ concentrations in freshwater sediments. Appl Environ Microbiol 53:217–223
Huang S, Chen C, Yang X, Wu Q, Zhang R (2011) Distribution of typical denitrifying functional genes and diversity of the nirS-encoding bacterial community related to environmental characteristics of river sediments. Biogeosciences 8:5251–5280
Jiang LJ, Zheng YP, Peng XT (2009) Vertical distribution and diversity of sulfate-reducing prokaryotes in the Pearl River estuarine sediments, Southern China. FEMS Microbiol Ecol 70:249–262
Jørgensen BB, Gallardo VA (1999) Thioploca spp.: filamentous sulfur bacteria with nitrate vacuoles. FEMS Microbiol Ecol 28:301–313
Kamp A, Stief P, Schulz-Vogt HN (2006) Anaerobic sulfide oxidation with nitrate by a freshwater Beggiatoa enrichment culture. Appl Environ Microb 72:4755–4760
Kojima H, Fukui M (2003) Phylogenetic analysis of Beggiatoa spp. from organic rich sediment of Tokyo Bay, Japan. Water Res 37:3216–3223
Korom SF (1992) Natural denitrification in the saturated zone: a review. Water Resour Res 28:1657–1668
Laverman AM, Van Cappellen P, van Rotterdam-Los D, Pallud C, Abell J (2006) Potential rates and pathways of microbial nitrate reduction in coastal sediments. FEMS Microbiol Ecol 58:179–192
Laverman AM, Canavan RW, Slomp CP, Van Cappellen P (2007) Potential nitrate removal in a coastal freshwater sediment (Haringvliet Lake, The Netherlands) and response to salinization. Water Res 41:3061–3068
Laverman AM, Garnier JA, Mounier EM, Roose-Amsaleg CL (2010) Nitrous oxide production kinetics during nitrate reduction in river sediment. Water Res 44:1753–1764
Li F, Yang R, Ti C, Lang M, Kimura SD, Yan X (2010) Denitrification characteristics of pond sediments in a Chinese agricultural watershed. Soil Sci Plant Nutr 56:66–71
Lu FH, Ni HG, Liu F, Zeng EY (2009) Occurrence of nutrients in riverine runoff of the Pearl River Delta, South China. J Hydrol 376:107–115
Ma HB, Aelion CM (2005) Ammonium production during microbial nitrate removal in soil microcosms from a developing marsh estuary. Soil Biol Biochem 37:1869–1878
Magalhães CM, Joye SB, Moreira RM, Wiebe WJ, Bordalo AA (2005) Effect of salinity and inorganic nitrogen concentration on nitrification and denitrification rates in intertidal sediments and rocky biofilms of the Douro River estuary, Portugal. Water Res 39:1783–1794
Mazéas L, Vigneron V, Le-Menach K, Budzinski H, Audic JM, Bernet N, Bouchez T (2008) Elucidation of nitrate reduction pathways in anaerobic bioreactors using a stable isotope approach. Rapid Commun Mass Spectrom 22:1746–1750
Megonigal JP, Hines ME, Visscher PT (2004) Anaerobic metabolism: linkages to trace gases and aerobic processes. In: Schlesinger WH (ed) Biogeochemistry. Elsevier-Pergamon, Oxford, pp 317–424
Pallud C, Meile C, Laverman AM, Abell J, Van Cappellen P (2007) The use of flow-through sediment reactors in biogeochemical kinetics: methodology and examples of applications. Mar Chem 106:256–271
Payne EK, Burgin AJ, Hamilton SK (2009) Sediment nitrate manipulation using porewater equilibrators reveals potential for N and S coupling in freshwaters. Aquat Microb Ecol 54:233–241
Piña-Ochoa E, Álvarez-Cobelas M (2006) Denitrification in aquatic environments: a cross-system analysis. Biogeochemistry 81:111–130
Qiu D, Huang L, Zhang J, Lin S (2010) Phytoplankton dynamics in and near the highly eutrophic Pearl River Estuary, South China Sea. Cont Shelf Res 30:177–186
Reyna L, Wunderlin DA, Genti-Raimondi S (2010) Identification and quantification of a novel nitrate-reducing community in sediments of Suquía River basin along a nitrate gradient. Environ Pollut 158:1608–1614
Sayama M, Risgaard-Petersen N, Nielsen LP, Fossing H, Christensen PB (2005) Impact of bacterial NO −3 transport on sediment biogeochemistry. Appl Environ Microb 71:7575–7577
Scala DJ, Kerkhof LJ (1998) Nitrous oxide reductase (nosZ) gene-specific PCR primers for detection of denitrifiers and three nosZ genes from marine sediments. FEMS Microbiol Lett 162:61–68
Seitzinger S, Harrison JA, Bohlke JK, Bouwman AF, Lowrance R, Peterson B, Tobias C, Van Drecht G (2006) Denitrification across landscapes and waterscapes: a synthesis. Ecol Appl 16:2064–2090
Senga Y, Mochida K, Fukumori R, Okamoto N, Seike Y (2006) N2O accumulation in estuarine and coastal sediments: the influence of H2S on dissimilatory nitrate reduction. Estuar Coast Shelf Sci 67:231–238
Shao M, Zhang T, Fang HH (2010) Sulfur-driven autotrophic denitrification: diversity, biochemistry, and engineering applications. Appl Microbiol Biotechnol 88:1027–1042
Sheng Y, Fu G, Chen F, Chen J (2011) Geochemical characteristics of inorganic sulfur in Shijing River, South China. J Environ Monit 13:807–812
Silvennoinen H, Liikanen A, Torssonen J, Stange CF, Martikainen PJ (2008) Denitrification and N2O effluxes in the Bothnian Bay (northern Baltic Sea) river sediments as affected by temperature under different oxygen concentrations. Biogeochemistry 88:63–72
Silver WL, Herman DJ, Firestone MK (2001) Dissimilatory nitrate reduction to ammonium in upland tropical forest soils. Ecology 82:2410–2416
Smith CJ, Nedwell DB, Dong LF, Osborn AM (2007) Diversity and abundance of nitrate reductase genes (narG and napA), nitrite reductase genes (nirS and nrfA), and their transcripts in estuarine sediments. Appl Environ Microb 73:3612–3622
Strous M, Pelletier E, Mangenot S et al (2006) Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 440:790–794
Thamdrup B, Dalsgaard T (2002) Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl Environ Microb 68:1312–1318
Thräback IN, Enwall K, Jarvis A, Hallin S (2004) Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol Ecol 49:401–417
Tiedje JM (1988) Ecology of denitrification and dissimilatory nitrate reduction to ammonium. In: Zehnder AJB (ed) Environmental microbiology of anaerobes. Wiley, New York, pp 179–244
Tiquia SM, Masson SA, Devol A (2006) Vertical distribution of nitrite reductase genes (nirS) in continental margin sediments of the Gulf of Mexico. FEMS Microbiol Ecol 58:464–475
Trimmer M, Nicholls JC, Deflandre B (2003) Anaerobic ammonium oxidation measured in sediments along the Thames estuary, United Kingdom. Appl Environ Microb 69:6447–6454
Weber KA, Urrutia MM, Churchill PF, Kukkadapu RK, Roden EE (2006) Anaerobic redox cycling of iron by freshwater sediment microorganisms. Environ Microbiol 8:100–113
Woese CR, Mandelco L, Yang D, Gherna R, Madigan MT (1990) The case for relationship of the flavobacteria and their relatives to the green sulfur bacteria. Syst Appl Microbiol 13:258–262
Zhang T, Zhang M, Shao MF, Fang HHP (2009) Autotrophic denitrification in nitrate-induced marine sediment remediation and Sulfurimonas denitrificans-like bacteria. Chemosphere 76:677–682
Zopfi J, Kjaer T, Nielsen LP, Jørgensen BB (2001) Ecology of Thioploca spp.: nitrate and sulfur storage in relation to chemical microgradients and influence of Thioploca spp. on the sedimentary nitrogen cycle. Appl Environ Microb 67:5530–5537
Acknowledgments
This work was partly supported by grants from the Chinese National Natural Science Foundation (Nos. 51039007 and 51179212) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Marcel van der Perk
Rights and permissions
About this article
Cite this article
Yang, X., Huang, S., Wu, Q. et al. Nitrate reduction coupled with microbial oxidation of sulfide in river sediment. J Soils Sediments 12, 1435–1444 (2012). https://doi.org/10.1007/s11368-012-0542-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-012-0542-9